The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells
Abstract
1. Introduction
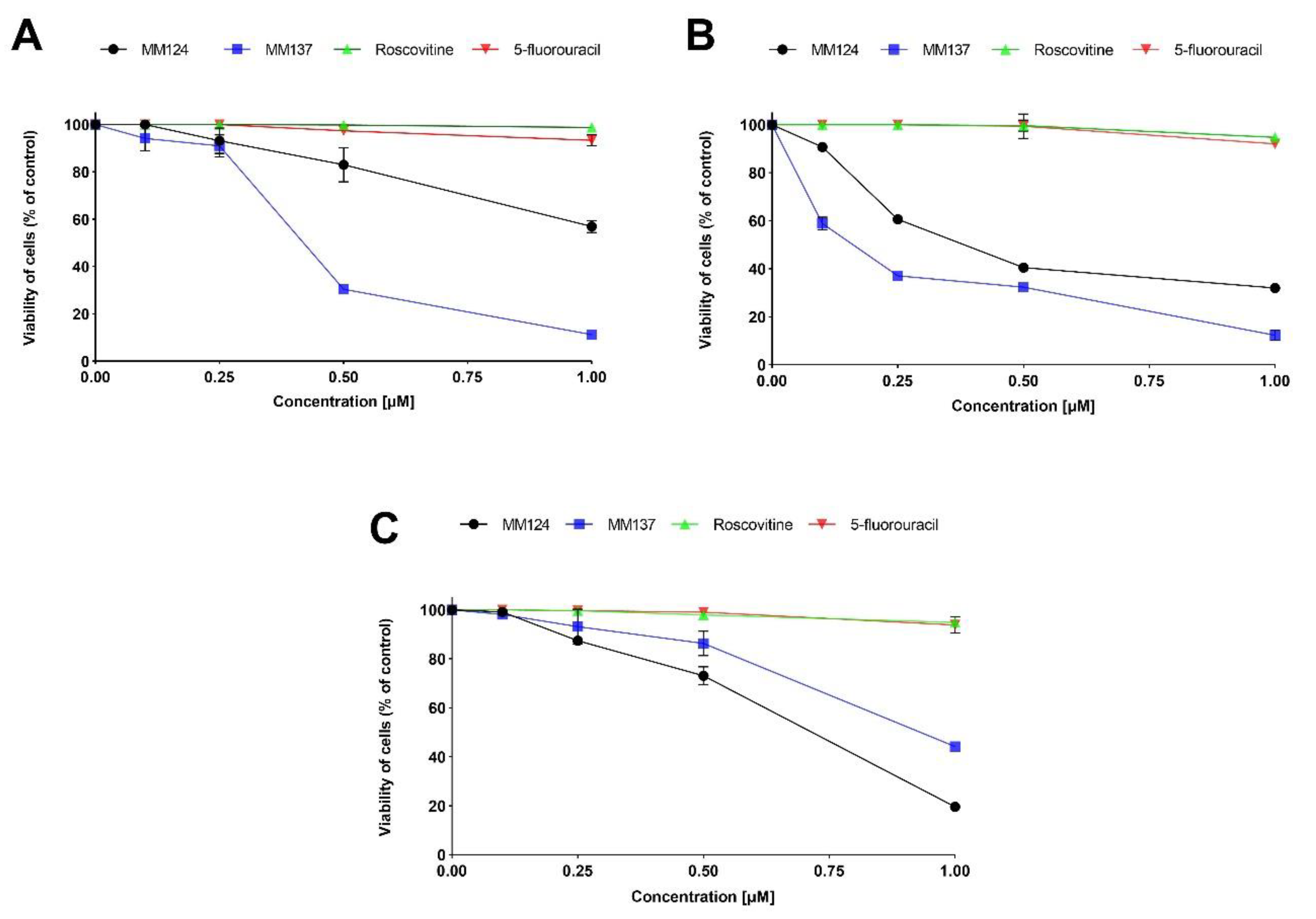
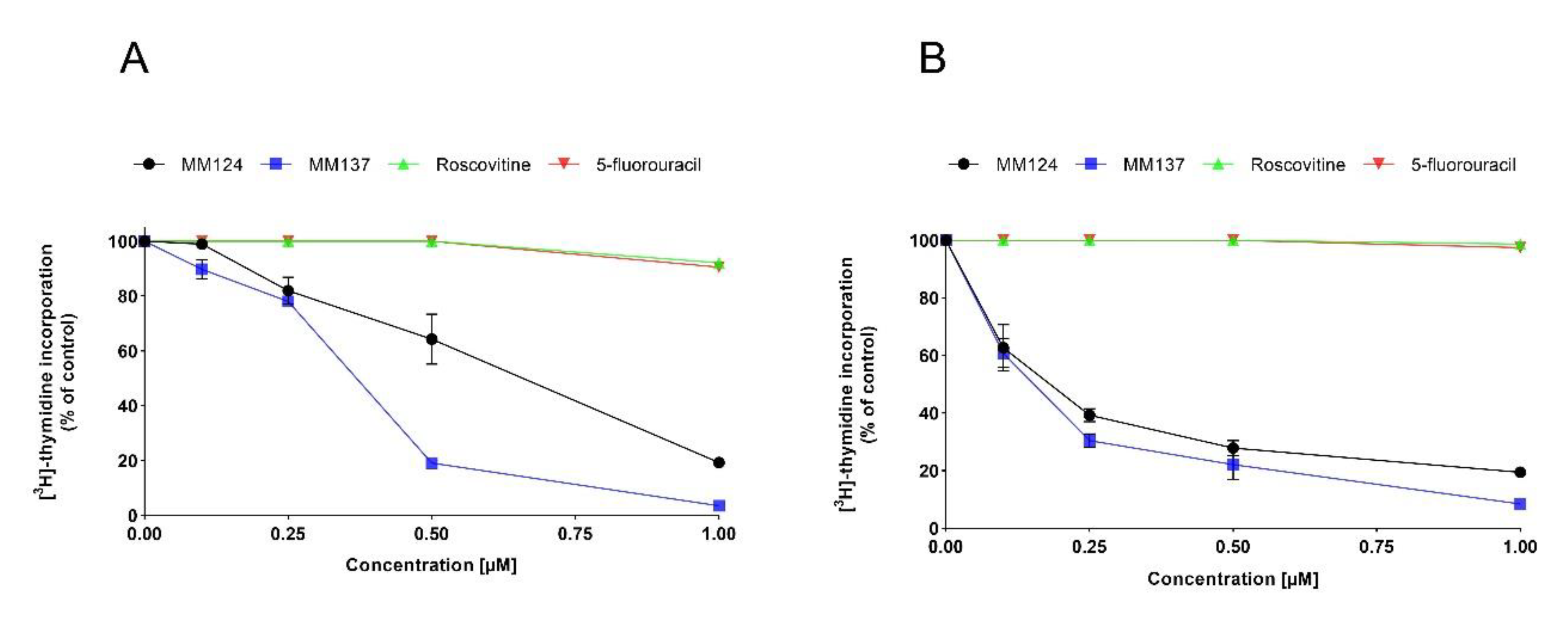
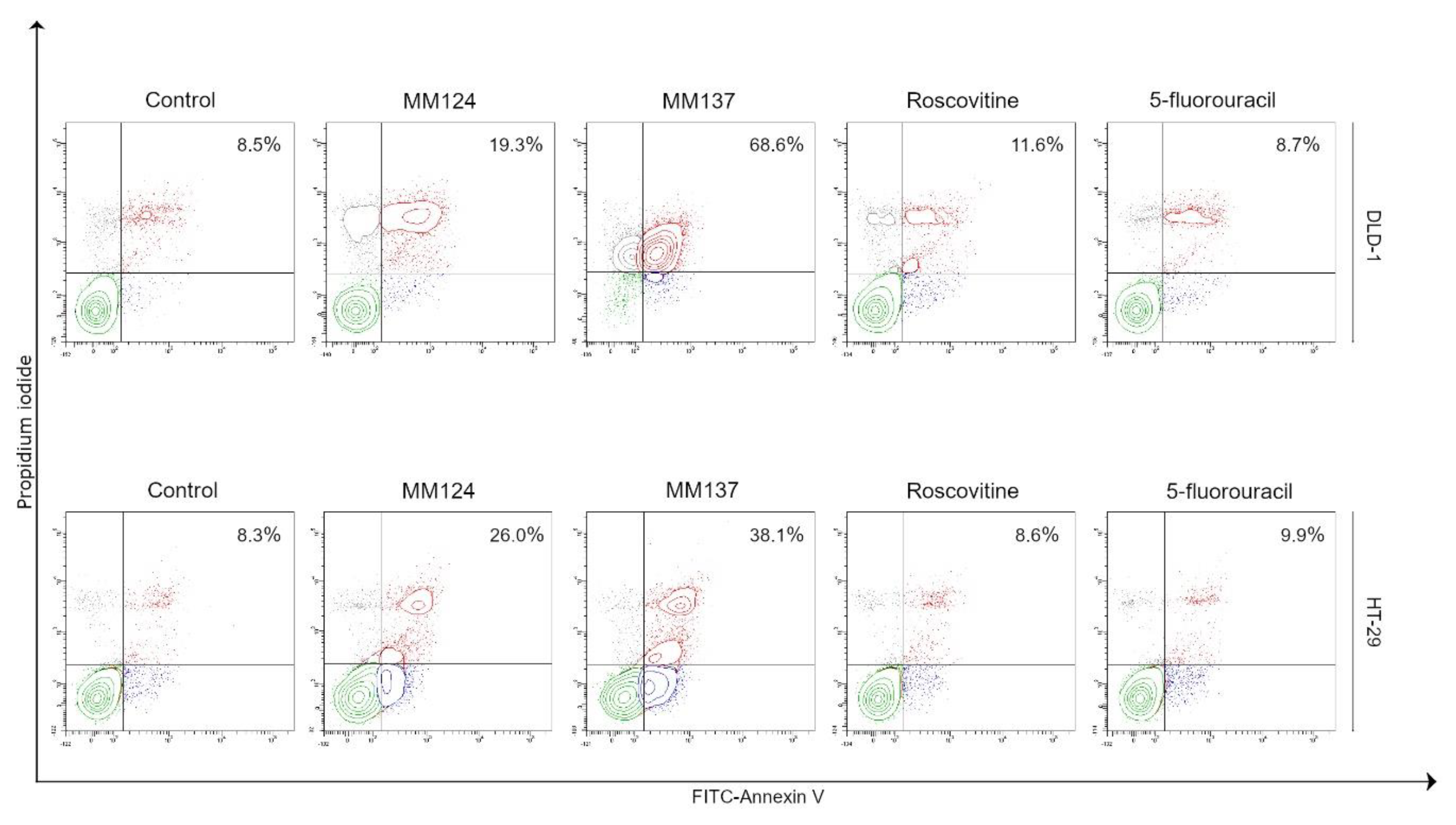
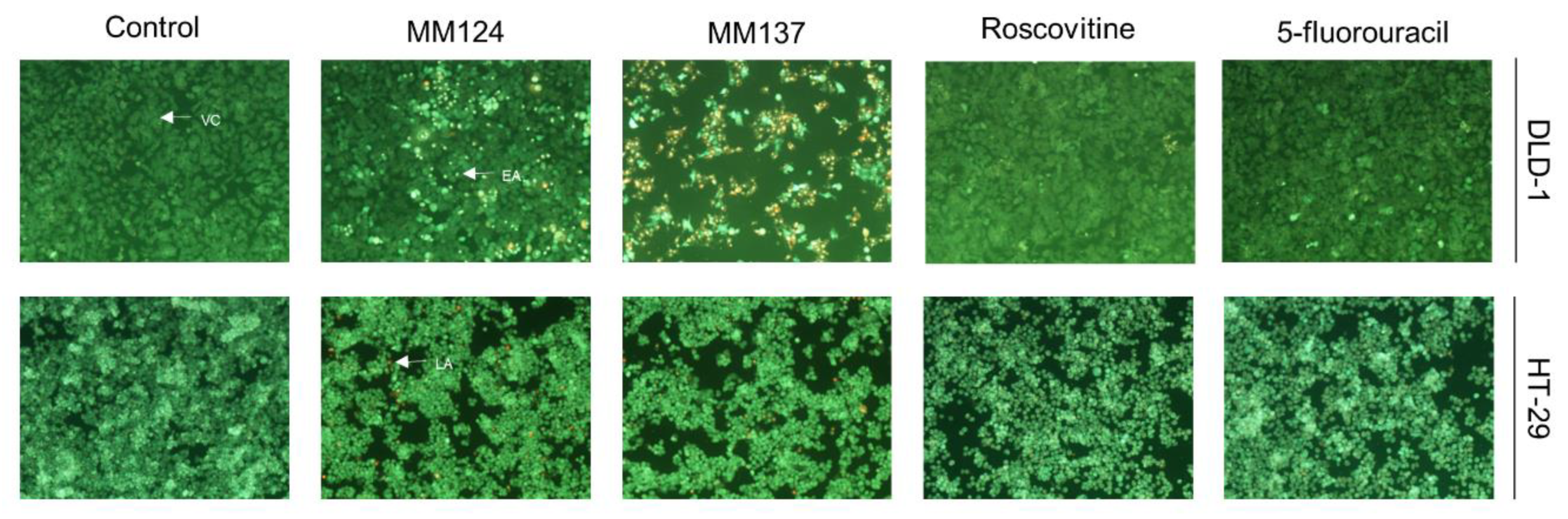
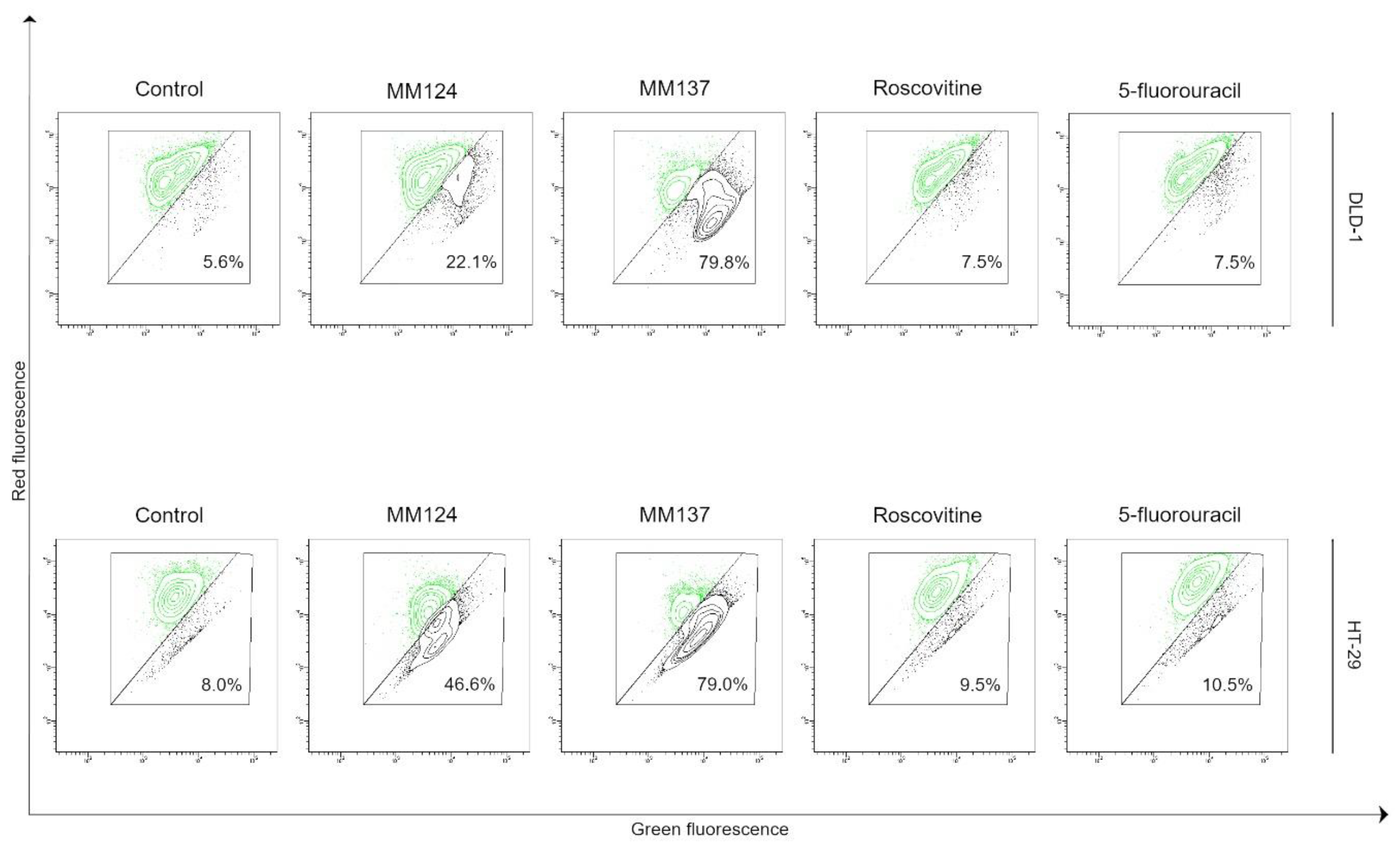
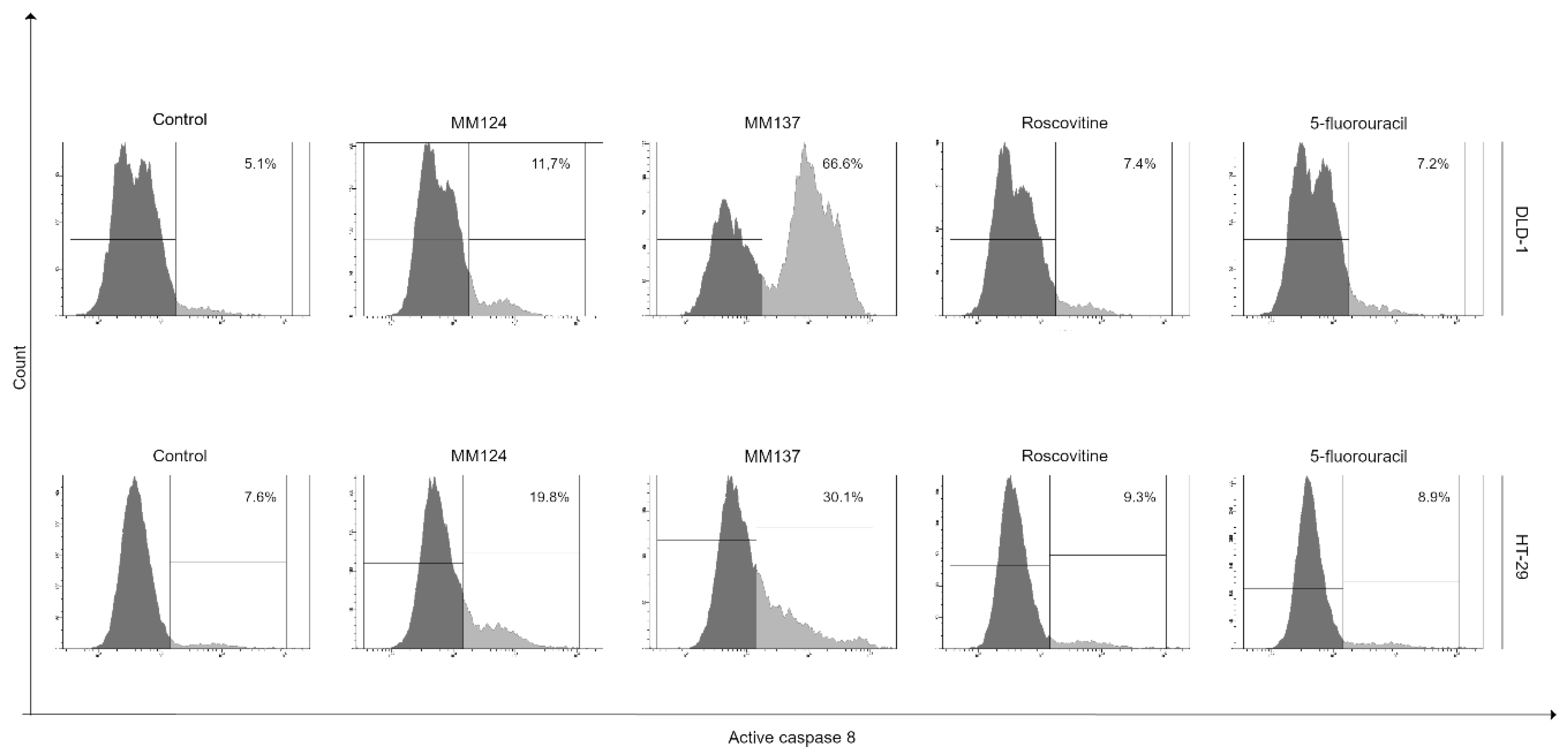
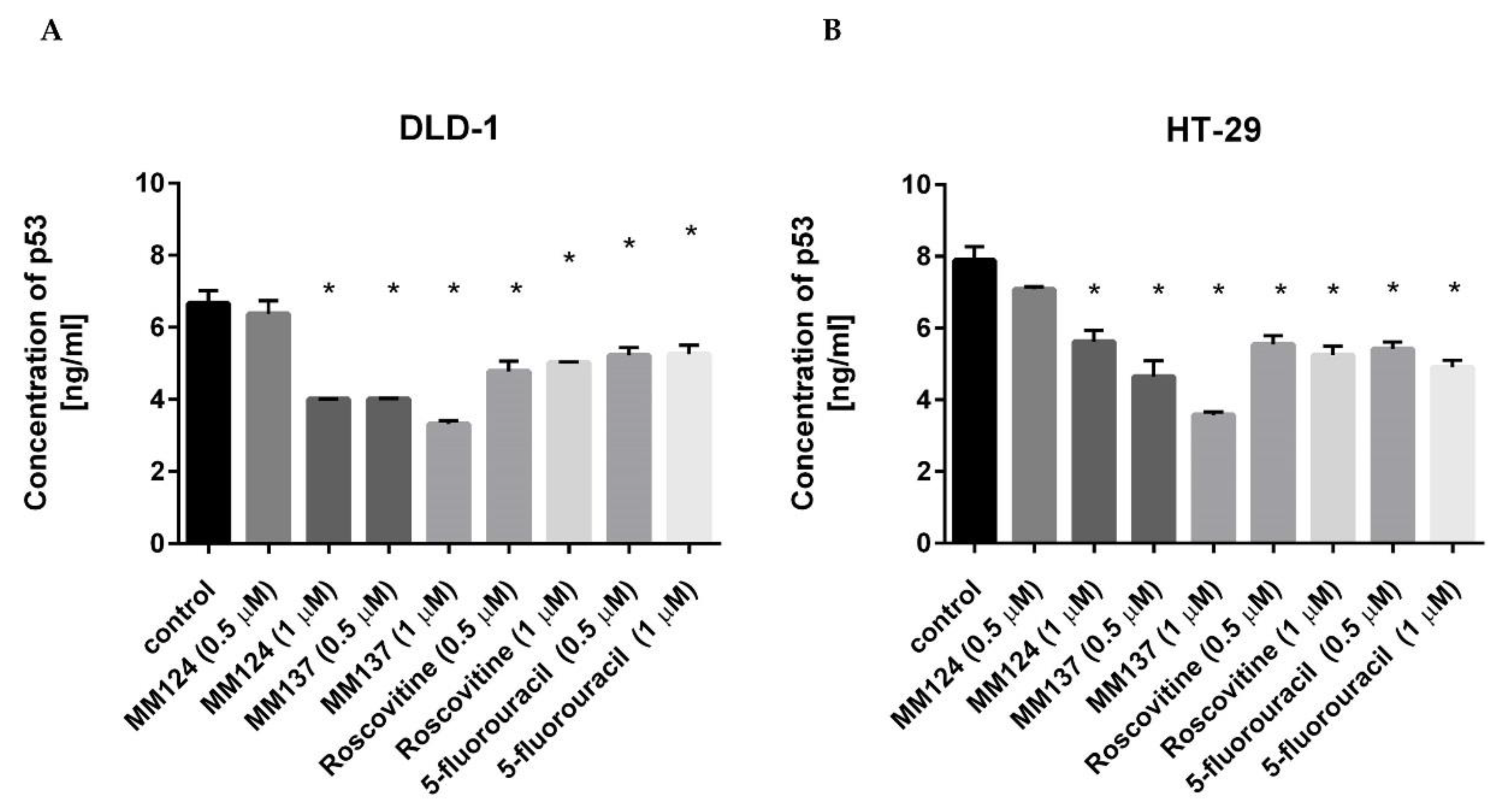
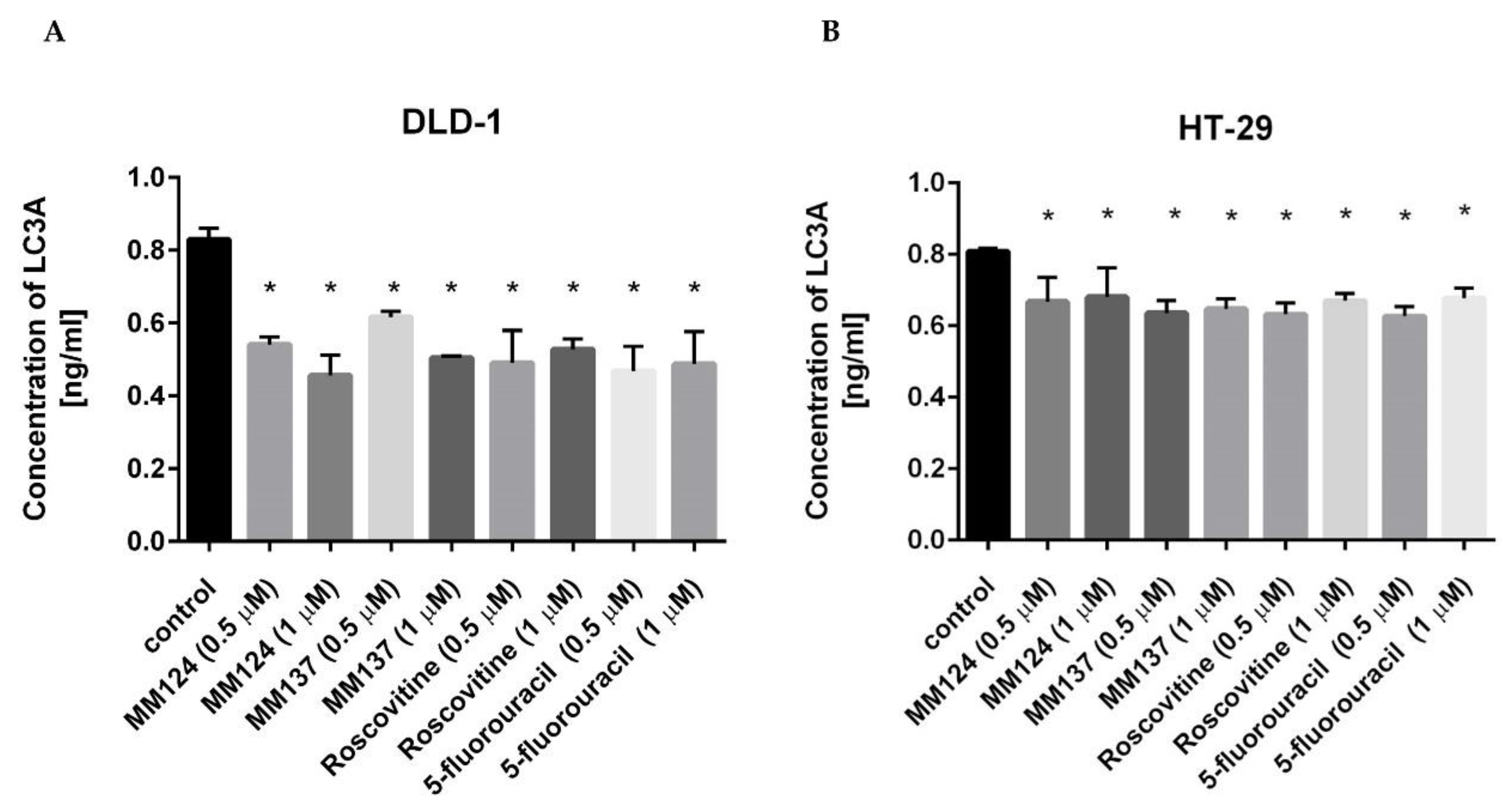
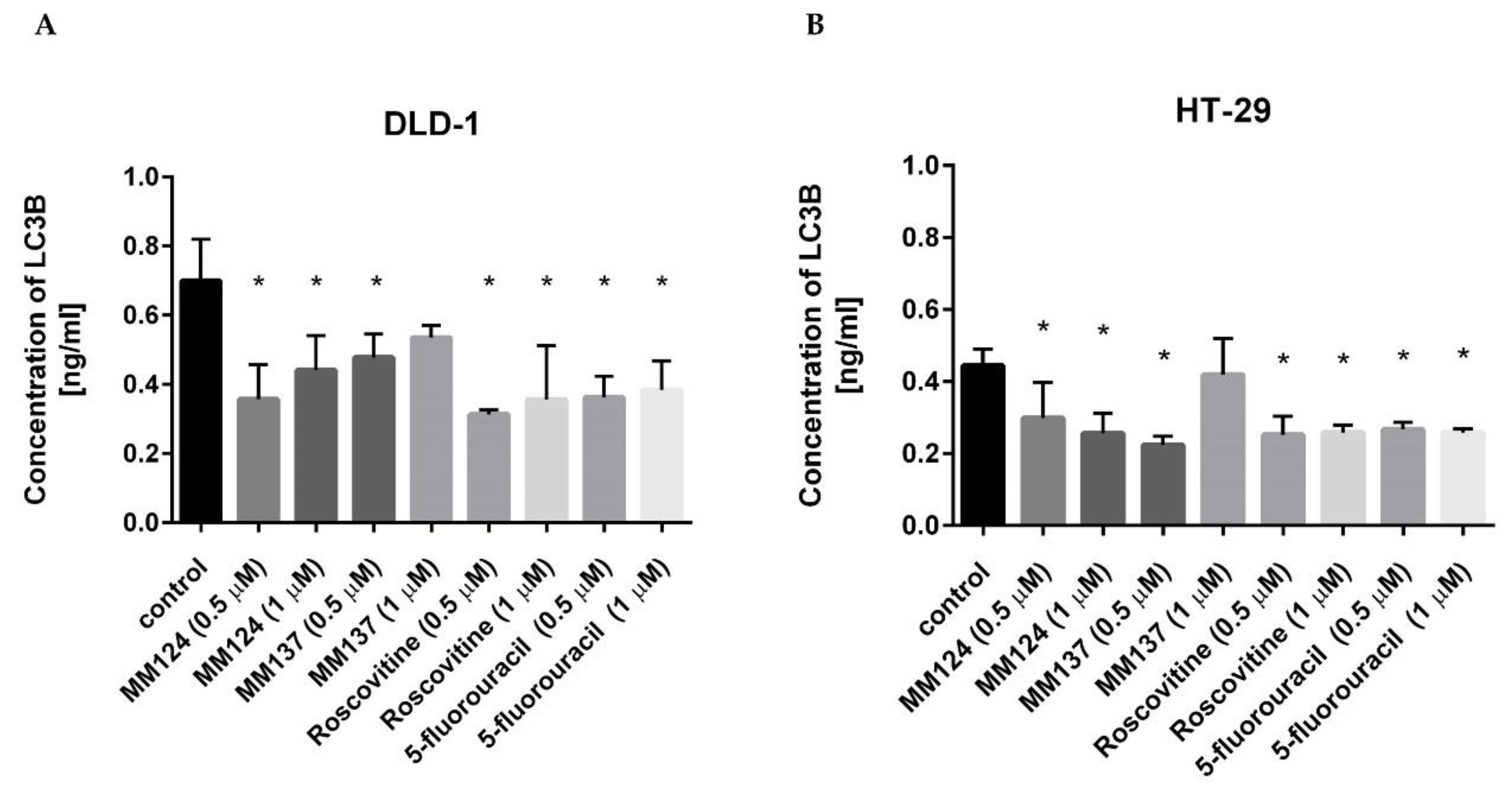
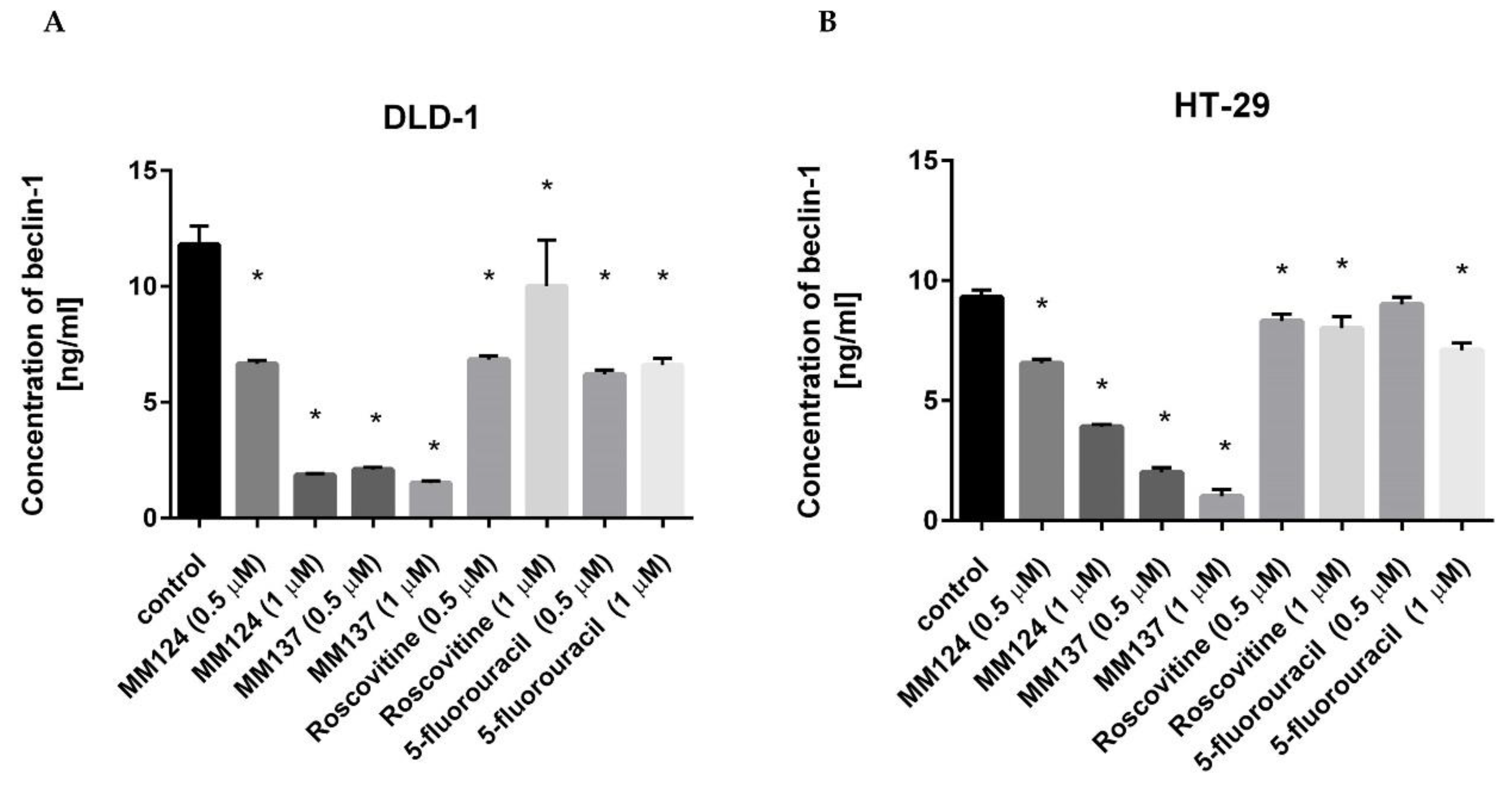
2. Results
3. Discussion
4. Materials and Methods
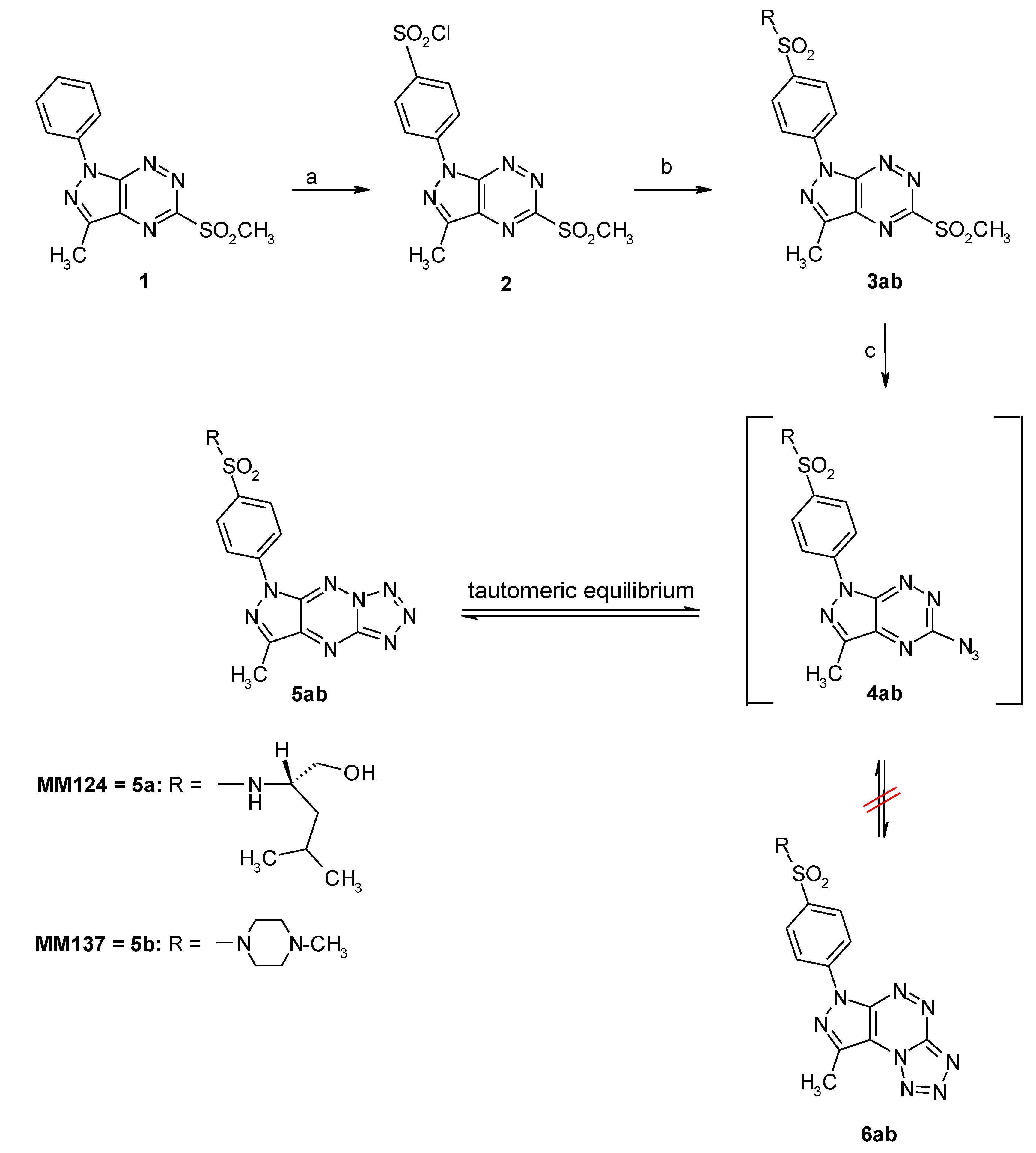
4.1. Synthesis of MM124 and MM137
4.1.1. General Method Synthesis of Derivative 2
4.1.2. Synthesis of Sulfonamides 3ab
4.1.3. Synthesis of Tricyclic Sulfonamides
4.2. Cell Culture of HT-29 and DLD-1 Cells
4.3. Cell Viability Assay
4.4. [3H]Thymidine Incorporation Assay
4.5. Flow Cytometry Assessment of Annexin V Binding
4.6. Acridine Orange/Ethidium Bromide Fluorescent Staining
4.7. Analysis of Mitochondrial Membrane Potential
4.8. Analysis of Caspase-8 Enzymatic Activity
4.9. Determination of p53, Beclin-1, LC3A and LC3B
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| 5-FU | 5-fluorouracil |
| EGFR | epidermal growth factor receptor |
| VEGF | vascular endothelial growth factor |
| PDE5 | phosphodiesterase 5 |
| CA | carbonic anhydrase |
| CAIX | carbonic anhydrase IX |
| CAXII | carbonic anhydrase XII |
| MMP | mitochondrial membrane potential |
| LC3A | microtubule-associated protein 1A/1B light chain 3A |
| LC3B | microtubule-associated protein 1A/1B light chain 3B |
| ClSO3H | chlorosulfonic acid |
| MeCN | acetonitrile |
| EtOH | ethanol |
| HCQ | hydroxychloroquine |
| mCRC | metastatic colorectal cancer |
