Trans-Axonal Signaling in Neural Circuit Wiring
Abstract
1. Introduction
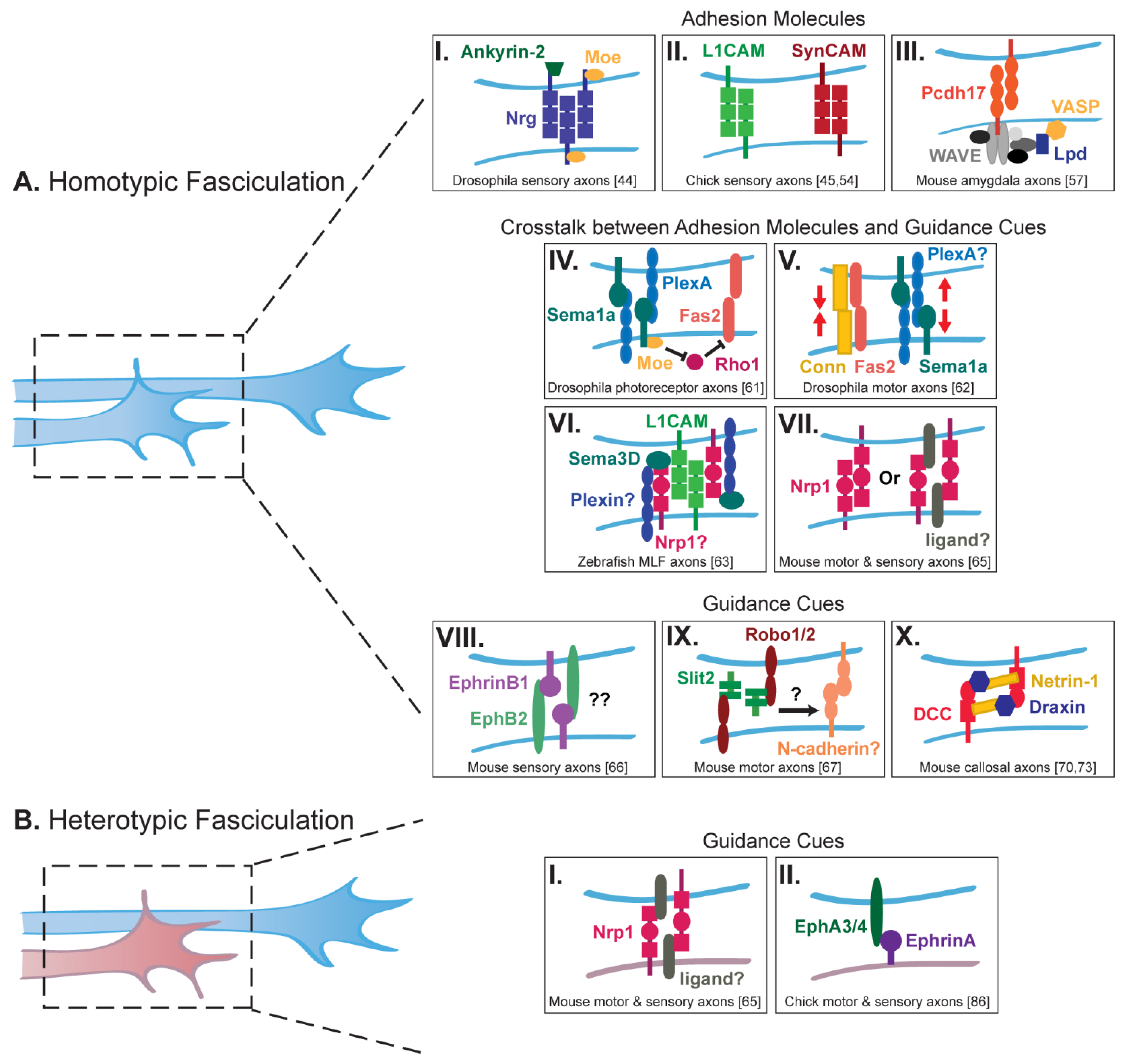
2. Axon Fasciculation and Adhesion
2.1. Homotypic Fasciculation
2.2. Heterotypic Fasciculation
3. Trans-Axonal Repulsion
3.1. Repulsion and Selective Defasciculation at Choice Points
3.2. Axon–Axon Repulsion between and within Tracts
3.3. Axon Repulsion at the Target
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALCAM | Activated leukocyte cell adhesion molecule |
| BMP | Bone morphogenetic protein |
| CAM | Cell adhesion molecule |
| cAMP | Cyclic adenosine monophosphate |
| Cas | Crk-associated substrate |
| CNS | Central nervous system |
| Conn | Connectin |
| CYFIP2 | Cytoplasmic FMR1-interacting protein 2 |
| DREZ | Dorsal root entry zone |
| DSCAM | Down syndrome cell adhesion molecule |
| ECM | Extracellular matrix |
| Fas2 | Fascilin 2 |
| Fmi | Flamingo |
| Gogo | Golden goal |
| HS | Heparan sulfate |
| HSPG | Heparan sulfate proteoglycan |
| ISN | Intersegmental nerve |
| LAMP | Limbic-system-associated protein |
| lHB | Lateral habenula |
| Lpd | Lamellipodin |
| MLF | Medial longitudinal fascicle |
| NCAM | Neural cell adhesion molecule |
| Nrg | Neuroglian |
| Nrp | Neuropilin |
| OB | Olfactory bulb |
| OE | Olfactory epithelium |
| OSN | Olfactory sensory neuron |
| Pbl | Pebble |
| Pcdh | Protocadherin |
| Plex | Plexin |
| PSA | Polysialic Acid |
| RGC | Retinal ganglion cell |
| SC | Superior colliculus |
| Sema | Semaphorin |
| Shh | Sonic hedgehog |
| SN | Segmental nerve |
| SynCAM | Synaptic cell adhesion molecule |
| TAG-1 | Transient axonal glycoprotein type-1 |
| VTA | Ventral tegmental area |
| WRC | WAVE regulatory complex |
References
- Cang, J.; Feldheim, D.A. Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 2013, 36, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Roig-Puiggros, S.; Vigouroux, R.J.; Beckman, D.; Bocai, N.I.; Chiou, B.; Davimes, J.; Gomez, G.; Grassi, S.; Hoque, A.; Karikari, T.K.; et al. Construction and reconstruction of brain circuits: Normal and pathological axon guidance. J. Neurochem. 2020, 153, 10–32. [Google Scholar] [CrossRef]
- McFadden, K.; Minshew, N.J. Evidence for dysregulation of axonal growth and guidance in the etiology of ASD. Front. Hum. Neurosci. 2013, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Schumann, C.M.; Nordahl, C.W. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Leinsinger, G.L.; Heiss, D.T.; Jassoy, A.G.; Pfluger, T.; Hahn, K.; Danek, A. Persistent mirror movements: Functional MR imaging of the hand motor cortex. Radiology 1997, 203, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.; Rivière, J.B.; Pham, J.M.; Dubé, M.P.; Girard, S.; Morin, S.; Dion, P.A.; Asselin, G.; Rochefort, D.; Hince, P.; et al. Mutations in DCC cause congenital mirror movements. Science 2010, 328, 592. [Google Scholar] [CrossRef]
- Volk, A.E.; Carter, O.; Fricke, J.; Herkenrath, P.; Poggenborg, J.; Borck, G.; Demant, A.W.; Ivo, R.; Eysel, P.; Kubisch, C.; et al. Horizontal gaze palsy with progressive scoliosis: Three novel ROBO3 mutations and descriptions of the phenotypes of four patients. Mol. Vis. 2011, 17, 1978–1986. [Google Scholar]
- Nugent, A.A.; Kolpak, A.L.; Engle, E.C. Human disorders of axon guidance. Curr. Opin. Neurobiol. 2012, 22, 837–843. [Google Scholar] [CrossRef]
- Van Battum, E.Y.; Brignani, S.; Pasterkamp, R.J. Axon guidance proteins in neurological disorders. Lancet Neurol. 2015, 14, 532–546. [Google Scholar] [CrossRef]
- Marsh, A.P.L.; Edwards, T.J.; Galea, C.; Cooper, H.M.; Engle, E.C.; Jamuar, S.S.; Méneret, A.; Moutard, M.L.; Nava, C.; Rastetter, A.; et al. DCC mutation update: Congenital mirror movements, isolated agenesis of the corpus callosum, and developmental split brain syndrome. Hum. Mutat. 2018, 39, 23–39. [Google Scholar] [CrossRef]
