β-Carbolines in Experiments on Laboratory Animals
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Standards
2.2. Raw Materials
2.3. The Experimental Diet
2.4. Animal Experiment
2.5. β-Carboline Determination in Feed, Chicory, and Animal Blood
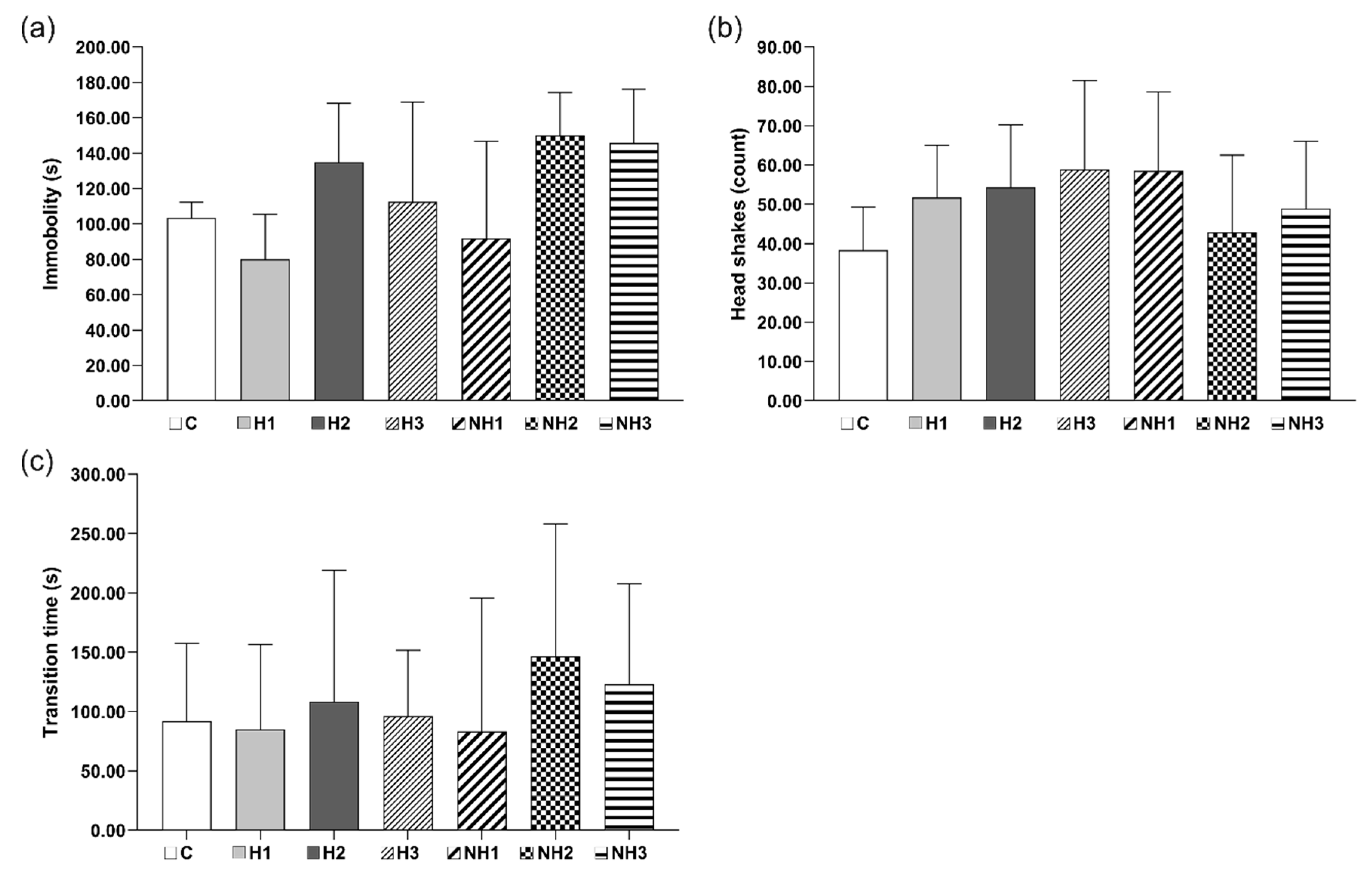
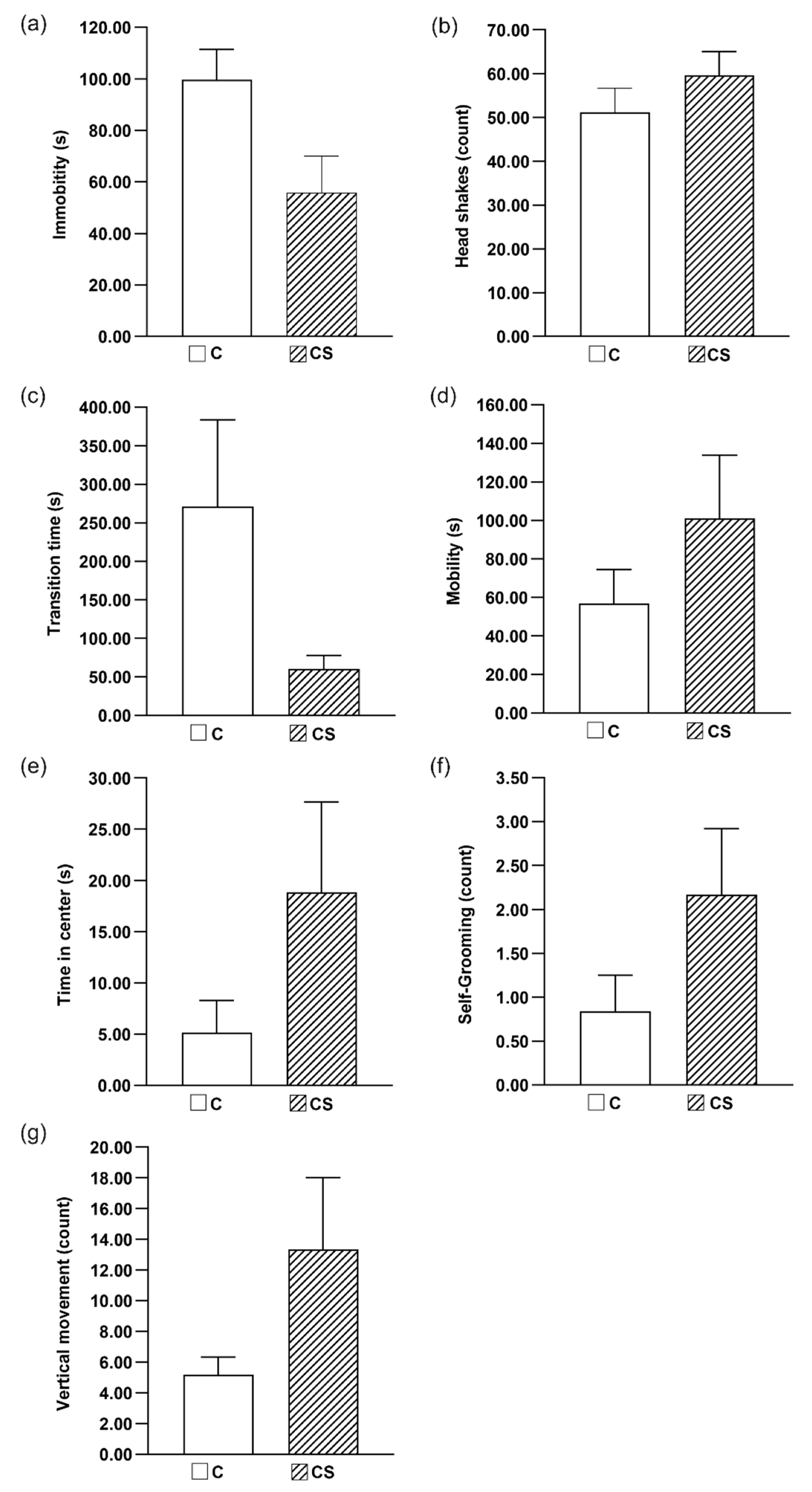
2.6. Behavioral Tests
2.6.1. Classical Labyrinth Test
2.6.2. Open Field Test (OFT)
2.6.3. The Forced Swim Test (FST)
2.7. Fecal Analysis
2.8. In Vitro Experiments
2.8.1. Cell Culture
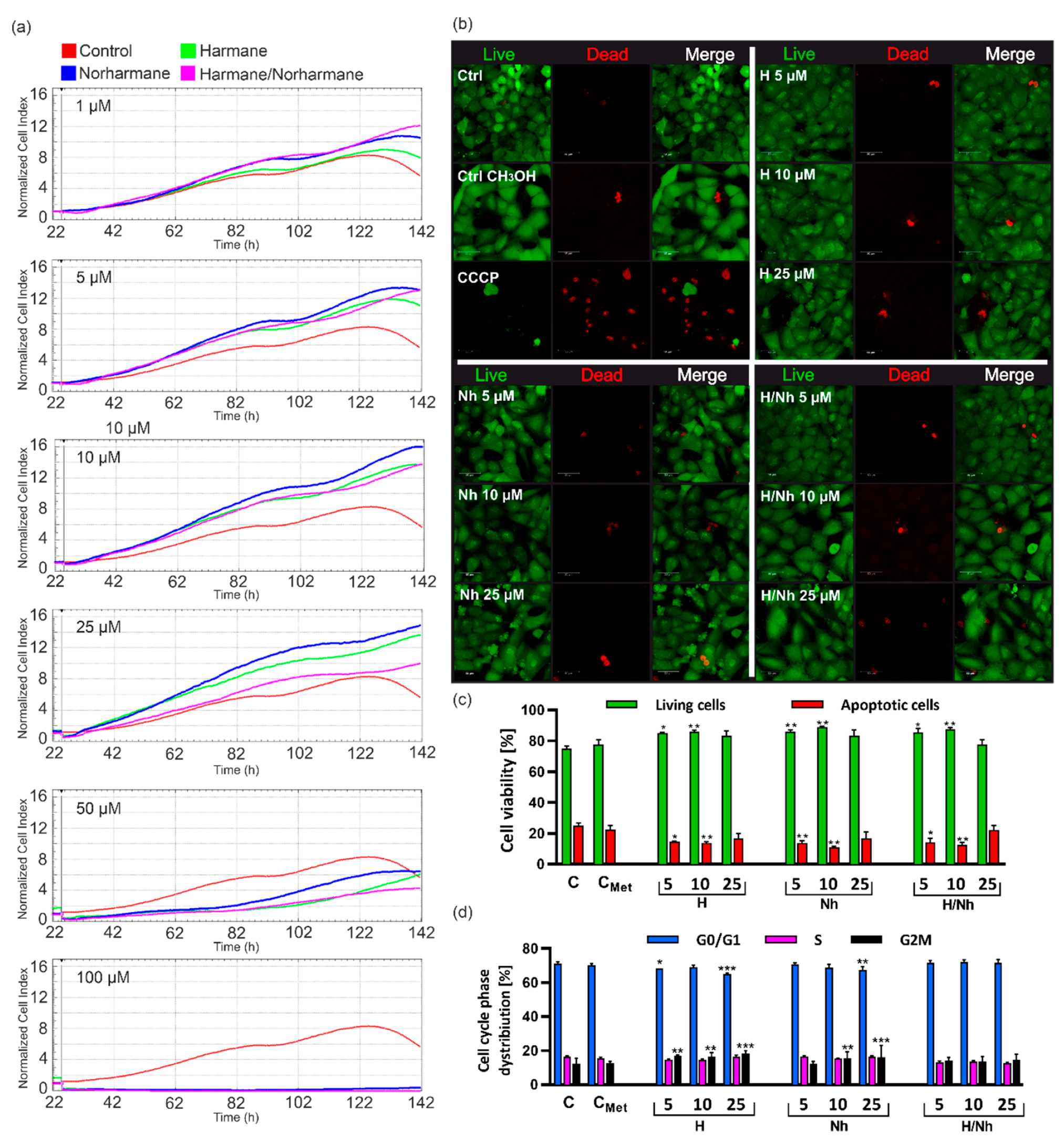
2.8.2. Real-Time Cell Proliferation Analysis by xCELLigence System
2.8.3. Confocal Microscopy Analysis of Cell Viability
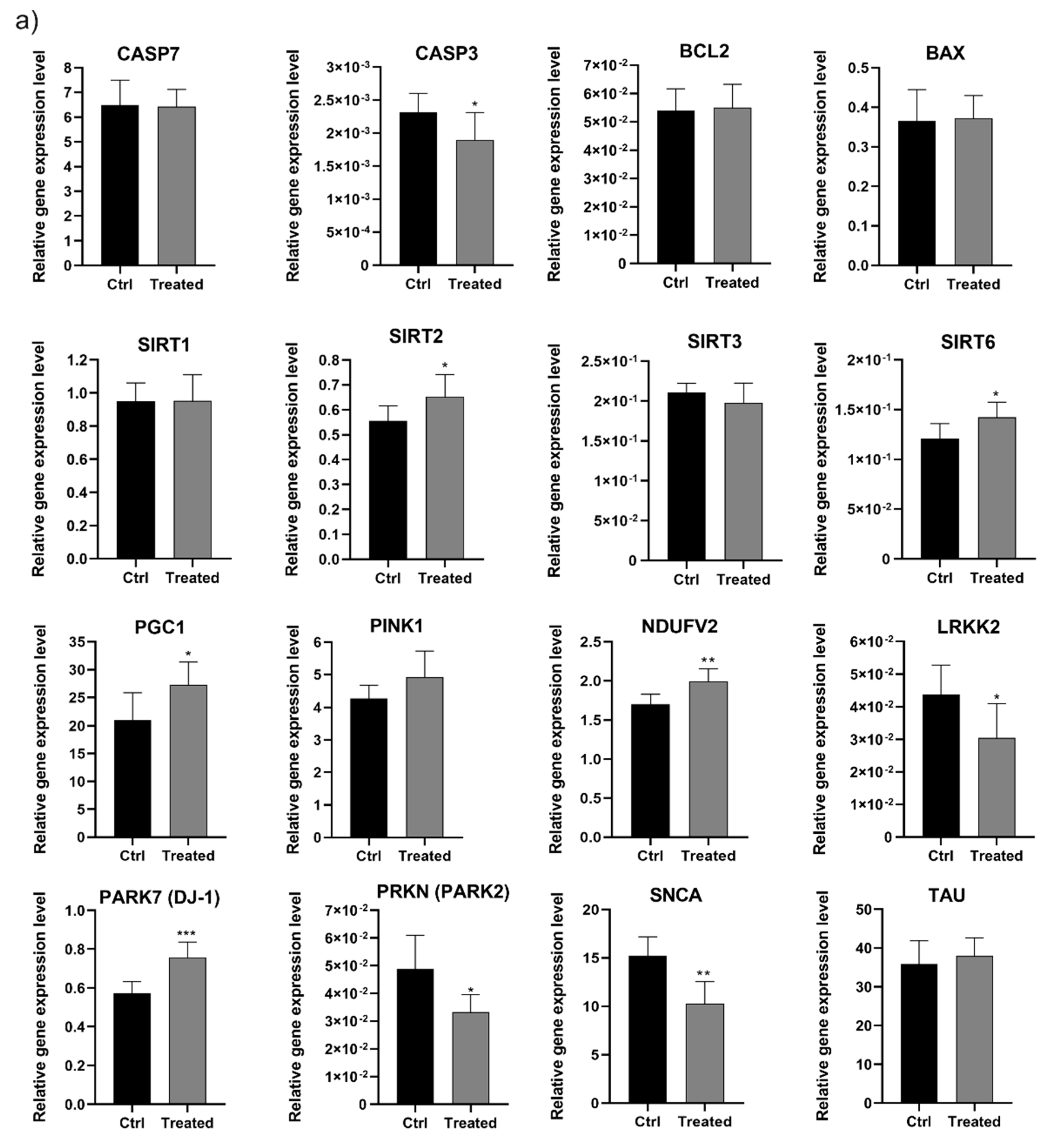
2.8.4. Apoptosis/Necrosis Assay
2.8.5. Cell Cycle Analysis
2.8.6. Mitochondrial Membrane Potential (ΔΨm) Analysis
2.8.7. Total RNA Isolation
2.8.8. cDNA Synthesis and Real-Time PCR
2.9. Statistical Methods
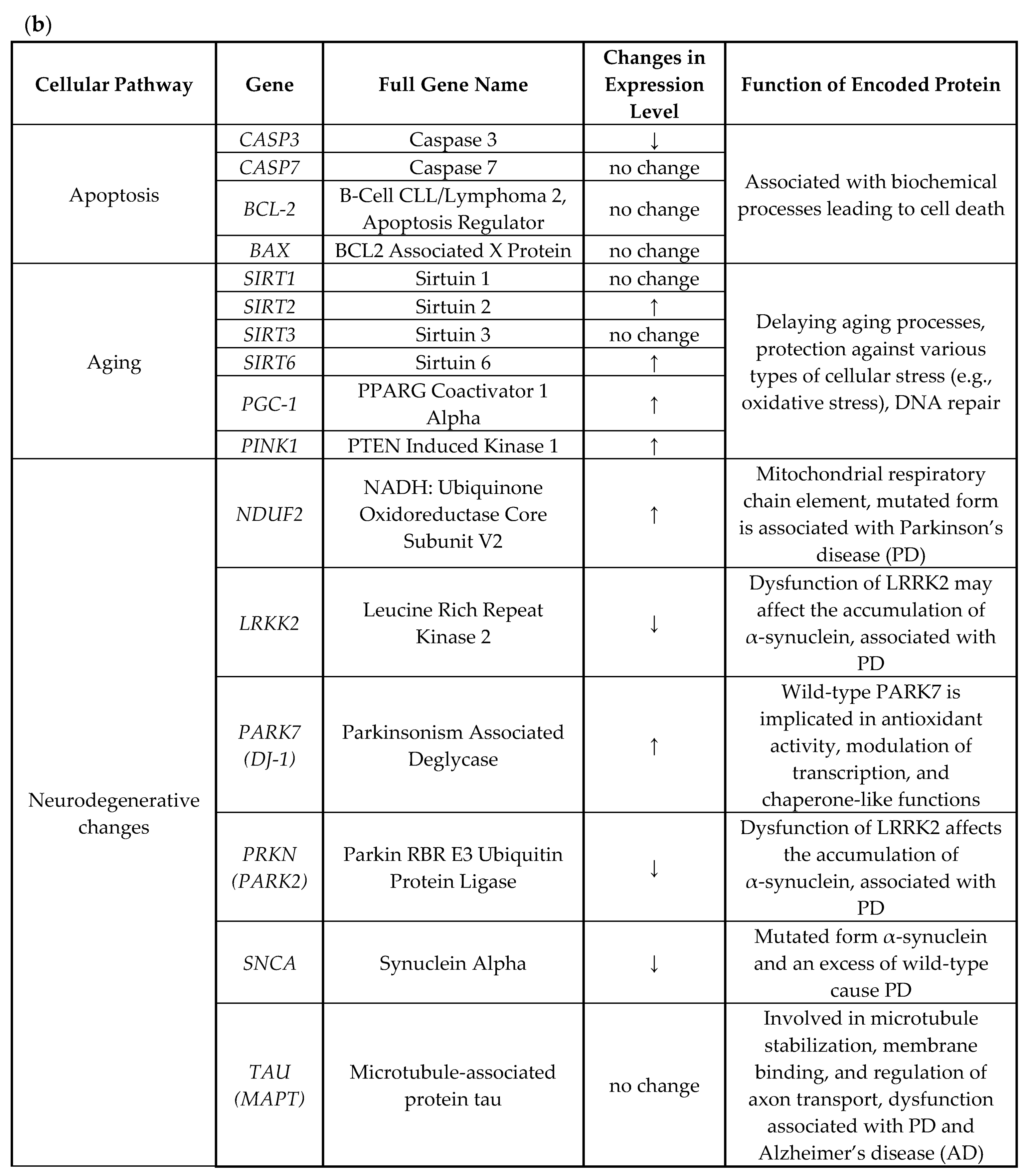
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfau, W.; Skog, K. Exposure to b-carbolines norharman and harman. J. Chromatogr. B 2004, 802, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Relative exposure to β-carbolines norharman and harman from foods and tobacco smoke. Food Addit. Contam. 