Cell-Based Therapeutic Approaches for Cystic Fibrosis
Abstract
1. Introduction
2. Etiology and Pathophysiology of CF
3. Animal Models of CF
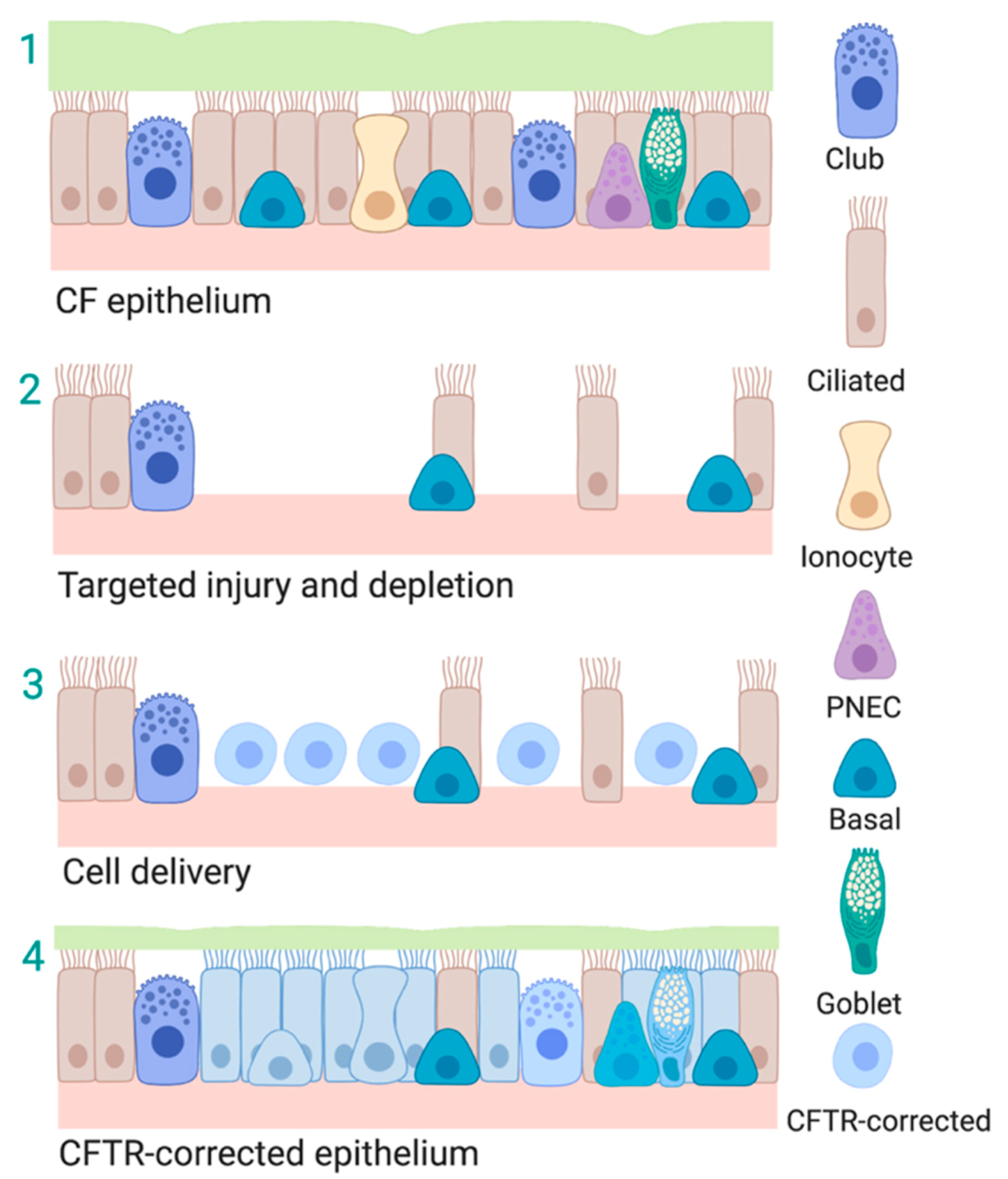
4. Overview of Cell Types Used in Cell-Based Therapeutic Approaches for the Lung
5. Mesenchymal Stromal Cells (MSC)
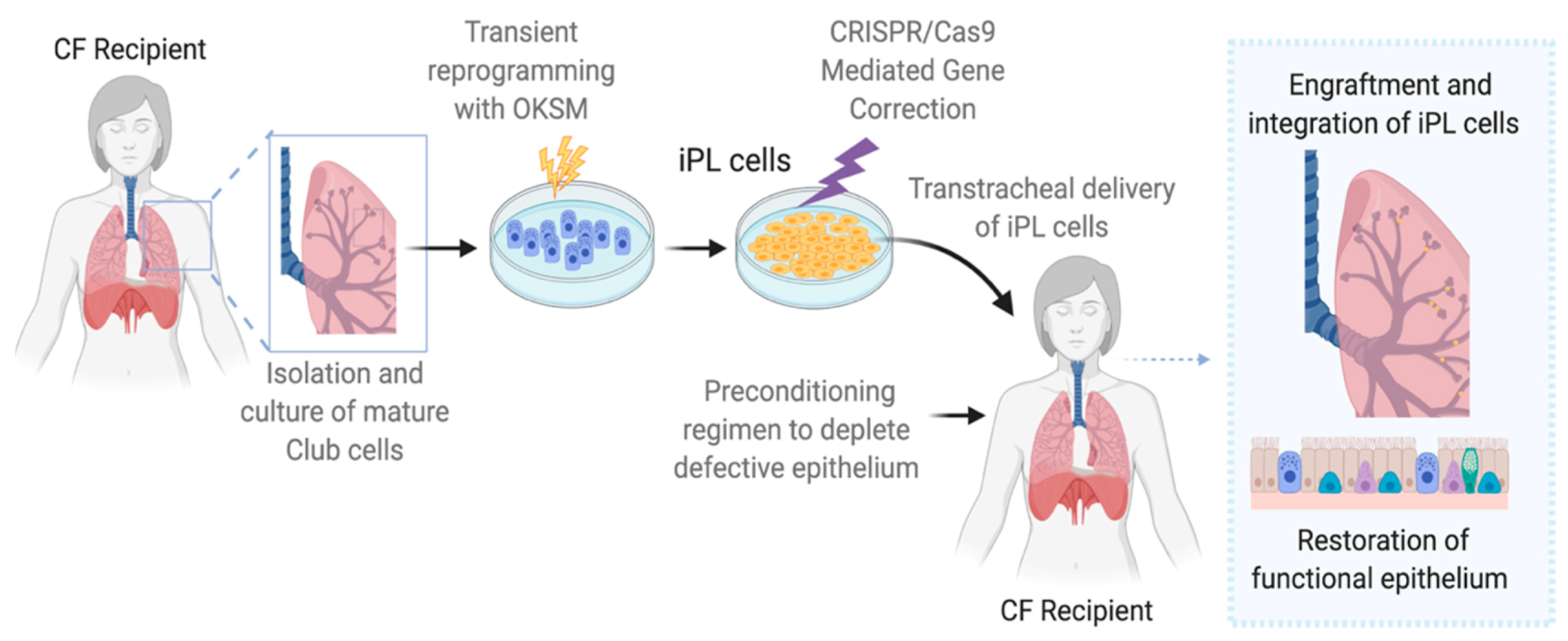
6. Induced Progenitor-Like Cells (iPL)
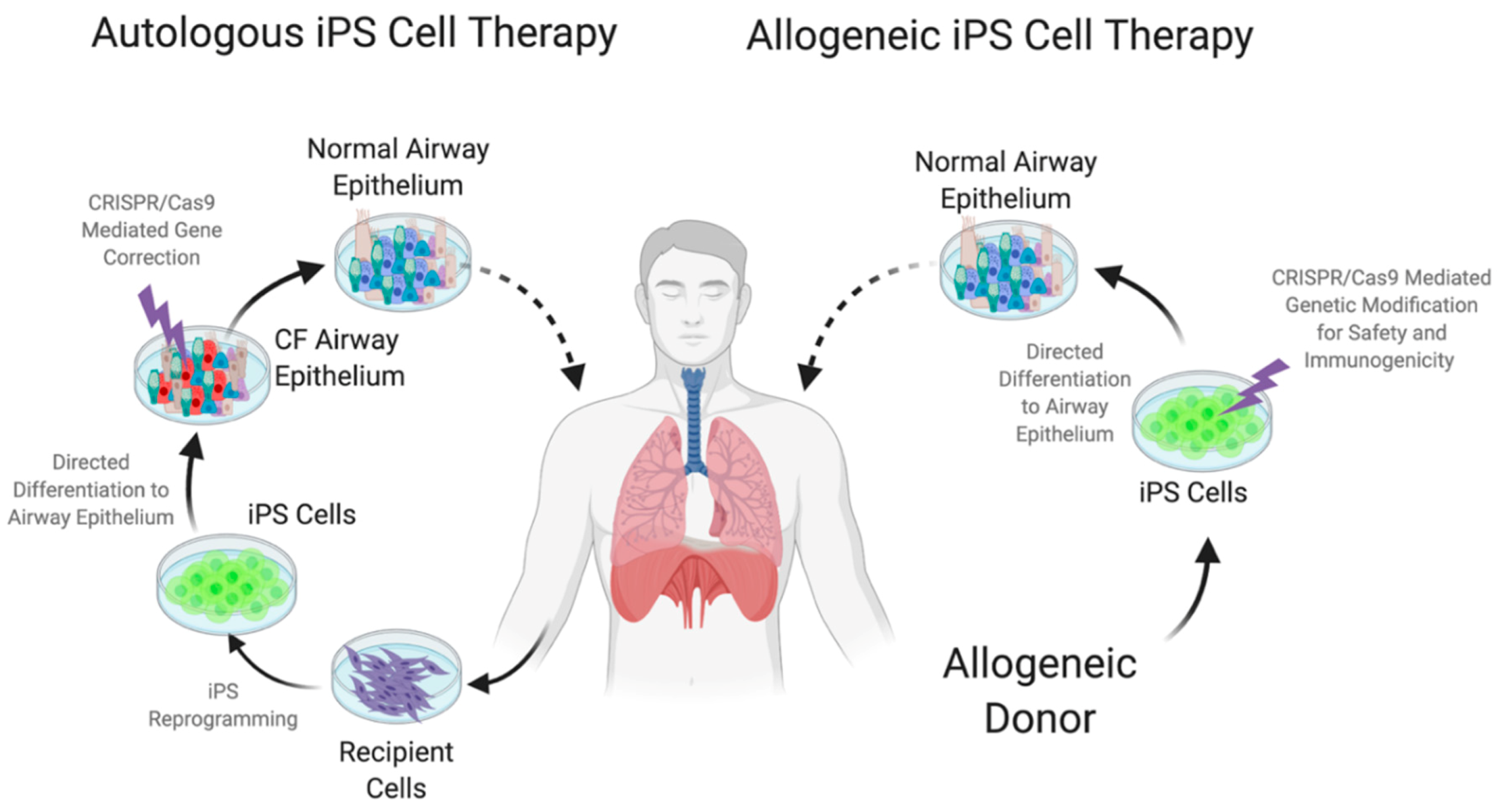
7. Embryonic and Induced Pluripotent Stem Cells (iPS)
8. Designer Pluripotent Cells
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEC-II | Alveolar type II epithelial cells |
| ALI | Air–liquid-interface |
| BMC | Bone marrow cells |
| BMP4 | Bone Morphogenetic Protein 4 |
| cAMP | Cyclic adenosine monophosphate |
| Cas9 | CRISPR associated protein 9 |
| CCSP | Club cell secretory protein |
| CFBE | CF bronchial epithelial |
| CF | Cystic Fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| Ciita | Class II, major histocompatibility complex, transactivator |
| Cl− | Chloride anion |
| cMyc | c-Myelocytomatosis oncogene cellular homolog |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CTLA4-Ig | Cytotoxic T-lymphocyte-associated protein 4-immunoglobulin |
| ES | Embryonic stem |
| EVs | Extracellular vesicles |
| FoxI1 | Forkhead box I1 |
| GCV | Ganciclovir |
| GSHs | Genomic safe harbors |
| HLA | Human leukocyte antigens |
| HO-1 | Heme oxygenase 1 |
| hPSCs | Human pluripotent stem cells |
| HSC | Hematopoietic stem cells |
| HSV-TK | Herpes Simplex Virus type 1 thymidine kinase |
| IBMX | 3-isobutyl-1-methylxanthine |
| IL-1β | Interleukin 1 beta |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-1ra | Interleukin-1 receptor antagonist |
| iPL | Induced Progenitor-Like Cells |
| iPS | Induced pluripotent stem |
| KLF4 | Kruppel Like Factor 4 |
| mRNA | Messenger Ribonucleic acid |
| MSC | Mesenchymal stem cells |
| MUC5AC | Mucin 5AC, Oligomeric Mucus/Gel-Forming |
| NF-κB | Nuclear factor-κB |
| NKX2-1 | NK2 Homeobox 1 |
| Oct-4 | Octamer-binding transcription factor 4 |
| ΔF508 | Deletion of phenylalanine 508 |
| PNEC | Pulmonary neuroendocrine cells |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| Sox2 | SRY (sex determining region Y)-box 2 |
| TNFα | Tumor necrosis factor alpha |
| wtCFTR | Wild-type cystic fibrosis transmembrane conductance regulator |
| ZO-1 | Zonula occludens-1 |
References
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, H.-M.; Collet, J.-P.; Penrod, J.R.; Ferraro, P.; Poirier, C. A cost-effectiveness and cost-utility study of lung transplantation. J. Heart Lung Transplant. 2005, 24, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic fibrosis. Nat. Rev. Dis. Primers 2015, 1, 15010–15019. [Google Scholar] [CrossRef]
- Gentzsch, M.; Mall, M.A. Ion Channel Modulators in Cystic Fibrosis. Chest 2018, 154, 383–393. [Google Scholar] [CrossRef]
- Burgener, E.B.; Moss, R.B. Cystic fibrosis transmembrane conductance regulator modulators: Precision medicine in cystic fibrosis. Curr. Opin. Pediatr. 2018, 30, 372–377. [Google Scholar] [CrossRef]
- Johnson, L.G.; Olsen, J.C.; Sarkadi, B.; Moore, K.L.; Swanstrom, R.; Boucher, R.C. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992, 2, 21–25. [Google Scholar] [CrossRef]
- Dorin, J.R.; Farley, R.; Webb, S.; Smith, S.N.; Farini, E.; Delaney, S.J.; Wainwright, B.J.; Alton, E.W.; Porteous, D.J. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther. 1996, 3, 797–801. [Google Scholar]
- Stoltz, D.A.; Meyerholz, D.K.; Pezzulo, A.A.; Ramachandran, S.; Rogan, M.P.; Davis, G.J.; Hanfland, R.A.; Wohlford-Lenane, C.; Dohrn, C.L.; Bartlett, J.A.; et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010, 2, 29ra31. [Google Scholar] [CrossRef]
- Moran, O.; Zegarra-Moran, O. On the measurement of the functional properties of the CFTR. J. Cyst. Fibros. 2008, 7, 483–494. [Google Scholar] [CrossRef]
- Davies, J.C.; Alton, E.W.F.W.; Bush, A. Cystic fibrosis. BMJ 2007, 335, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Raia, V.; Kroemer, G. Strategies for the etiological therapy of cystic fibrosis. Cell Death Differ. 2017, 24, 1825–1844. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Cymberknoh, M.; Kerem, E.; Ferkol, T.; Elizur, A. Airway inflammation in cystic fibrosis: Molecular mechanisms and clinical implications. Thorax 2013, 68, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- De Rose, V.; Burgel, P.-R.; Gaggar, A.; Greene, C. Airway Inflammatory/Immune Responses in COPD and Cystic Fibrosis. Mediat. Inflamm. 2018, 2018, 7280747-3. [Google Scholar] [CrossRef]