| CDK1 | cyclin-dependent kinase 1 |
| CDK2 | cyclin-dependent kinase 2 |
References
- Bracht, K.; Nicholls, A.M.; Liu, Y.; Bodmer, W.F. 5-Fluorouracil response in a large panel of colorectal cancer cell lines is associated with mismatch repair deficiency. Br. J. Cancer 2010, 103, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Mullick, P.; Khan, S.A.; Begum, T.; Verma, S.; Kaushik, D.; Alam, O. Synthesis of 1,2,4-triazine derivatives as potential anti-anxiety and anti-inflammatory agents. Acta Pol. Pharm. Drug Res. 2009, 66, 379–385. [Google Scholar]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of some 4H-1,2,4-triazin-5-one derivatives. Il Farm. 2001, 55, 590–595. [Google Scholar] [CrossRef]
- Mojzych, M. Antiviral activity evaluation of pyrazolo[4,3-e][1,2,4]triazines. JCSP 2011, 33, 698–702. [Google Scholar]
- Kumar, R.; Sirohi, T.; Singh, H.; Yadav, R.; Roy, R.; Chaudhary, A.; Pandeya, S. 1,2,4-triazine analogs as novel class of therapeutic agents. Mini-Rev. Med. Chem. 2014, 14, 168–207. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds. Eur. J. Med. Chem. 2017, 142, 328–375. [Google Scholar] [CrossRef]
- Lindner, H.J.; Schaden, G. [Pyrazolo(4.3-e)as-triazine, a new heterocyclic system from Pseudomonas fluorescens Var. Pseudoiodinum]. Pseudoidinum. Chem. Ber. 1972, 105, 1949–1955. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hirata, K.; Nakagami, H.; Takashina, J.; Mahmud, T.; Kobayashi, M.; In, Y.; Ishida, T. Novel Violet Pigment, Nostocine A, an Extracellular Metabolite from Cyanobacterium Nostoc spongiaeforme. HETEROCYCLES 1996, 43, 1513. [Google Scholar] [CrossRef]