2004, 21, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Celikyurt, I.K.; Utkan, T.; Gocmez, S.S.; Hudson, A.; Aricioglu, F. Effect of harmane, an endogenous β-carboline, on learning and memory in rats. Pharmacol. Biochem. Behav. 2013, 103, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Michalec, M.; Jiang, W.; Factor-Litvak, P.; Zheng, W. Elevated blood harmane (1-methyl-9H-pyrido(3,4-b)indole) concentrations in Parkinson’s disease. Neurotoxicology 2014, 40, 52–56. [Google Scholar] [CrossRef][Green Version]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness: Monoamine oxidase. Br. J. Pharmacol. 2009, 147, S287–S296. [Google Scholar] [CrossRef]
- Baum, S.S.; Hill, R.; Rommelspacher, H. Harman-induced changes of extracellular concentrations of neurotransmitters in the nucleus accumbens of rats. Eur. J. Pharmacol. 1996, 314, 75–82. [Google Scholar] [CrossRef]
- Farzin, D.; Mansouri, N. Antidepressant-like effect of harmane and other β-carbolines in the mouse forced swim test. Eur. Neuropsychopharmcol. 2016, 16, 324–328. [Google Scholar] [CrossRef]
- Li, S.; Teng, L.; Liu, W.; Cheng, X.; Jiang, B.; Wang, Z.; Wang, C. Pharmacokinetic study of harmane and its 10 metabolites in rat after intravenous and oral administration by UPLC-ESI-MS/MS. Pharm. Biol. 2016, 54, 1768–1781. [Google Scholar] [CrossRef]
- Paul, H.A.; Bomhof, M.R.; Vogel, H.J.; Reimer, R.A. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci. Rep. 2016, 6, 20683. [Google Scholar] [CrossRef]