- De Rose, V.; Molloy, K.; Gohy, S.; Pilette, C.; Greene, C.M. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediat. Inflamm. 2018, 2018, 1309746. [Google Scholar] [CrossRef]
- Wine, J.J.; Joo, N.S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004, 1, 47–53. [Google Scholar] [CrossRef]
- Dajani, R.; Zhang, Y.; Taft, P.J.; Travis, S.M.; Starner, T.D.; Olsen, A.; Zabner, J.; Welsh, M.J.; Engelhardt, J.F. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am. J. Respir. Cell Mol. Biol. 2005, 32, 548–552. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.L. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Snouwaert, J.N.; Brigman, K.K.; Latour, A.M.; Malouf, N.N.; Boucher, R.C.; Smithies, O.; Koller, B.H. An animal model for cystic fibrosis made by gene targeting. Science 1992, 257, 1083–1088. [Google Scholar] [CrossRef]
- Zhou, L.; Dey, C.R.; Wert, S.E.; DuVall, M.D.; Frizzell, R.A.; Whitsett, J.A. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science 1994, 266, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.S.; Hao, Y.; Rokhlina, T.; Samuel, M.; Stoltz, D.A.; Li, Y.; Petroff, E.; Vermeer, D.W.; Kabel, A.C.; Yan, Z.; et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Investig. 2008, 118, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Navis, A.; Marjoram, L.; Bagnat, M. Cftr controls lumen expansion and function of Kupffer’s vesicle in zebrafish. Development 2013, 140, 1703–1712. [Google Scholar] [CrossRef]
- Tuggle, K.L.; Birket, S.E.; Cui, X.; Hong, J.; Warren, J.; Reid, L.; Chambers, A.; Ji, D.; Gamber, K.; Chu, K.K.; et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS ONE 2014, 9, e91253. [Google Scholar] [CrossRef]
- Fan, Z.; Perisse, I.V.; Cotton, C.U.; Regouski, M.; Meng, Q.; Domb, C.; Van Wettere, A.J.; Wang, Z.; Harris, A.; White, K.L.; et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight 2018, 3, 1542. [Google Scholar] [CrossRef]
- Hodges, C.A.; Cotton, C.U.; Palmert, M.R.; Drumm, M.L. Generation of a conditional null allele for Cftr in mice. Genesis 2008, 46, 546–552. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, Z.; Engelhardt, J.F. Viral Vectors, Animal Models, and Cellular Targets for Gene Therapy of Cystic Fibrosis Lung Disease. Hum. Gene Ther. 2020, 31, 524–537. [Google Scholar] [CrossRef]
- Ostedgaard, L.S.; Meyerholz, D.K.; Chen, J.-H.; Pezzulo, A.A.; Karp, P.H.; Rokhlina, T.; Ernst, S.E.; Hanfland, R.A.; Reznikov, L.R.; Ludwig, P.S.; et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci. Transl. Med. 2011, 3, 74ra24. [Google Scholar] [CrossRef]
- Stoltz, D.A.; Rokhlina, T.; Ernst, S.E.; Pezzulo, A.A.; Ostedgaard, L.S.; Karp, P.H.; Samuel, M.S.; Reznikov, L.R.; Rector, M.V.; Gansemer, N.D.; et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J. Clin. Investig. 2013, 123, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sui, H.; Fisher, J.T.; Yan, Z.; Liu, X.; Cho, H.-J.; Joo, N.S.; Zhang, Y.; Zhou, W.; Yi, Y.; et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Investig. 2010, 120, 3149–3160. [Google Scholar] [CrossRef]
- Sun, X.; Olivier, A.K.; Yi, Y.; Pope, C.E.; Hayden, H.S.; Liang, B.; Sui, H.; Zhou, W.; Hager, K.R.; Zhang, Y.; et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am. J. Pathol. 2014, 184, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, E.L.; Clark, C.P.; Xue, Y.; Hogan, B.L.M. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development 2009, 136, 3741–3745. [Google Scholar] [CrossRef]
- Rawlins, E.L.; Ostrowski, L.E.; Randell, S.H.; Hogan, B.L.M. Lung development and repair: Contribution of the ciliated lineage. Proc. Natl. Acad. Sci. USA 2007, 104, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.J.; Koch, S.; Müller, B.; Unterstab, F.; Unterstab, F.; von Wichert, P.; Moll, R. Proliferation and number of Clara cell 10-kDa protein (CC10)-reactive epithelial cells and basal cells in normal, hyperplastic and metaplastic bronchial mucosa. Virchows Arch. 2000, 437, 648–655. [Google Scholar] [CrossRef]
- Borthwick, D.W.; Shahbazian, M.; Krantz, Q.T.; Dorin, J.R.; Randell, S.H. Evidence for stem-cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 2001, 24, 662–670. [Google Scholar] [CrossRef]
- Giangreco, A.; Shen, H.; Reynolds, S.D.; Stripp, B.R. Molecular phenotype of airway side population cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L624–L630. [Google Scholar] [CrossRef]