- Smirnov, V.; Kiprianova, E.A.; Garagulya, A.D.; Esipov, S.E.; Dovjenko, S.A. Fluviols, bicyclic nitrogen-rich antibiotics produced by Pseudomonas fluorescens. FEMS Microbiol. Lett. 1997, 153, 357–361. [Google Scholar] [CrossRef]
- Gucký, T.; Fryšová, I.; Slouka, J.; Hajduch, M.; Dzubak, P. Cyclocondensation reaction of heterocyclic carbonyl compounds, Part XIII: Synthesis and cytotoxic activity of some 3,7-diaryl-5-(3,4,5-trimethoxyphenyl)pyrazolo[4,3-e][1,2,4]triazines. Eur. J. Med. Chem. 2009, 44, 891–900. [Google Scholar] [CrossRef]
- Mojzych, M.; Rykowski, A.; Smyk, B. Pyrazolo[4,3-e][1,2,4]triazines: Purine Analogues with Electronic Absorption in the Visible Region. Molecules 2005, 10, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Mojzych, M.; Bernat, Z.; Karczmarzyk, Z.; Matysiak, J.; Fruziński, A. Synthesis, Structural Characterization, and Biological Activity of New Pyrazolo[4,3-e][1,2,4]triazine Acyclonucleosides. Molecules 2020, 25, 221. [Google Scholar] [CrossRef] [PubMed]
- Mojzych, M.; Bielawska, A.; Bielawski, K.; Ceruso, M.; Supuran, C. Pyrazolo[4,3-e][1,2,4]triazine sulfonamides as carbonic anhydrase inhibitors with antitumor activity. Bioorg. Med. Chem. 2014, 22, 2643–2647. [Google Scholar] [CrossRef]
- Mojzych, M. Cytotoxic activity of some pyrazolo[4,3-e][1,2,4]triazines against human cancer cell lines. JCSP 2011, 33, 123–128. [Google Scholar]
- Mojzych, M.; Tarasiuk, P.; Karczmarzyk, Z.; Juszczak, M.; Rzeski, W.; Fruziński, A.; Woźny, A. Synthesis, Structure and Antiproliferative Activity of New pyrazolo[4,3- e]triazolo[4,5-b][1,2,4]triazine Derivatives. Med. Chem. 2018, 14, 53–59. [Google Scholar] [CrossRef]