- Bellon, A.; Mann, F. Keeping up with advances in axon guidance. Cur. Opin. Neurobiol. 2018, 53, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.J. Molecular mechanisms of axon guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Missaire, M.; Hindges, R. The role of cell adhesion molecules in visual circuit formation: From neurite outgrowth to maps and synaptic specificity. Dev. Neurobiol. 2015, 75, 569–583. [Google Scholar] [CrossRef]
- Frei, J.A.; Stoeckli, E.T. SynCAMs—From axon guidance to neurodevelopmental disorders. Mol. Cell Neurosci. 2017, 81, 41–48. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ruiz de Almodovar, C. VEGF ligands and receptors: Implications in neurodevelopment and neurodegeneration. Cell Mol. Life Sci. 2013, 70, 1763–1778. [Google Scholar] [CrossRef]
- Sánchez-Camacho, C.; Bovolenta, P. Emerging mechanisms in morphogen-mediated axon guidance. Bioessays 2009, 31, 1013–1025. [Google Scholar] [CrossRef]
- Yam, P.T.; Charron, F. Signaling mechanisms of non-conventional axon guidance cues: The Shh, BMP and Wnt morphogens. Curr. Opin. Neurobiol. 2013, 23, 965–973. [Google Scholar] [CrossRef]
- Bonanomi, D. Axon pathfinding for locomotion. Semin. Cell Dev. Biol. 2019, 85, 26–35. [Google Scholar] [CrossRef]
- Howard, L.J.; Brown, H.E.; Wadsworth, B.C.; Evans, T.A. Midline axon guidance in the Drosophila embryonic central nervous system. Semin. Cell Dev. Biol. 2019, 85, 13–25. [Google Scholar] [CrossRef]
- Comer, J.D.; Alvarez, S.; Butler, S.J.; Kaltschmidt, J.A. Commissural axon guidance in the developing spinal cord: From Cajal to the present day. Neural. Dev. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Feldheim, D.A.; O’Leary, D.D. Visual map development: Bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb. Perspect. Biol. 2010, 2, a001768. [Google Scholar] [CrossRef] [PubMed]
- Erskine, L.; Herrera, E. Connecting the retina to the brain. ASN Neuro 2014, 6, 1759091414562107. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Luo, L. Development of wiring specificity in the olfactory system. Curr. Opin. Neurobiol. 2006, 16, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Imai, T. Positional information in neural map development: Lessons from the olfactory system. Dev. Growth Differ. 2012, 54, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Sperry, R.W. Chemoaffinity in the Orderly Growth of Nerve Fiber Patterns and Connections. Proc. Natl. Acad. Sci. USA 1963, 50, 703–710. [Google Scholar] [CrossRef]
- Petrovic, M.; Schmucker, D. Axonal wiring in neural development: Target-independent mechanisms help to establish precision and complexity. Bioessays News Rev. Mol. Cell. Dev. Biol. 2015, 37, 996–1004. [Google Scholar] [CrossRef]
- Wang, L.; Marquardt, T. What axons tell each other: Axon-axon signaling in nerve and circuit assembly. Curr. Opin. Neurobiol. 2013, 23, 974–982. [Google Scholar] [CrossRef]
- Landmesser, L.; Honig, M.G. Altered sensory projections in the chick hind limb following the early removal of motoneurons. Dev. Biol. 1986, 118, 511–531. [Google Scholar] [CrossRef]
- Honig, M.G.; Lance-Jones, C.; Landmesser, L. The development of sensory projection patterns in embryonic chick hindlimb under experimental conditions. Dev. Biol. 1986, 118, 532–548. [Google Scholar] [CrossRef]
- Swanson, G.J.; Lewis, J. Sensory nerve routes in chick wing buds deprived of motor innervation. J. Embryol. Exp. Morphol. 1986, 95, 37–52. [Google Scholar] [PubMed]
- Reh, T.A.; Pitts, E.; Constantine-Paton, M. The organization of the fibers in the optic nerve of normal and tectum-less Rana pipiens. J. Comp. Neurol. 1983, 218, 282–296. [Google Scholar] [CrossRef] [PubMed]