- Bustos, M.L.; Mura, M.; Marcus, P.; Hwang, D.; Ludkovski, O.; Wong, A.P.; Waddell, T.K. Bone marrow cells expressing clara cell secretory protein increase epithelial repair after ablation of pulmonary clara cells. Mol. Ther. 2013, 21, 1251–1258. [Google Scholar] [CrossRef]
- Duchesneau, P.; Besla, R.; Derouet, M.F.; Guo, L.; Karoubi, G.; Silberberg, A.; Wong, A.P.; Waddell, T.K. Partial Restoration of CFTR Function in cftr-Null Mice following Targeted Cell Replacement Therapy. Mol. Ther. 2017, 25, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J. Concise review: Current status of stem cells and regenerative medicine in lung biology and diseases. Stem Cells 2014, 32, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.A.; Laffey, J.G.; Pelosi, P.; Rocco, P.R.M. Mesenchymal stem cell trials for pulmonary diseases. J. Cell. Biochem. 2014, 115, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, J.; Zhang, D.; Song, Y.; She, J.; Bai, C. Bone marrow-derived mesenchymal stem cells enhance autophagy via PI3K/AKT signalling to reduce the severity of ischaemia/reperfusion-induced lung injury. J. Cell. Mol. Med. 2015, 19, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.S.H.; Mei, S.H.J.; Stewart, D.J. The Immunomodulatory and Therapeutic Effects of Mesenchymal Stromal Cells for Acute Lung Injury and Sepsis. J. Cell. Physiol. 2015, 230, 2606–2617. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.-G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.-J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef]
- Gupta, N.; Sinha, R.; Krasnodembskaya, A.; Xu, X.; Nizet, V.; Matthay, M.A.; Griffin, J.H. The TLR4-PAR1 Axis Regulates Bone Marrow Mesenchymal Stromal Cell Survival and Therapeutic Capacity in Experimental Bacterial Pneumonia. Stem Cells 2018, 36, 796–806. [Google Scholar] [CrossRef]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef]
- Kitoko, J.Z.; de Castro, L.L.; Nascimento, A.P.; Abreu, S.C.; Cruz, F.F.; Arantes, A.C.; Xisto, D.G.; Martins, M.A.; Morales, M.M.; Rocco, P.R.M.; et al. Therapeutic administration of bone marrow-derived mesenchymal stromal cells reduces airway inflammation without up-regulating Tregs in experimental asthma. Clin. Exp. Allergy 2018, 48, 205–216. [Google Scholar] [CrossRef]
- Kennelly, H.; Mahon, B.P.; English, K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci. Rep. 2016, 6, 38207–38211. [Google Scholar] [CrossRef]
- Eisenberg, L.M.; Eisenberg, C.A. Stem cell plasticity, cell fusion, and transdifferentiation. Birth Defects Res. C Embryo Today 2003, 69, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Perán, M.; Marchal, J.A.; Rodríguez-Serrano, F.; Alvarez, P.; Aránega, A. Transdifferentiation: Why and how? Cell Biol. Int. 2011, 35, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Sanges, D.; Lluis, F.; Cosma, M.P. Cell-fusion-mediated reprogramming: Pluripotency or transdifferentiation? Implications for regenerative medicine. Adv. Exp. Med. Biol. 2011, 713, 137–159. [Google Scholar] [PubMed]
- Moodley, Y.; Manuelpillai, U.; Weiss, D.J. Cellular therapies for lung disease: A distant horizon. Respirology 2011, 16, 223–237. [Google Scholar] [CrossRef]
- Rippon, H.J.; Lane, S.; Qin, M.; Ismail, N.-S.; Wilson, M.R.; Takata, M.; Bishop, A.E. Embryonic stem cells as a source of pulmonary epithelium in vitro and in vivo. Proc. Am. Thorac. Soc. 2008, 5, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Van Vranken, B.E.; Rippon, H.J.; Samadikuchaksaraei, A.; Trounson, A.O.; Bishop, A.E. The differentiation of distal lung epithelium from embryonic stem cells. Curr. Protoc. Stem Cell Biol. 2007, 2, 1G.1.1–1G.1.22. [Google Scholar]
- Huang, S.X.L.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.-G.; Chen, Y.-W.; Mumau, M.; Green, M.D.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 84–91. [Google Scholar] [CrossRef]
- Wong, A.P.; Bear, C.E.; Chin, S.; Pasceri, P.; Thompson, T.O.; Huan, L.-J.; Ratjen, F.; Ellis, J.; Rossant, J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol. 2012, 30, 876–882. [Google Scholar] [CrossRef]
- Longmire, T.A.; Ikonomou, L.; Hawkins, F.; Christodoulou, C.; Cao, Y.; Jean, J.C.; Kwok, L.W.; Mou, H.; Rajagopal, J.; Shen, S.S.; et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 2012, 10, 398–411. [Google Scholar] [CrossRef]