- Mojzych, M.; Šubertová, V.; Bielawska, A.; Bielawski, K.; Bazgier, V.; Berka, K.; Gucký, T.; Fornal, E.; Krystof, V. Synthesis and kinase inhibitory activity of new sulfonamide derivatives of pyrazolo[4,3-e][1,2,4]triazines. Eur. J. Med. Chem. 2014, 78, 217–224. [Google Scholar] [CrossRef]
- Mojzych, M.; Dolashki, A.; Voelter, W. Synthesis of pyrazolo[4,3- e ][1,2,4]triazine sulfonamides, novel Sildenafil analogs with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6616–6624. [Google Scholar] [CrossRef]
- Mojzych, M.; Tarasiuk, P.; Kotwica-Mojzych, K.; Rafiq, M.; Seo, S.-Y.; Nicewicz, M.; Fornal, E. Synthesis of chiral pyrazolo[4,3-e][1,2,4]triazine sulfonamides with tyrosinase and urease inhibitory activity. J. Enzym. Inhib. Med. Chem. 2016, 32, 99–105. [Google Scholar] [CrossRef]
- Matysiak, J.; Skrzypek, A.; Tarasiuk, P.; Mojzych, M. QSAR study of pyrazolo[4,3-e][1,2,4]triazine sulfonamides against tumor-associated human carbonic anhydrase isoforms IX and XII. Comput. Biol. Chem. 2017, 71, 57–62. [Google Scholar] [CrossRef]
- Mojzych, M.; Karczmarzyk, Z.; Wysocki, W.; Ceruso, M.; Supuran, C.T.; Krystof, V.; Urbanczyk-Lipkowska, Z.; Kalicki, P. New approaches to the synthesis of sildenafil analogues and their enzyme inhibitory activity. Bioorg. Med. Chem. 2015, 23, 1421–1429. [Google Scholar] [CrossRef]
- Mojzych, M.; Bielawska, A.; Bielawski, K.; Kotwica-Mojzych, K.; Pawlak, D.; Hermanowicz, J.M.; Tankiewicz-Kwedlo, A.; Szymanowska, A. Novel L-proline sulphonamide Derivatives Comprising pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine System, Method of Manufacturing Thereof, Uses Thereof and Pharmaceutical Composition Comprising the Same. Polish Patent Application no. PCT/PL2019/000110, 27 November 2019. [Google Scholar]
- Supuran, C. Special Issue: Sulfonamides. Molecules 2017, 22, 1642. [Google Scholar] [CrossRef] [PubMed]