- St John, J.A.; Clarris, H.J.; McKeown, S.; Royal, S.; Key, B. Sorting and convergence of primary olfactory axons are independent of the olfactory bulb. J. Comp. Neurol. 2003, 464, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brand, A.H. Targeted neuronal ablation: The role of pioneer neurons in guidance and fasciculation in the CNS of Drosophila. Development 1997, 124, 3253–3262. [Google Scholar] [PubMed]
- Pike, S.H.; Melancon, E.F.; Eisen, J.S. Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons. Development 1992, 114, 825–831. [Google Scholar] [PubMed]
- Pittman, A.J.; Law, M.Y.; Chien, C.B. Pathfinding in a large vertebrate axon tract: Isotypic interactions guide retinotectal axons at multiple choice points. Development 2008, 135, 2865–2871. [Google Scholar] [CrossRef]
- Osterhout, J.A.; El-Danaf, R.N.; Nguyen, P.L.; Huberman, A.D. Birthdate and outgrowth timing predict cellular mechanisms of axon target matching in the developing visual pathway. Cell Rep. 2014, 8, 1006–1017. [Google Scholar] [CrossRef]
- Pignata, A.; Ducuing, H.; Boubakar, L.; Gardette, T.; Kindbeiter, K.; Bozon, M.; Tauszig-Delamasure, S.; Falk, J.; Thoumine, O.; Castellani, V. A Spatiotemporal Sequence of Sensitization to Slits and Semaphorins Orchestrates Commissural Axon Navigation. Cell Rep. 2019, 29, 347–362.e345. [Google Scholar] [CrossRef]
- Okumura, M.; Kato, T.; Miura, M.; Chihara, T. Hierarchical axon targeting of Drosophila olfactory receptor neurons specified by the proneural transcription factors Atonal and Amos. Genes Cells Devoted Mol. Cell. Mech. 2016, 21, 53–64. [Google Scholar] [CrossRef]
- Whitlock, K.E.; Westerfield, M. A transient population of neurons pioneers the olfactory pathway in the zebrafish. J. Neurosci. 1998, 18, 8919–8927. [Google Scholar] [CrossRef]
- Bak, M.; Fraser, S.E. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development (Camb. Engl.) 2003, 130, 4999–5008. [Google Scholar] [CrossRef] [PubMed]
- Šmít, D.; Fouquet, C.; Pincet, F.; Zapotocky, M.; Trembleau, A. Axon tension regulates fasciculation/defasciculation through the control of axon shaft zippering. eLife 2017, 6, e19907. [Google Scholar] [CrossRef] [PubMed]
- Schwarting, G.A.; Henion, T.R. Regulation and function of axon guidance and adhesion molecules during olfactory map formation. J. Cell Biochem. 2011, 112, 2663–2671. [Google Scholar] [CrossRef][Green Version]
- Siegenthaler, D.; Enneking, E.-M.; Moreno, E.; Pielage, J. L1CAM/Neuroglian controls the axon-axon interactions establishing layered and lobular mushroom body architecture. J. Cell Biol. 2015, 208, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; Camilli, S.J.; Xue, Q.-S. Effects of L1 blockade on sensory axon outgrowth and pathfinding in the chick hindlimb. Dev. Biol. 2002, 243, 137–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Honig, M.G.; Rutishauser, U.S. Changes in the segmental pattern of sensory neuron projections in the chick hindlimb under conditions of altered cell adhesion molecule function. Dev. Biol. 1996, 175, 325–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Landmesser, L.; Dahm, L.; Schultz, K.; Rutishauser, U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev. Biol. 1988, 130, 645–670. [Google Scholar] [CrossRef]
- Thanos, S.; Bonhoeffer, F.; Rutishauser, U. Fiber-fiber interaction and tectal cues influence the development of the chicken retinotectal projection. Proc. Natl. Acad. Sci. USA 1984, 81, 1906–1910. [Google Scholar] [CrossRef]
- Pollerberg, G.E.; Mack, T.G. Cell adhesion molecule SC1/DMGRASP is expressed on growing axons of retina ganglion cells and is involved in mediating their extension on axons. Dev. Biol. 1994, 165, 670–687. [Google Scholar] [CrossRef]
- Ott, H.; Bastmeyer, M.; Stuermer, C.A. Neurolin, the goldfish homolog of DM-GRASP, is involved in retinal axon pathfinding to the optic disk. J. Neurosci. 1998, 18, 3363–3372. [Google Scholar] [CrossRef]