- Mou, H.; Zhao, R.; Sherwood, R.; Ahfeldt, T.; Lapey, A.; Wain, J.; Sicilian, L.; Izvolsky, K.; Musunuru, K.; Cowan, C.; et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 2012, 10, 385–397. [Google Scholar] [CrossRef]
- Firth, A.L.; Dargitz, C.T.; Qualls, S.J.; Menon, T.; Wright, R.; Singer, O.; Gage, F.H.; Khanna, A.; Verma, I.M. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1723–E1730. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Gotoh, S.; Tateishi, K.; Yamamoto, Y.; Korogi, Y.; Nagasaki, T.; Matsumoto, H.; Muro, S.; Hirai, T.; Ito, I.; et al. Directed Induction of Functional Multi-ciliated Cells in Proximal Airway Epithelial Spheroids from Human Pluripotent Stem Cells. Stem Cell Rep. 2016, 6, 18–25. [Google Scholar] [CrossRef] [PubMed]
- McCauley, K.B.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 2017, 20, 844–857.e6. [Google Scholar] [CrossRef] [PubMed]
- McCauley, K.B.; Hawkins, F.; Kotton, D.N. Derivation of Epithelial-Only Airway Organoids from Human Pluripotent Stem Cells. Curr. Protoc. Stem Cell Biol. 2018, 45, e51. [Google Scholar] [CrossRef]
- Miller, A.J.; Hill, D.R.; Nagy, M.S.; Aoki, Y.; Dye, B.R.; Chin, A.M.; Huang, S.; Zhu, F.; White, E.S.; Lama, V.; et al. In Vitro Induction and In Vivo Engraftment of Lung Bud Tip Progenitor Cells Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 101–119. [Google Scholar] [CrossRef]
- Litvack, M.L.; Wigle, T.J.; Lee, J.; Wang, J.; Ackerley, C.; Grunebaum, E.; Post, M. Alveolar-like Stem Cell-derived Myb(-) Macrophages Promote Recovery and Survival in Airway Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 1219–1229. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Nakagawa, M.; Garitaonandia, I.; Slavin, I.; Altun, G.; Lacharite, R.M.; Nazor, K.L.; Tran, H.T.; Lynch, C.L.; Leonardo, T.R.; et al. Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 2011, 21, 1551–1563. [Google Scholar] [CrossRef]
- Salven, P.; Mustjoki, S.; Alitalo, R.; Alitalo, K.; Rafii, S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood 2003, 101, 168–172. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Lee, M.W.; Choi, J.; Yang, M.S.; Moon, Y.J.; Park, J.S.; Kim, H.C.; Kim, Y.J. Mesenchymal stem cells from cryopreserved human umbilical cord blood. Biochem. Biophys. Res. Commun. 2004, 320, 273–278. [Google Scholar] [CrossRef]
- Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Fujita, J.; Kinjo, K.; Matsuzaki, Y.; Tsuma, M.; Miyatake, H.; Muguruma, Y.; Tsuboi, K.; Itabashi, Y.; Ikeda, Y.; et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood 2004, 104, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Kopen, G.C.; Prockop, D.J.; Phinney, D.G. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc. Natl. Acad. Sci. USA 1999, 96, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Bassler, P.; Lioznov, M.-V.; Bruns, H.; Kluth, D.; Zander, A.-R.; Fiegel, H.-C. Liver-specific gene expression in mesenchymal stem cells is induced by liver cells. World J. Gastroenterol. 2005, 11, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Izumida, Y.; Aoki, T.; Yasuda, D.; Koizumi, T.; Suganuma, C.; Saito, K.; Murai, N.; Shimizu, Y.; Hayashi, K.; Odaira, M.; et al. Hepatocyte growth factor is constitutively produced by donor-derived bone marrow cells and promotes regeneration of pancreatic beta-cells. Biochem. Biophys. Res. Commun. 2005, 333, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Borue, X.; Lee, S.; Grove, J.; Herzog, E.L.; Harris, R.; Diflo, T.; Glusac, E.; Hyman, K.; Theise, N.D.; Krause, D.S. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am. J. Pathol. 2004, 165, 1767–1772. [Google Scholar] [CrossRef]
- Krause, D.S.; Theise, N.D.; Collector, M.I.; Henegariu, O.; Hwang, S.; Gardner, R.; Neutzel, S.; Sharkis, S.J. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001, 105, 369–377. [Google Scholar] [CrossRef]
- Kotton, D.N.; Ma, B.Y.; Cardoso, W.V.; Sanderson, E.A.; Summer, R.S.; Williams, M.C.; Fine, A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001, 128, 5181–5188. [Google Scholar]
- Xu, J.; Woods, C.R.; Mora, A.L.; Joodi, R.; Brigham, K.L.; Iyer, S.; Rojas, M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L131–L141. [Google Scholar] [CrossRef]
- Ma, N.; Gai, H.; Mei, J.; Ding, F.-B.; Bao, C.-R.; Nguyen, D.M.; Zhong, H. Bone marrow mesenchymal stem cells can differentiate into type II alveolar epithelial cells in vitro. Cell Biol. Int. 2011, 35, 1261–1266. [Google Scholar] [CrossRef]