- Rykowski, A.; Mojzych, M. Synthesis of Functionalized 1H-Pyrazolo[4,3-e][1,2,4]triazines and Their Fused Derivatives via Ipso-Substitution of Methylsulfonyl Group with O-, N-, S- and C-Nucleophiles. HETEROCYCLES 2004, 63, 1829. [Google Scholar] [CrossRef]
- Karczmarzyk, Z.; Mojzych, M.; Rykowski, A. Synthesis and structure of a novel mesomeric betaine 6,7-dimethyl-2H-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine. J. Mol. Struct. 2007, 829, 22–28. [Google Scholar] [CrossRef]
- Mojzych, M.; Karczmarzyk, Z.; Wysocki, W.; Urbanczyk-Lipkowska, Z.; Żaczek, N. Valence tautomerism of new pyrazolo[4,3-e]tetrazole[4,5-b][1,2,4]triazines. J. Mol. Struct. 2014, 1067, 147–153. [Google Scholar] [CrossRef]
- Gornowicz, A.; Kałuża, Z.; Bielawska, A.; Gabryel, H.; Czarnomysy, R.; Bielawski, K.S. Cytotoxic efficacy of a novel dinuclear platinum(II) complex used with anti-MUC1 in human breast cancer cells. Mol. Cell. Biochem. 2014, 392, 161–174. [Google Scholar] [CrossRef][Green Version]
- Hu, T.; Wang, L.; Zhang, L.; Lu, L.; Shen, J.; Chan, R.L.; Li, M.; Wu, W.K.; To, K.K.W.; Cho, C.H. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine 2015, 22, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Varghese, V.; Magnani, L.; Harada-Shoji, N.; Mauri, F.; Szydlo, R.M.; Yao, S.; Lam, E.W.-F.; Kenny, L.M. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Jin, S.; White, E. Role of Autophagy in Cancer. Autophagy 2007, 3, 28–31. [Google Scholar] [CrossRef]
- Koustas, E.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. Upgraded role of autophagy in colorectal carcinomas. World J. Gastrointest. Oncol. 2018, 10, 367–369. [Google Scholar] [CrossRef]