- Gonzalez-Fuentes, J.; Moya, C.; Castro-Vázquez, L.; Lozano, M.; Marcos, P.; Plaza-Oliver, M.; Rodríguez-Robledo, V.; Santander-Ortega, M.; Villaseca-González, N.; Arroyo-Jimenez, M. Neuroprotective natural molecules, from food to brain. Front. Neurosci. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Morales, P.L.; Liste, I. Stem cells as in Vitro model of Parkinson’s disease. Stem Cells Int. 2012, 2012, 980941. [Google Scholar]
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-carbolines in food: A review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Shmatova, J.; Nenajdenko, V.; Bhadra, K. Trifluoromethylaterd carboline compounds targetin DNA: Synthesis, binding and anti-proliferative effects on human cancer cell lines. Bioorg. Chem. 2019, 80, 61–79. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Wojtowicz, E.; Zawirska-Wojtasiak, R.; Przygoński, K.; Mildner-Szkudlarz, S. Bioactive β-carbolines norharman and harman in traditional and novel raw materials for chicory coffee. Food Chem. 2015, 175, 280–283. [Google Scholar] [CrossRef]
- Adachi, J.; Mizoi, Y.; Naito, T.; Yamamoto, K.; Fujiwara, S.; Ninomiya, I. Determination of β-carbolines in foodstuffs by high-performance liquid chromatography and high-performance liquid chromatography-mass spectrometry. J. Chromatogr. A 1991, 538, 331–339. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.; Barnes, L.F.; Guan, Y.; Louis, E.D. Determination of harmane and harmine in human blood using reversed-phased high-performance liquid chromatography and fluorescence detection. Anal. Biochem. 2000, 279, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, L.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljeström, M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Piechowska, P.; Wojtowicz, E.; Przygoński, K.; Mildner-Szkudlarz, S. Bioactivity of selected materials for coffee substitute. PLoS ONE 2018, 13, e0206762. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Li, S.; Zhang, Y.; Deng, G.; Wang, Y.; Qi, S.; Cheng, X.; Ma, Y.; Xie, Y.; Wang, C. Exposure Characteristics of the Analogous β-Carboline Alkaloids Harmaline and Harmine Based on the Efflux Transporter of Multidrug Resistance Protein 2. Front. Pharmacol. 2017, 8, 541. [Google Scholar] [CrossRef]
- Dai, J.; Dan, W.; Schneider, U.; Wang, J. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.; Wang, H.-K.; Bastow, K.F.; Hu, C.-Q.; Lee, K.-H. Antitumor agents 201.1 Cytotoxicity of harmine and β-carboline analogs. Bioorg. Med. Chem. Lett. 1999, 9, 3319–3324. [Google Scholar] [CrossRef]