- Thelen, K.; Maier, B.; Faber, M.; Albrecht, C.; Fischer, P.; Pollerberg, G.E. Translation of the cell adhesion molecule ALCAM in axonal growth cones—Regulation and functional importance. J. Cell Sci. 2012, 125, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.A.; Koo, S.J.; Nicolas, S.; Fraboulet, S.; Pfaff, S.L.; Pourquié, O.; Sanes, J.R. Axon fasciculation defects and retinal dysplasias in mice lacking the immunoglobulin superfamily adhesion molecule BEN/ALCAM/SC1. Mol. Cell Neurosci. 2004, 27, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bruce, F.M.; Brown, S.; Smith, J.N.; Fuerst, P.G.; Erskine, L. DSCAM promotes axon fasciculation and growth in the developing optic pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Frei, J.A.; Andermatt, I.; Gesemann, M.; Stoeckli, E.T. The SynCAM synaptic cell adhesion molecules are involved in sensory axon pathfinding by regulating axon-axon contacts. J. Cell Sci. 2014, 127, 5288–5302. [Google Scholar] [CrossRef]
- Suter, T.; Blagburn, S.V.; Fisher, S.E.; Anderson-Keightly, H.M.; D’Elia, K.P.; Jaworski, A. TAG-1 Multifunctionality Coordinates Neuronal Migration, Axon Guidance, and Fasciculation. Cell Rep. 2020, 30, 1164–1177. [Google Scholar] [CrossRef]
- Steimel, A.; Wong, L.; Najarro, E.H.; Ackley, B.D.; Garriga, G.; Hutter, H. The Flamingo ortholog FMI-1 controls pioneer-dependent navigation of follower axons in C. elegans. Development 2010, 137, 3663–3673. [Google Scholar] [CrossRef]
- Hayashi, S.; Inoue, Y.; Kiyonari, H.; Abe, T.; Misaki, K.; Moriguchi, H.; Tanaka, Y.; Takeichi, M. Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts. Dev. Cell 2014, 30, 673–687. [Google Scholar] [CrossRef]
- Yoshida, Y. Semaphorin signaling in vertebrate neural circuit assembly. Front. Mol. Neurosci. 2012, 5, 71. [Google Scholar] [CrossRef]
- Jongbloets, B.C.; Pasterkamp, R.J. Semaphorin signalling during development. Development 2014, 141, 3292–3297. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, Y.; Cheng, S.; Rao, Y. Plexin a-semaphorin-1a reverse signaling regulates photoreceptor axon guidance in Drosophila. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 12151–12156. [Google Scholar] [CrossRef]
- Hsieh, H.-H.; Chang, W.-T.; Yu, L.; Rao, Y. Control of axon-axon attraction by Semaphorin reverse signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 11383–11388. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Huang, A.S.; Kolodkin, A.L. Semaphorin-1a acts in concert with the cell adhesion molecules fasciclin II and connectin to regulate axon fasciculation in Drosophila. Genetics 2000, 156, 723–731. [Google Scholar] [PubMed]
- Wolman, M.A.; Regnery, A.M.; Becker, T.; Becker, C.G.; Halloran, M.C. Semaphorin3D regulates axon axon interactions by modulating levels of L1 cell adhesion molecule. J. Neurosci. 2007, 27, 9653–9663. [Google Scholar] [CrossRef] [PubMed]
- Castellani, V.; Chédotal, A.; Schachner, M.; Faivre-Sarrailh, C.; Rougon, G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron 2000, 27, 237–249. [Google Scholar] [CrossRef]
- Huettl, R.E.; Soellner, H.; Bianchi, E.; Novitch, B.G.; Huber, A.B. Npn-1 contributes to axon-axon interactions that differentially control sensory and motor innervation of the limb. PLoS Biol. 2011, 9, e1001020. [Google Scholar] [CrossRef] [PubMed]
- Luxey, M.; Jungas, T.; Laussu, J.; Audouard, C.; Garces, A.; Davy, A. Eph:ephrin-B1 forward signaling controls fasciculation of sensory and motor axons. Dev. Biol. 2013, 383, 264–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaworski, A.; Tessier-Lavigne, M. Autocrine/juxtaparacrine regulation of axon fasciculation by Slit-Robo signaling. Nat. Neurosci. 2012, 15, 367–369. [Google Scholar] [CrossRef]
- Shiau, C.E.; Bronner-Fraser, M. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development 2009, 136, 4155–4164. [Google Scholar] [CrossRef]