- Li, Y.-J.; Lei, Y.-H.; Yao, N.; Wang, C.-R.; Hu, N.; Ye, W.; Zhang, D.; Chen, Z.-S. Autophagy and multidrug resistance in cancer. Chin. J. Cancer 2017, 36, 52. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Fang, X.; Wang, X. Autophagy and inflammation. Clin. Transl. Med. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Gariboldi, M.B.; Taiana, E.; Bonzi, M.C.; Craparotta, I.; Giovannardi, S.; Mancini, M.; Monti, E. The BH3-mimetic obatoclax reduces HIF-1α levels and HIF-1 transcriptional activity and sensitizes hypoxic colon adenocarcinoma cells to 5-fluorouracil. Cancer Lett. 2015, 364, 156–164. [Google Scholar] [CrossRef]
- Traini, R.; Ben-Josef, G.; Pastrana, D.V.; Moskatel, E.; Sharma, A.K.; Antignani, A.; Fitzgerald, D.J. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol. Cancer Ther. 2010, 9, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Billard, C. BH3 Mimetics: Status of the Field and New Developments. Mol. Cancer Ther. 2013, 12, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, T.; Shen, J.; Zhang, L.; Chan, R.L.-Y.; Lu, L.; Li, M.; Cho, C.H.; Wu, W.K. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine 2015, 22, 1079–1087. [Google Scholar] [CrossRef]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Wang, L.; Yeung, J.; Hu, T.; Lee, W.Y.-W.; Lu, L.; Zhang, L.; Shen, J.; Chan, R.; Wu, W.K.; Cho, C.H. Dihydrotanshinone induces p53-independent but ROS-dependent apoptosis in colon cancer cells. Life Sci. 2013, 93, 344–351. [Google Scholar] [CrossRef]
- Li, J.; Hou, N.; Faried, A.; Tsutsumi, S.; Takeuchi, T.; Kuwano, H. Inhibition of Autophagy by 3-MA Enhances the Effect of 5-FU-Induced Apoptosis in Colon Cancer Cells. Ann. Surg. Oncol. 2008, 16, 761–771. [Google Scholar] [CrossRef]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar]