- Hamann, J.; Wernicke, C.; Lehmann, J.; Reichmann, H.; Rommelspacher, H.; Gille, G. 9-Methyl-β-carboline up-regulates the appearance of differentiated dopaminergic neurones in primary mesencephalic culture. Neurochem. Int. 2008, 52, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Polanski, W.; Enzensperger, C.; Reichmann, H.; Gille, G. The exceptional properties of 9-methyl-β-carboline: Stimulation, protection and regeneration of dopaminergic neurons coupled with anti-inflammatory effects. J. Neurochem. 2010, 113, 1659–1675. [Google Scholar] [CrossRef]
- Hamann, J.; Rommelspacher, H.; Storch, A.; Reichmann, H.; Gille, G. Neurotoxic mechanisms of 2,9-dimethyl-beta-carbolinium ion in primary dopaminergic culture. J. Neurochem. 2006, 98, 1185–1199. [Google Scholar] [CrossRef]
- Poon, R.Y. Cell cycle control: A system of interlinking oscillators. Methods Mol. Biol. 2016, 1342, 3–19. [Google Scholar]
- Kitagishi, Y.; Nakano, N.; Ogino, M.; Ichimura, M.; Minami, A.; Matsuda, S. PINK1 signaling in mitochondrial homeostasis and in aging (Review). Int. J. Mol. Med. 2016, 39, 3–8. [Google Scholar] [CrossRef]
- Pan, Y.; Nishida, Y.; Wang, M.; Verdin, E. Metabolic regulation, mitochondria and the life-prolonging effect of rapamycin: A mini-review. Gerontology 2012, 58, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. Antioxidant properties of β-carbolines. In