- Cerrada, A.; de la Torre, P.; Grande, J.; Haller, T.; Flores, A.I.; Pérez-Gil, J. Human decidua-derived mesenchymal stem cells differentiate into functional alveolar type II-like cells that synthesize and secrete pulmonary surfactant complexes. PLoS ONE 2014, 9, e110195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Yan, J.; Xia, Y.; Gu, C.; Ma, Y.; Tao, H. Differentiation of human amniotic fluid-derived mesenchymal stem cells into type II alveolar epithelial cells in vitro. Int. J. Mol. Med. 2014, 33, 1507–1513. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, H.; Keir, P.A.; Edwards, C.J.; Webb, S.; Dorin, J.R. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir. Res. 2006, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, J.M.; Keaney, P.; Passero, M.; Dooner, M.S.; Pimentel, J.; Greer, D.; Demers, D.; Foster, B.; Peterson, A.; Dooner, G.; et al. Bone marrow production of lung cells: The impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp. Hematol. 2006, 34, 230–241. [Google Scholar] [CrossRef]
- Kassmer, S.H.; Krause, D.S. Detection of bone marrow-derived lung epithelial cells. Exp. Hematol. 2010, 38, 564–573. [Google Scholar] [CrossRef]
- Kleeberger, W.; Versmold, A.; Rothämel, T.; Glöckner, S.; Bredt, M.; Haverich, A.; Lehmann, U.; Kreipe, H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am. J. Pathol. 2003, 162, 1487–1494. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef]
- Iyer, S.S.; Torres-Gonzalez, E.; Neujahr, D.C.; Kwon, M.; Brigham, K.L.; Jones, D.P.; Mora, A.L.; Rojas, M. Effect of bone marrow-derived mesenchymal stem cells on endotoxin-induced oxidation of plasma cysteine and glutathione in mice. Stem Cells Int. 2010, 2010, 868076. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef]
- Tai, W.-L.; Dong, Z.-X.; Zhang, D.-D.; Wang, D.-H. Therapeutic effect of intravenous bone marrow-derived mesenchymal stem cell transplantation on early-stage LPS-induced acute lung injury in mice. Nan Fang Yi Ke Da Xue Xue Bao 2012, 32, 283–290. [Google Scholar] [PubMed]
- Xu, J.; Qu, J.; Cao, L.; Sai, Y.; Chen, C.; He, L.; Yu, L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J. Pathol. 2008, 214, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.-X.; Sun, J.-P.; Wang, P.; Tian, Q.; Yang, Z.; Chen, L.-A. Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chin. Med. J. 2011, 124, 2715–2722. [Google Scholar]
- Bonfield, T.L.; Koloze, M.; Lennon, D.P.; Zuchowski, B.; Yang, S.E.; Caplan, A.I. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L760–L770. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Keane-Myers, A.; Brown, J.M.; Metcalfe, D.D.; Gorham, J.D.; Gorham, J.D.; Bundoc, V.G.; Bundoc, V.G.; Hodges, M.G.; Jelinek, I.; et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl. Acad. Sci. USA 2010, 107, 5652–5657. [Google Scholar] [CrossRef]
- Goodwin, M.; Sueblinvong, V.; Eisenhauer, P.; Ziats, N.P.; LeClair, L.; Poynter, M.E.; Steele, C.; Rincon, M.; Weiss, D.J. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011, 29, 1137–1148. [Google Scholar] [CrossRef]
- van Haaften, T.; Byrne, R.; Bonnet, S.; Rochefort, G.Y.; Akabutu, J.; Bouchentouf, M.; Rey-Parra, G.J.; Galipeau, J.; Haromy, A.; Eaton, F.; et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am. J. Respir. Crit. Care Med. 2009, 180, 1131–1142. [Google Scholar] [CrossRef]
- Tropea, K.A.; Leder, E.; Aslam, M.; Lau, A.N.; Raiser, D.M.; Lee, J.-H.; Balasubramaniam, V.; Fredenburgh, L.E.; Alex Mitsialis, S.; Kourembanas, S.; et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L829–L837. [Google Scholar] [CrossRef]
- Ishizawa, K.; Kubo, H.; Yamada, M.; Kobayashi, S.; Numasaki, M.; Ueda, S.; Suzuki, T.; Sasaki, H. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004, 556, 249–252. [Google Scholar] [CrossRef]
- Zhen, G.; Liu, H.; Gu, N.; Zhang, H.; Xu, Y.; Zhang, Z. Mesenchymal stem cells transplantation protects against rat pulmonary emphysema. Front. Biosci. 2008, 13, 3415–3422. [Google Scholar] [CrossRef]
- Aguilar, S.; Scotton, C.J.; McNulty, K.; Nye, E.; Stamp, G.; Laurent, G.; Bonnet, D.; Janes, S.M. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS ONE 2009, 4, e8013. [Google Scholar] [CrossRef]