- Lambert, L.A.; Qiao, N.; Hunt, K.K.; Lambert, D.H.; Mills, G.B.; Meijer, L.; Keyomarsi, K. Autophagy: A novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res. 2008, 68, 7966–7974. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, S.R. The Cyclin-dependent Kinase Inhibitor CYC202 (R-Roscovitine) Inhibits Retinoblastoma Protein Phosphorylation, Causes Loss of Cyclin D1, and Activates the Mitogen-activated Protein Kinase Pathway. Cancer Res. 2004, 64, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, N.; Gornowicz, A.; Bielawska, A.; Surażyński, A.; Szymanowska, A.; Czarnomysy, R.; Bielawski, K.S. The molecular mechanism of anticancer action of novel octahydropyrazino[2,1-a:5,4-a’]diisoquinoline derivatives in human gastric cancer cells. Investig. New Drugs 2018, 36, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, M.; Kałuża, Z.; Bielawska, A.; Czarnomysy, R.; Gornowicz, A.; Bielawski, K.S. Cytotoxic activity of octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives in human breast cancer cells. Arch. Pharmacal Res. 2014, 38, 628–641. [Google Scholar] [CrossRef]
- Gornowicz, A.; Bielawska, A.; Czarnomysy, R.; Gabryel, H.; Muszyńska, A.; Bielawski, K.S. The combined treatment with novel platinum(II) complex and anti-MUC1 increases apoptotic response in MDA-MB-231 breast cancer cells. Mol. Cell. Biochem. 2015, 408, 103–113. [Google Scholar] [CrossRef]
- Czajkowska, A.; Gornowicz, A.; Pawłowska, N.; Czarnomysy, R.; Nazaruk, J.; Szymanowski, W.; Bielawska, A.; Bielawski, K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a′]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. BioMed Res. Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Gornowicz, A.; Pawłowska, N.; Czajkowska, A.; Czarnomysy, R.; Bielawska, A.; Bielawski, K.S.; Michalak, O.; Staszewska-Krajewska, O.; Kałuża, Z. Biological evaluation of octahydropyrazin[2,1-a:5,4-a′]diisoquinoline derivatives as potent anticancer agents. Tumor Biol. 2017, 39, 1010428317701641. [Google Scholar] [CrossRef]
- Gornowicz, A.; Szymanowski, W.; Bielawska, A.; Szymanowska, A.; Czarnomysy, R.; Kałuża, Z.; Bielawski, K. Monoclonal anti-MUC1 antibody with novel octahydropyrazino[2,1-a:5,4-a’]diisoquinoline derivative as a potential multi-targeted strategy in MCF-7 breast cancer cells. Oncol. Rep. 2019, 42, 1391–1403. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Bielawski, K.; Bielawska, A. The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5221. https://doi.org/10.3390/ijms21155221
Gornowicz A, Szymanowska A, Mojzych M, Bielawski K, Bielawska A. The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells. International Journal of Molecular Sciences. 2020; 21(15):5221. https://doi.org/10.3390/ijms21155221
Chicago/Turabian StyleGornowicz, Agnieszka, Anna Szymanowska, Mariusz Mojzych, Krzysztof Bielawski, and Anna Bielawska. 2020. "The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells" International Journal of Molecular Sciences 21, no. 15: 5221. https://doi.org/10.3390/ijms21155221
APA StyleGornowicz, A., Szymanowska, A., Mojzych, M., Bielawski, K., & Bielawska, A. (2020). The Effect of Novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine Sulfonamide Derivatives on Apoptosis and Autophagy in DLD-1 and HT-29 Colon Cancer Cells. International Journal of Molecular Sciences, 21(15), 5221. https://doi.org/10.3390/ijms21155221