Isoquinolines and Beta-Carbolines as Neurotoxins and Neuroprotectants: New Vistas in Parkinson’s Disease Therapy. Current Topics in Neurotoxicity, 1st ed.; Springer: Boston, MA, USA, 2012; pp. 133–144. [Google Scholar]
- Moura, D.J.; Richter, M.F.; Boeira, J.M.; Pêgas Henriques, J.A.; Saffi, J. Antioxidant properties of β-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 2007, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wang, Y.W.; Qi, S.L.; Zhang, Y.P.; Deng, G.; Ding, W.Z.; Ma, C.; Lin, Q.Y.; Guan, H.D.; Liu, W.; et al. Analogous β-Carboline Alkaloids Harmaline and Harmine Ameliorate Scopolamine-Induced Cognition Dysfunction by Attenuating Acetylcholinesterase Activity, Oxidative Stress, and Inflammation in Mice. Front. Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, W.; Müller, T.; Große, H.; Dierks, T.; Rommelspacher, H. Plasma levels of the beta-carbolines harman and norharman in Parkinson’s disease. Acta Neurol. Scand. 1995, 92, 451–454. [Google Scholar] [CrossRef]
- Rommelspacher, H.; Schmidt, L.G.; May, T. Plasma norharman (beta-carboline) levels are elevated in chronic alcoholics. Alcohol. Clin. Exp. Res. 1991, 15, 553–559. [Google Scholar] [CrossRef]