- Boyer, N.P.; Gupton, S.L. Revisiting Netrin-1: One Who Guides (Axons). Front. Cell Neurosci. 2018, 12, 221. [Google Scholar] [CrossRef]
- Islam, S.M.; Shinmyo, Y.; Okafuji, T.; Su, Y.; Naser, I.B.; Ahmed, G.; Zhang, S.; Chen, S.; Ohta, K.; Kiyonari, H.; et al. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science 2009, 323, 388–393. [Google Scholar] [CrossRef]
- Ahmed, G.; Shinmyo, Y.; Ohta, K.; Islam, S.M.; Hossain, M.; Naser, I.B.; Riyadh, M.A.; Su, Y.; Zhang, S.; Tessier-Lavigne, M.; et al. Draxin inhibits axonal outgrowth through the netrin receptor DCC. J. Neurosci. 2011, 31, 14018–14023. [Google Scholar] [CrossRef]
- Gao, X.; Metzger, U.; Panza, P.; Mahalwar, P.; Alsheimer, S.; Geiger, H.; Maischein, H.M.; Levesque, M.P.; Templin, M.; Söllner, C. A Floor-Plate Extracellular Protein-Protein Interaction Screen Identifies Draxin as a Secreted Netrin-1 Antagonist. Cell Rep. 2015, 12, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bhowmick, T.; Liu, Y.; Gao, X.; Mertens, H.D.T.; Svergun, D.I.; Xiao, J.; Zhang, Y.; Wang, J.-H.; Meijers, R. Structural Basis for Draxin-Modulated Axon Guidance and Fasciculation by Netrin-1 through DCC. Neuron 2018, 97, 1261–1267.e1264. [Google Scholar] [CrossRef] [PubMed]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urbán, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 2007, 316, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.; Aguado, T.; Keimpema, E.; Barabas, K.; Ballester Rosado, C.J.; Nguyen, L.; Monory, K.; Marsicano, G.; Di Marzo, V.; Hurd, Y.L.; et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. USA 2008, 105, 8760–8765. [Google Scholar] [CrossRef]
- Saez, T.M.; Fernandez Bessone, I.; Rodriguez, M.S.; Alloatti, M.; Otero, M.G.; Cromberg, L.E.; Pozo Devoto, V.M.; Oubina, G.; Sosa, L.; Buffone, M.G.; et al. Kinesin-1-mediated axonal transport of CB1 receptors is required for cannabinoid-dependent axonal growth and guidance. Development 2020. [Google Scholar] [CrossRef]
- Rash, B.G.; Richards, L.J. A role for cingulate pioneering axons in the development of the corpus callosum. J. Comp. Neurol. 2001, 434, 147–157. [Google Scholar] [CrossRef]
- Bianco, I.H.; Wilson, S.W. The habenular nuclei: A conserved asymmetric relay station in the vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1005–1020. [Google Scholar] [CrossRef]
- Kowski, A.B.; Veh, R.W.; Weiss, T. Dopaminergic activation excites rat lateral habenular neurons in vivo. Neuroscience 2009, 161, 1154–1165. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Jennings, J.H.; Ung, R.L.; Blair, G.A.; Weinberg, R.J.; Neve, R.L.; Boyce, F.; Mattis, J.; Ramakrishnan, C.; Deisseroth, K.; et al. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 2013, 80, 1039–1053. [Google Scholar] [CrossRef]
- Schmidt, E.R.E.; Brignani, S.; Adolfs, Y.; Lemstra, S.; Demmers, J.; Vidaki, M.; Donahoo, A.-L.S.; Lilleväli, K.; Vasar, E.; Richards, L.J.; et al. Subdomain-mediated axon-axon signaling and chemoattraction cooperate to regulate afferent innervation of the lateral habenula. Neuron 2014, 83, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Molnar, Z.; Adams, R.; Blakemore, C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J. Neurosci. 1998, 18, 5723–5745. [Google Scholar] [CrossRef] [PubMed]
- Hevner, R.F.; Miyashita-Lin, E.; Rubenstein, J.L.R. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: Evidence that cortical and thalamic axons interact and guide each other. J. Comp. Neurol. 2002, 447, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Deck, M.; Lokmane, L.; Chauvet, S.; Mailhes, C.; Keita, M.; Niquille, M.; Yoshida, M.; Yoshida, Y.; Lebrand, C.; Mann, F.; et al. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron 2013, 77, 472–484. [Google Scholar] [CrossRef]