- Cargnoni, A.; Ressel, L.; Rossi, D.; Poli, A.; Arienti, D.; Lombardi, G.; Parolini, O. Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosis. Cytotherapy 2012, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Jang, A.-S.; Kim, Y.-E.; Cha, J.-Y.; Kim, T.-H.; Jung, S.; Park, S.-K.; Lee, Y.-K.; Won, J.-H.; Kim, Y.-H.; et al. Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respir. Res. 2010, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.A.; Dutreil, M.; Fattman, C.; Pandey, A.C.; Torres, G.; Go, K.; Phinney, D.G. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. USA 2007, 104, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef]
- Shigemura, N.; Okumura, M.; Mizuno, S.; Imanishi, Y.; Nakamura, T.; Sawa, Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am. J. Transplant. 2006, 6, 2592–2600. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Anderson, P.; González, M.A.; Rico, L.; Büscher, D.; Delgado, M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009, 58, 929–939. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Navarro-Alvarez, N.; Nahmias, Y.; Goldwasser, Y.; Kitagawa, Y.; Tilles, A.W.; Tompkins, R.G.; Parekkadan, B.; Yarmush, M.L. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol. Ther. 2010, 18, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2012, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteomics 2013, 13, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Caretti, A.; Peli, V.; Colombo, M.; Zulueta, A. Lights and Shadows in the Use of Mesenchymal Stem Cells in Lung Inflammation, a Poorly Investigated Topic in Cystic Fibrosis. Cells 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Dutly, A.E.; Sacher, A.; Lee, H.; Hwang, D.M.; Liu, M.; Keshavjee, S.; Hu, J.; Waddell, T.K. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L740–L752. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Keating, A.; Lu, W.-Y.; Duchesneau, P.; Wang, X.; Sacher, A.; Hu, J.; Waddell, T.K. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J. Clin. Investig. 2009, 119, 336–348. [Google Scholar] [CrossRef]
- Gilpin, S.E.; Lung, K.C.; Sato, M.; Singer, L.G.; Keshavjee, S.; Waddell, T.K. Altered progenitor cell and cytokine profiles in bronchiolitis obliterans syndrome. J. Heart Lung Transplant. 2012, 31, 222–228. [Google Scholar] [CrossRef]
- Duchesneau, P.; Wong, A.P.; Waddell, T.K. Optimization of targeted cell replacement therapy: A new approach for lung disease. Mol. Ther. 2010, 18, 1830–1836. [Google Scholar] [CrossRef]
- Wang, G.; Bunnell, B.A.; Painter, R.G.; Quiniones, B.C.; Tom, S.; Lanson, N.A.; Spees, J.L.; Bertucci, D.; Peister, A.; Weiss, D.J.; et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: Potential therapy for cystic fibrosis. Proc. Natl. Acad. Sci. USA 2005, 102, 186–191. [Google Scholar] [CrossRef]
- Paracchini, V.; Carbone, A.; Colombo, F.; Castellani, S.; Mazzucchelli, S.; Gioia, S.D.; Degiorgio, D.; Seia, M.; Porretti, L.; Colombo, C.; et al. Amniotic mesenchymal stem cells: A new source for hepatocyte-like cells and induction of CFTR expression by coculture with cystic fibrosis airway epithelial cells. J. Biomed. Biotechnol. 2012, 2012, 575471. [Google Scholar] [CrossRef]
- Carbone, A.; Castellani, S.; Favia, M.; Diana, A.; Paracchini, V.; Di Gioia, S.; Seia, M.; Casavola, V.; Colombo, C.; Conese, M. Correction of defective CFTR/ENaC function and tightness of cystic fibrosis airway epithelium by amniotic mesenchymal stromal (stem) cells. J. Cell. Mol. Med. 2014, 18, 1631–1643. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int 2016, 2016, 5303048. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.T.; Fletcher, D.; Episalla, N.; Auster, L.; Kaur, S.; Gwin, M.C.; Folz, M.; Velasquez, D.; Roy, V.; van Heeckeren, R.; et al. Mesenchymal Stem Cell Soluble Mediators and Cystic Fibrosis. J. Stem Cell Res. Ther 2017, 7. [Google Scholar] [CrossRef]
- Li, M.; Pascual, G.; Glass, C.K. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol. Cell. Biol. 2000, 20, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Mitsialis, S.A.; Aslam, M.; Vitali, S.H.; Vergadi, E.; Konstantinou, G.; Sdrimas, K.; Fernandez-Gonzalez, A.; Kourembanas, S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 2012, 126, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-G.; Feng, X.-M.; Abbott, J.; Fang, X.-H.; Hao, Q.; Monsel, A.; Qu, J.-M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Liu, X.-B.; Huang, S.; Bi, X.-Y.; Wang, H.-X.; Xie, L.-X.; Wang, Y.-Q.; Cao, X.-F.; Lv, J.; Xiao, F.-J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Zulueta, A.; Colombo, M.; Peli, V.; Falleni, M.; Tosi, D.; Ricciardi, M.; Baisi, A.; Bulfamante, G.; Chiaramonte, R.; Caretti, A. Lung mesenchymal stem cells-derived extracellular vesicles attenuate the inflammatory profile of Cystic Fibrosis epithelial cells. Cell. Signal 2018, 51, 110–118. [Google Scholar] [CrossRef]