- Gruss, M.; Appenroth, D.; Flubacher, A.; Enzensperger, C.; Bock, J.; Fleck, C.; Gille, G.; Braun, K. 9-Methyl-β-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendritic and synaptic proliferation. J. Neurochem. 2012, 121, 924–931. [Google Scholar] [CrossRef]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef]
- Giblin, W.; Skinner, M.E.; Lombard, D.B. Sirtuins: Guardians of mammalian healthspan. Trends Genet. 2014, 30, 271–286. [Google Scholar] [CrossRef]
- Anderson, R.; Prolla, T. PGC-1α in aging and anti-aging interventions. BBA Gen. Subj. 2009, 1790, 1059–1066. [Google Scholar] [CrossRef]
- Stefanis, L. α-Synuclein in Parkinson’s disease. CSH Perspect. Med. 2012, 4, a009399. [Google Scholar]
- Zhang, X.; Gao, F.; Wang, D.; Li, C.; Fu, Y.; He, W.; Zhang, J. Tau pathology in Parkinson’s disease. Front. Neurol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nguyen, M.; Qin, F.X.; Tong, Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007, 6, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Su, L.; Singhal, S.; Liu, X. Emerging roles of SIRT6 on telomere maintenance, DNA repair, metabolism and mammalian aging. Mol. Cell. Biochem. 2012, 364, 345–350. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Galindo, M.F.; Solesio, M.E.; Jordan, J. Role of mitochondrial fission and mitophagy in Parkinson’s disease. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging, 1st ed.; Academic Press: Main St. Salt Lake City, UT, USA; Volume 4, pp. 213–225.