- Chen, Y.; Magnani, D.; Theil, T.; Pratt, T.; Price, D.J. Evidence that descending cortical axons are essential for thalamocortical axons to cross the pallial-subpallial boundary in the embryonic forebrain. PLoS ONE 2012, 7, e33105. [Google Scholar] [CrossRef]
- Wang, L.; Klein, R.; Zheng, B.; Marquardt, T. Anatomical coupling of sensory and motor nerve trajectory via axon tracking. Neuron 2011, 71, 263–277. [Google Scholar] [CrossRef]
- Grueber, W.B.; Sagasti, A. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb. Perspect. Biol. 2010, 2, a001750. [Google Scholar] [CrossRef]
- Peng, J.; Fabre, P.J.; Dolique, T.; Swikert, S.M.; Kermasson, L.; Shimogori, T.; Charron, F. Sonic Hedgehog Is a Remotely Produced Cue that Controls Axon Guidance Trans-axonally at a Midline Choice Point. Neuron 2018, 97, 326–340.e324. [Google Scholar] [CrossRef]
- Araújo, S.J.; Tear, G. Axon guidance mechanisms and molecules: Lessons from invertebrates. Nat. Rev. Neurosci. 2003, 4, 910–922. [Google Scholar] [CrossRef]
- Yu, H.H.; Araj, H.H.; Ralls, S.A.; Kolodkin, A.L. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron 1998, 20, 207–220. [Google Scholar] [CrossRef]
- Jeong, S.; Juhaszova, K.; Kolodkin, A.L. The Control of semaphorin-1a-mediated reverse signaling by opposing pebble and RhoGAPp190 functions in drosophila. Neuron 2012, 76, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Chak, K.; Andreone, B.J.; Wooley, J.R.; Kolodkin, A.L. The extracellular matrix proteoglycan perlecan facilitates transmembrane semaphorin-mediated repulsive guidance. Genes Dev. 2012, 26, 2222–2235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yazdani, U.; Thompson-Peer, K.L.; Kolodkin, A.L.; Terman, J.R. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development 2007, 134, 2337–2347. [Google Scholar] [CrossRef]
- Gallarda, B.W.; Bonanomi, D.; Muller, D.; Brown, A.; Alaynick, W.A.; Andrews, S.E.; Lemke, G.; Pfaff, S.L.; Marquardt, T. Segregation of axial motor and sensory pathways via heterotypic trans-axonal signaling. Science 2008, 320, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Oishi, K.; Tabata, H.; Torii, K.; Nakajima, K. Segregation and pathfinding of callosal axons through EphA3 signaling. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 16251–16260. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; She, L.; Sui, Y.N.; Liu, L.; Richards, L.J.; Poo, M.M. Axon position within the corpus callosum determines contralateral cortical projection. Proc. Natl. Acad. Sci. USA 2013, 110, E2714–E2723. [Google Scholar] [CrossRef]
- Molnar, Z.; Garel, S.; Lopez-Bendito, G.; Maness, P.; Price, D.J. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. Eur. J. Neurosci. 2012, 35, 1573–1585. [Google Scholar] [CrossRef]
- Lokmane, L.; Proville, R.; Narboux-Nême, N.; Györy, I.; Keita, M.; Mailhes, C.; Léna, C.; Gaspar, P.; Grosschedl, R.; Garel, S. Sensory map transfer to the neocortex relies on pretarget ordering of thalamic axons. Curr. Biol. CB 2013, 23, 810–816. [Google Scholar] [CrossRef]
- Imai, T.; Yamazaki, T.; Kobayakawa, R.; Kobayakawa, K.; Abe, T.; Suzuki, M.; Sakano, H. Pre-target axon sorting establishes the neural map topography. Science 2009, 325, 585–590. [Google Scholar] [CrossRef]
- Sakano, H. Developmental regulation of olfactory circuit formation in mice. Dev. Growth Differ. 2020, 62, 199–213. [Google Scholar] [CrossRef]
- Imai, T.; Suzuki, M.; Sakano, H. Odorant receptor-derived cAMP signals direct axonal targeting. Science 2006, 314, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Chesler, A.T.; Zou, D.J.; Le Pichon, C.E.; Peterlin, Z.A.; Matthews, G.A.; Pei, X.; Miller, M.C.; Firestein, S. A G protein/cAMP signal cascade is required for axonal convergence into olfactory glomeruli. Proc. Natl. Acad. Sci. USA 2007, 104, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.; Fisher, S.A.; Stefanik, D.J.; Kim, J.; Raper, J.A. Coordination of olfactory receptor choice with guidance receptor expression and function in olfactory sensory neurons. PLoS Genet. 2018, 14, e1007164. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Takeuchi, H.; Imai, T.; Saito, H.; Kiyonari, H.; Abe, T.; Chen, M.; Weinstein, L.S.; Yu, C.R.; Storm, D.R.; et al. Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell 2013, 154, 1314–1325. [Google Scholar] [CrossRef]