- Guo, L.; Karoubi, G.; Duchesneau, P.; Shutova, M.V.; Sung, H.-K.; Tonge, P.; Bear, C.; Rogers, I.; Nagy, A.; Waddell, T.K. Generation of Induced Progenitor-like Cells from Mature Epithelial Cells Using Interrupted Reprogramming. Stem Cell Rep. 2017, 9, 1780–1795. [Google Scholar] [CrossRef]
- Guo, L.; Karoubi, G.; Duchesneau, P.; Aoki, F.G.; Shutova, M.V.; Rogers, I.; Nagy, A.; Waddell, T.K. Interrupted reprogramming of alveolar type II cells induces progenitor-like cells that ameliorate pulmonary fibrosis. NPJ Regen Med. 2018, 3, 14. [Google Scholar] [CrossRef]
- Firth, A.L.; Menon, T.; Parker, G.S.; Qualls, S.J.; Lewis, B.M.; Ke, E.; Dargitz, C.T.; Wright, R.; Khanna, A.; Gage, F.H.; et al. Functional Gene Correction for Cystic Fibrosis in Lung Epithelial Cells Generated from Patient iPSCs. Cell Rep. 2015, 12, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Soh, B.S.; Zheng, D.; Li Yeo, J.S.; Yang, H.H.; Ng, S.Y.; Wong, L.H.; Zhang, W.; Li, P.; Nichane, M.; Asmat, A.; et al. CD166(pos) subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Mol. Ther. 2012, 20, 2335–2346. [Google Scholar] [CrossRef]
- Wang, R.; McCauley, K.B.; Kotton, D.N.; Hawkins, F. Differentiation of human airway-organoids from induced pluripotent stem cells (iPSCs). Methods Cell Biol. 2020, 159, 95–114. [Google Scholar] [PubMed]
- Shafa, M.; Ionescu, L.I.; Vadivel, A.; Collins, J.J.P.; Xu, L.; Zhong, S.; Kang, M.; de Caen, G.; Daneshmand, M.; Shi , J.; et al. Human induced pluripotent stem cell-derived lung progenitor and alveolar epithelial cells attenuate hyperoxia-induced lung injury. Cytotherapy 2018, 20, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.-F.; Yang, K.-Y.; Chiou, S.-H.; Chen, N.-J.; Mo, M.-H.; Lin, C.-S.; Wang, C.-T. Induced Pluripotent Stem Cells Regulate Triggering Receptor Expressed on Myeloid Cell-1 Expression and the p38 Mitogen-Activated Protein Kinase Pathway in Endotoxin-Induced Acute Lung Injury. Stem Cells 2019, 37, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Fan, X.-L.; Jiang, D.; Zhang, Y.; Li, X.; Xu, Z.-B.; Fang, S.-B.; Chiu, S.; Tse, H.-F.; Lian, Q.; et al. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Rep. 2018, 11, 1120–1135. [Google Scholar] [CrossRef]
- Tamò, L.; Simillion, C.; Hibaoui, Y.; Feki, A.; Gugger, M.; Prasse, A.; Jäger, B.; Goldmann, T.; Geiser, T.; Gazdhar, A. Gene Network Analysis of Interstitial Macrophages After Treatment with Induced Pluripotent Stem Cells Secretome (iPSC-cm) in the Bleomycin Injured Rat Lung. Stem Cell Rev. Rep. 2018, 14, 412–424. [Google Scholar] [CrossRef]
- Mitchell, A.; Wanczyk, H.; Jensen, T.; Finck, C. Human induced pluripotent stem cells ameliorate hyperoxia-induced lung injury in a mouse model. Am. J. Transl. Res. 2020, 12, 292–307. [Google Scholar]
- de Miguel-Beriain, I. The ethics of stem cells revisited. Adv. Drug Deliv. Rev. 2015, 82-83, 176–180. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Concise Review: Human Embryonic Stem Cells-What Have We Done? What Are We Doing? Where Are We Going? Stem Cells 2017, 35, 17–25. [Google Scholar] [CrossRef]
- Moodley, Y.; Thompson, P.; Warburton, D. Stem cells: A recapitulation of development. Respirology 2013, 18, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Alysandratos, K.-D.; Hawkins, F.; McCauley, K.B.; Jacob, A.; Choi, J.; Caballero, I.S.; Vedaie, M.; Kurmann, A.A.; Ikonomou, L.; et al. Pluripotent stem cell differentiation reveals distinct developmental pathways regulating lung- versus thyroid-lineage specification. Development 2017, 144, 3879–3893. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Shofuda, T.; Higuchi, Y.; Nagamori, I.; Oda, M.; Nakamori, M.; Onodera, M.; Kanematsu, D.; Yamamoto, A.; Katsuma, A.; et al. Human Genomic Safe Harbors and the Suicide Gene-Based Safeguard System for iPSC-Based Cell Therapy. Stem Cells Transl. Med. 2019, 8, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. Chemical ablation of tumor-initiating human pluripotent stem cells. Nat. Protoc. 2014, 9, 729–740. [Google Scholar] [CrossRef]
- Tan, H.L.; Fong, W.J.; Lee, E.H.; Yap, M.; Choo, A. mAb 84, a cytotoxic antibody that kills undifferentiated human embryonic stem cells via oncosis. Stem Cells 2009, 27, 1792–1801. [Google Scholar] [CrossRef]