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The role of LRRK2 in neurodegeneration of Parkinson disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef]
- Dawson, T.M.; Dawson, V.L. The role of parkin in familial and sporadic Parkinson’s disease. Mov. Disord. 2010, 25, 32–39. [Google Scholar] [CrossRef]
- Kahle, P.J.; Waak, J.; Gasser, T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radical Biol. Med. 2009, 47, 1354–1361. [Google Scholar] [CrossRef]
- Lev, N.; Barhum, Y.; Pilosof, N.S.; Ickowicz, D.; Cohen, H.Y.; Melamed, E.; Offen, D. DJ-1 protects against dopamine toxicity: Implications for Parkinson’s disease and aging. J. Gerontol. A Biol. 2013, 68, 215–225. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Kovalska, M.; Timkova, V.; Racay, P.; Lehotsky, J.; Kaplan, P. The effect of aging on mitochondrial complex I and the extent of oxidative stress in the rat brain cortex. Neurochem. Res. 2016, 41, 2160–2172. [Google Scholar] [CrossRef]
- Keller, S.; Polanski, W.H.; Enzensperger, C.; Reichmann, H.; Hermann, A.; Gille, G. 9-Methyl-β-carboline inhibits monoamine oxidase activity and stimulates the expression of neurotrophic factors by astrocytes. J. Neural. Transm. 2020, 127, 999–1012. [Google Scholar] [CrossRef] [PubMed]

| Animal Group | Harman (ng/mL) | SD | Norharman (ng/mL) | SD |
|---|---|---|---|---|
| Stage I | ||||
| C | 0.020 | 0.003 | 0.060 | 0.004 |
| H1 | 0.038 | 0.004 | 0.049 | 0.006 |
| H2 | 0.052 | 0.010 | 0.054 | 0.011 |
| H3 | 0.084 | 0.009 | 0.046 | 0.005 |
| NH1 | 0.018 | 0.008 | 0.080 | 0.039 |
| NH2 | 0.021 | 0.010 | 0.089 | 0.013 |
| NH3 | 0.018 | 0.005 | 0.107 | 0.022 |
| Stage II | ||||
| C | 0.010 | 0.001 | 0.067 | 0.007 |
| CS | 0.016 | 0.004 | 0.110 | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawirska-Wojtasiak, R.; Fedoruk-Wyszomirska, A.; Piechowska, P.; Mildner-Szkudlarz, S.; Bajerska, J.; Wojtowicz, E.; Przygoński, K.; Gurda, D.; Kubicka, W.; Wyszko, E. β-Carbolines in Experiments on Laboratory Animals. Int. J. Mol. Sci. 2020, 21, 5245. https://doi.org/10.3390/ijms21155245
Zawirska-Wojtasiak R, Fedoruk-Wyszomirska A, Piechowska P, Mildner-Szkudlarz S, Bajerska J, Wojtowicz E, Przygoński K, Gurda D, Kubicka W, Wyszko E. β-Carbolines in Experiments on Laboratory Animals. International Journal of Molecular Sciences. 2020; 21(15):5245. https://doi.org/10.3390/ijms21155245
Chicago/Turabian StyleZawirska-Wojtasiak, Renata, Agnieszka Fedoruk-Wyszomirska, Paulina Piechowska, Sylwia Mildner-Szkudlarz, Joanna Bajerska, Elżbieta Wojtowicz, Krzysztof Przygoński, Dorota Gurda, Wiktoria Kubicka, and Eliza Wyszko. 2020. "β-Carbolines in Experiments on Laboratory Animals" International Journal of Molecular Sciences 21, no. 15: 5245. https://doi.org/10.3390/ijms21155245
APA StyleZawirska-Wojtasiak, R., Fedoruk-Wyszomirska, A., Piechowska, P., Mildner-Szkudlarz, S., Bajerska, J., Wojtowicz, E., Przygoński, K., Gurda, D., Kubicka, W., & Wyszko, E. (2020). β-Carbolines in Experiments on Laboratory Animals. International Journal of Molecular Sciences, 21(15), 5245. https://doi.org/10.3390/ijms21155245