- Kaneko, M.; Nighorn, A. Interaxonal Eph-ephrin signaling may mediate sorting of olfactory sensory axons in Manduca sexta. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 11523–11538. [Google Scholar] [CrossRef][Green Version]
- Scholes, J.H. Nerve fibre topography in the retinal projection to the tectum. Nature 1979, 278, 620–624. [Google Scholar] [CrossRef]
- Chan, S.O.; Guillery, R.W. Changes in fiber order in the optic nerve and tract of rat embryos. J. Comp. Neurol. 1994, 344, 20–32. [Google Scholar] [CrossRef]
- Plas, D.T.; Lopez, J.E.; Crair, M.C. Pretarget sorting of retinocollicular axons in the mouse. J. Comp. Neurol. 2005, 491, 305–319. [Google Scholar] [CrossRef]
- Stuermer, C.A. Retinotopic organization of the developing retinotectal projection in the zebrafish embryo. J. Neurosci. 1988, 8, 4513–4530. [Google Scholar] [CrossRef]
- Simon, D.K.; O’Leary, D.D. Relationship of retinotopic ordering of axons in the optic pathway to the formation of visual maps in central targets. J. Comp. Neurol. 1991, 307, 393–404. [Google Scholar] [CrossRef]
- Lee, J.S.; von der Hardt, S.; Rusch, M.A.; Stringer, S.E.; Stickney, H.L.; Talbot, W.S.; Geisler, R.; Nusslein-Volhard, C.; Selleck, S.B.; Chien, C.B.; et al. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer). Neuron 2004, 44, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Trowe, T.; Klostermann, S.; Baier, H.; Granato, M.; Crawford, A.D.; Grunewald, B.; Hoffmann, H.; Karlstrom, R.O.; Meyer, S.U.; Muller, B.; et al. Mutations disrupting the ordering and topographic mapping of axons in the retinotectal projection of the zebrafish, Danio rerio. Development 1996, 123, 439–450. [Google Scholar] [PubMed]
- Poulain, F.E.; Chien, C.B. Proteoglycan-mediated axon degeneration corrects pretarget topographic sorting errors. Neuron 2013, 78, 49–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hornberg, H.; Cioni, J.M.; Harris, W.A.; Holt, C.E. Hermes Regulates Axon Sorting in the Optic Tract by Post-Trancriptional Regulation of Neuropilin 1. J. Neurosci. 2016, 36, 12697–12706. [Google Scholar] [CrossRef] [PubMed]
- Baudet, M.L.; Zivraj, K.H.; Abreu-Goodger, C.; Muldal, A.; Armisen, J.; Blenkiron, C.; Goldstein, L.D.; Miska, E.A.; Holt, C.E. miR-124 acts through CoREST to control onset of Sema3A sensitivity in navigating retinal growth cones. Nat. Neurosci. 2011, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Cioni, J.M.; Wong, H.H.; Bressan, D.; Kodama, L.; Harris, W.A.; Holt, C.E. Axon-Axon Interactions Regulate Topographic Optic Tract Sorting via CYFIP2-Dependent WAVE Complex Function. Neuron 2018, 97, 1078–1093.e1076. [Google Scholar] [CrossRef] [PubMed]
- Pittman, A.J.; Gaynes, J.A.; Chien, C.B. nev (cyfip2) is required for retinal lamination and axon guidance in the zebrafish retinotectal system. Dev. Biol. 2010, 344, 784–794. [Google Scholar] [CrossRef][Green Version]
- Luo, L.; Flanagan, J.G. Development of continuous and discrete neural maps. Neuron 2007, 56, 284–300. [Google Scholar] [CrossRef]
- Imai, T.; Sakano, H. Axon-axon interactions in neuronal circuit assembly: Lessons from olfactory map formation. Eur. J. Neurosci. 2011, 34, 1647–1654. [Google Scholar] [CrossRef]
- Goyal, G.; Zierau, A.; Lattemann, M.; Bergkirchner, B.; Javorski, D.; Kaur, R.; Hummel, T. Inter-axonal recognition organizes Drosophila olfactory map formation. Sci. Rep. 2019, 9, 11554. [Google Scholar] [CrossRef]