- Blum, B.; Bar-Nur, O.; Golan-Lev, T.; Benvenisty, N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat. Biotechnol. 2009, 27, 281–287. [Google Scholar] [CrossRef]
- Rong, Z.; Fu, X.; Wang, M.; Xu, Y. A scalable approach to prevent teratoma formation of human embryonic stem cells. J. Biol. Chem. 2012, 287, 32338–32345. [Google Scholar] [CrossRef]
- Lee, M.-O.; Moon, S.H.; Jeong, H.-C.; Yi, J.-Y.; Lee, T.-H.; Shim, S.H.; Rhee, Y.-H.; Lee, S.-H.; Oh, S.-J.; Lee, M.-Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef]
- Liang, Q.; Monetti, C.; Shutova, M.V.; Neely, E.J.; Hacibekiroglu, S.; Yang, H.; Kim, C.; Zhang, P.; Li, C.; Nagy, K.; et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 2018, 563, 701–704. [Google Scholar] [CrossRef]
- Lanza, R.; Russell, D.W.; Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 2019, 19, 723–733. [Google Scholar] [CrossRef]
- Taylor, C.J.; Peacock, S.; Chaudhry, A.N.; Bradley, J.A.; Bolton, E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Riolobos, L.; Hirata, R.K.; Turtle, C.J.; Wang, P.-R.; Gornalusse, G.G.; Zavajlevski, M.; Riddell, S.R.; Russell, D.W. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013, 21, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.-G.; Xu, Y.; et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S.; Salahudeen, A.A.; Sellers, Z.M.; Bravo, D.T.; Choi, S.S.; Batish, A.; Le, W.; Baik, R.; Kaushik, M.P.; Galper, N.; et al. High-Efficiency, Selection-free Gene Repair in Airway Stem Cells from Cystic Fibrosis Patients Rescues CFTR Function in Differentiated Epithelia. Cell Stem Cell 2020, 26, 161–171.e4. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.D.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef]
- Lin, Y.; Cradick, T.J.; Brown, M.T.; Deshmukh, H.; Ranjan, P.; Sarode, N.; Wile, B.M.; Vertino, P.M.; Stewart, F.J.; Bao, G. CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res. 2014, 42, 7473–7485. [Google Scholar] [CrossRef]
- Qadir, M.M.F.; Álvarez-Cubela, S.; Belle, K.; Sapir, T.; Messaggio, F.; Johnson, K.B.; Umland, O.; Hardin, D.; Klein, D.; Pérez-Álvarez, I.; et al. A Double Fail-Safe Approach to Prevent Tumorigenesis and Select Pancreatic β Cells from Human Embryonic Stem Cells. Stem Cell Rep. 2019, 12, 611–623. [Google Scholar] [CrossRef]
- Rosen, C.; Shezen, E.; Aronovich, A.; Klionsky, Y.Z.; Yaakov, Y.; Assayag, M.; Biton, I.E.; Tal, O.; Shakhar, G.; Ben-Hur, H.; et al. Preconditioning allows engraftment of mouse and human embryonic lung cells, enabling lung repair in mice. Nat. Med. 2015, 21, 869–879. [Google Scholar] [CrossRef]
- Farrow, N.; Cmielewski, P.; Donnelley, M.; Rout-Pitt, N.; Moodley, Y.; Bertoncello, I.; Parsons, D. Epithelial disruption: A new paradigm enabling human airway stem cell transplantation. Stem Cell Res. Ther. 2018, 9, 153–158. [Google Scholar] [CrossRef]
- Shiraishi, K.; Shichino, S.; Tsukui, T.; Hashimoto, S.; Ueha, S.; Matsushima, K. Engraftment and proliferation potential of embryonic lung tissue cells in irradiated mice with emphysema. Sci. Rep. 2019, 9, 3657. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Nichane, M.; Javed, A.; Sivakamasundari, V.; Ganesan, M.; Ang, L.T.; Kraus, P.; Lufkin, T.; Loh, K.M.; Lim, B. Isolation and 3D expansion of multipotent Sox9+ mouse lung progenitors. Nat. Methods 2017, 14, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.E.; Brumwell, A.N.; Xi, Y.; Gotts, J.E.; Brownfield, D.G.; Treutlein, B.; Tan, K.; Tan, V.; Liu, F.C.; Looney, M.R.; et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015, 517, 621–625. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchesneau, P.; Waddell, T.K.; Karoubi, G. Cell-Based Therapeutic Approaches for Cystic Fibrosis. Int. J. Mol. Sci. 2020, 21, 5219. https://doi.org/10.3390/ijms21155219
Duchesneau P, Waddell TK, Karoubi G. Cell-Based Therapeutic Approaches for Cystic Fibrosis. International Journal of Molecular Sciences. 2020; 21(15):5219. https://doi.org/10.3390/ijms21155219
Chicago/Turabian StyleDuchesneau, Pascal, Thomas K. Waddell, and Golnaz Karoubi. 2020. "Cell-Based Therapeutic Approaches for Cystic Fibrosis" International Journal of Molecular Sciences 21, no. 15: 5219. https://doi.org/10.3390/ijms21155219
APA StyleDuchesneau, P., Waddell, T. K., & Karoubi, G. (2020). Cell-Based Therapeutic Approaches for Cystic Fibrosis. International Journal of Molecular Sciences, 21(15), 5219. https://doi.org/10.3390/ijms21155219