- Takeuchi, H.; Inokuchi, K.; Aoki, M.; Suto, F.; Tsuboi, A.; Matsuda, I.; Suzuki, M.; Aiba, A.; Serizawa, S.; Yoshihara, Y.; et al. Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell 2010, 141, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.B.; Couto, A.; Chou, Y.-H.; Berdnik, D.; Dickson, B.J.; Luo, L.; Komiyama, T. Temporal target restriction of olfactory receptor neurons by Semaphorin-1a/PlexinA-mediated axon-axon interactions. Neuron 2007, 53, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Sagasti, A.; Guido, M.R.; Raible, D.W.; Schier, A.F. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr. Biol. 2005, 15, 804–814. [Google Scholar] [CrossRef]
- Gosse, N.J.; Nevin, L.M.; Baier, H. Retinotopic order in the absence of axon competition. Nature 2008, 452, 892–895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Triplett, J.W.; Pfeiffenberger, C.; Yamada, J.; Stafford, B.K.; Sweeney, N.T.; Litke, A.M.; Sher, A.; Koulakov, A.A.; Feldheim, D.A. Competition is a driving force in topographic mapping. Proc. Natl. Acad. Sci. USA 2011, 108, 19060–19065. [Google Scholar] [CrossRef]
- Suetterlin, P.; Drescher, U. Target-independent ephrina/EphA-mediated axon-axon repulsion as a novel element in retinocollicular mapping. Neuron 2014, 84, 740–752. [Google Scholar] [CrossRef]
- Senti, K.-A.; Usui, T.; Boucke, K.; Greber, U.; Uemura, T.; Dickson, B.J. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Curr. Biol. CB 2003, 13, 828–832. [Google Scholar] [CrossRef]
- Hakeda-Suzuki, S.; Berger-Müller, S.; Tomasi, T.; Usui, T.; Horiuchi, S.Y.; Uemura, T.; Suzuki, T. Golden Goal collaborates with Flamingo in conferring synaptic-layer specificity in the visual system. Nat. Neurosci. 2011, 14, 314–323. [Google Scholar] [CrossRef]
- Tomasi, T.; Hakeda-Suzuki, S.; Ohler, S.; Schleiffer, A.; Suzuki, T. The transmembrane protein Golden goal regulates R8 photoreceptor axon-axon and axon-target interactions. Neuron 2008, 57, 691–704. [Google Scholar] [CrossRef]
- Millard, S.S.; Flanagan, J.J.; Pappu, K.S.; Wu, W.; Zipursky, S.L. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature 2007, 447, 720–724. [Google Scholar] [CrossRef]
- Katori, S.; Noguchi-Katori, Y.; Okayama, A.; Kawamura, Y.; Luo, W.; Sakimura, K.; Hirabayashi, T.; Iwasato, T.; Yagi, T. Protocadherin-αC2 is required for diffuse projections of serotonergic axons. Sci. Rep. 2017, 7, 15908. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.V.; Nwakeze, C.L.; Denny, C.A.; O’Keeffe, S.; Rieger, M.A.; Mountoufaris, G.; Kirner, A.; Dougherty, J.D.; Hen, R.; Wu, Q.; et al. Pcdhαc2 is required for axonal tiling and assembly of serotonergic circuitries in mice. Science 2017, 356, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Neukomm, L.J.; Freeman, M.R. Diverse cellular and molecular modes of axon degeneration. Trends Cell Biol. 2014, 24, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Burrill, J.D.; Easter, S.S., Jr. The first retinal axons and their microenvironment in zebrafish: Cryptic pioneers and the pretract. J. Neurosci. 1995, 15, 2935–2947. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spead, O.; Poulain, F.E. Trans-Axonal Signaling in Neural Circuit Wiring. Int. J. Mol. Sci. 2020, 21, 5170. https://doi.org/10.3390/ijms21145170
Spead O, Poulain FE. Trans-Axonal Signaling in Neural Circuit Wiring. International Journal of Molecular Sciences. 2020; 21(14):5170. https://doi.org/10.3390/ijms21145170
Chicago/Turabian StyleSpead, Olivia, and Fabienne E. Poulain. 2020. "Trans-Axonal Signaling in Neural Circuit Wiring" International Journal of Molecular Sciences 21, no. 14: 5170. https://doi.org/10.3390/ijms21145170
APA StyleSpead, O., & Poulain, F. E. (2020). Trans-Axonal Signaling in Neural Circuit Wiring. International Journal of Molecular Sciences, 21(14), 5170. https://doi.org/10.3390/ijms21145170




