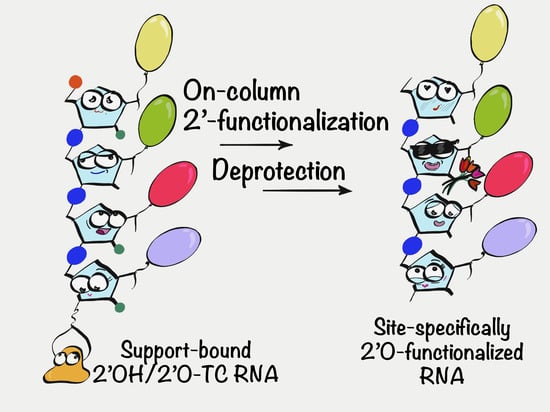
Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method
Abstract
1. Introduction
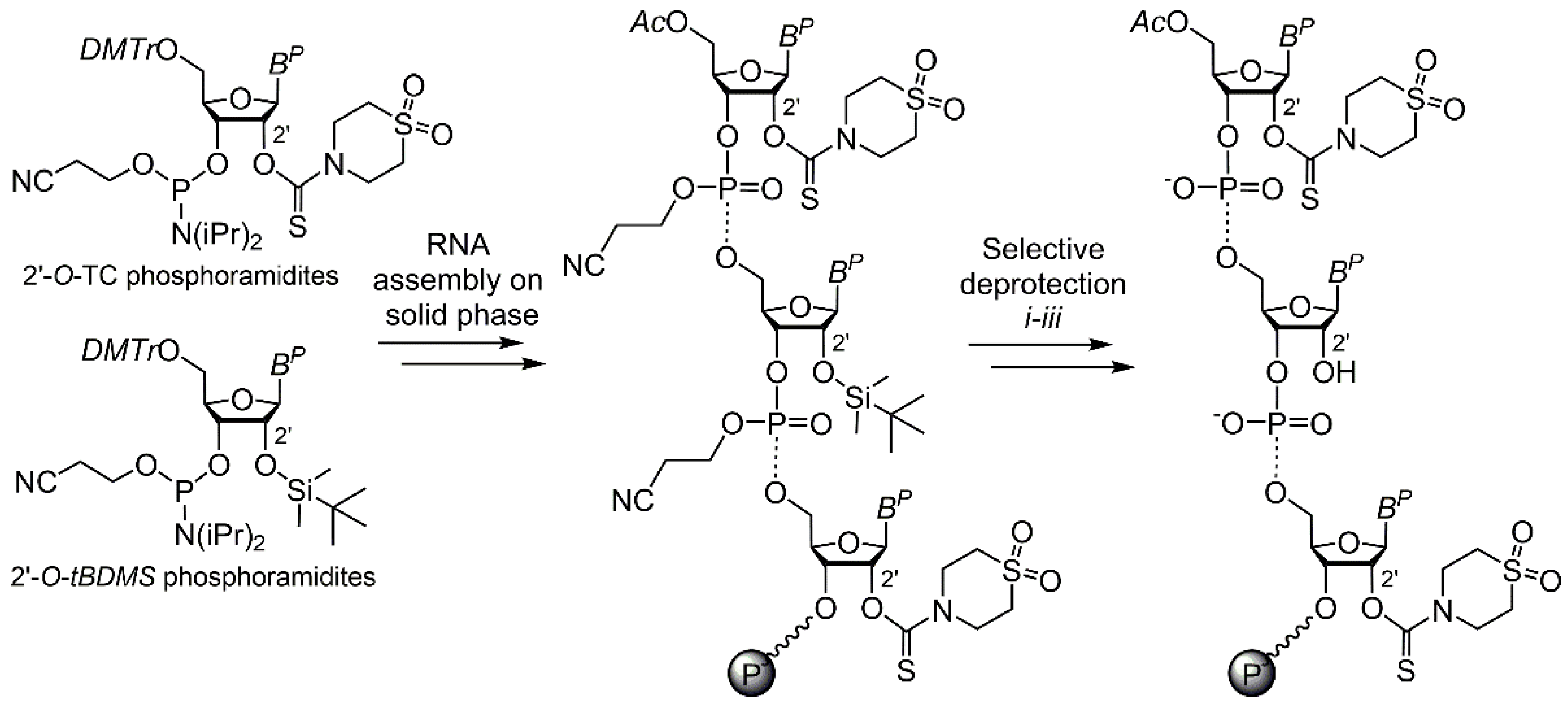
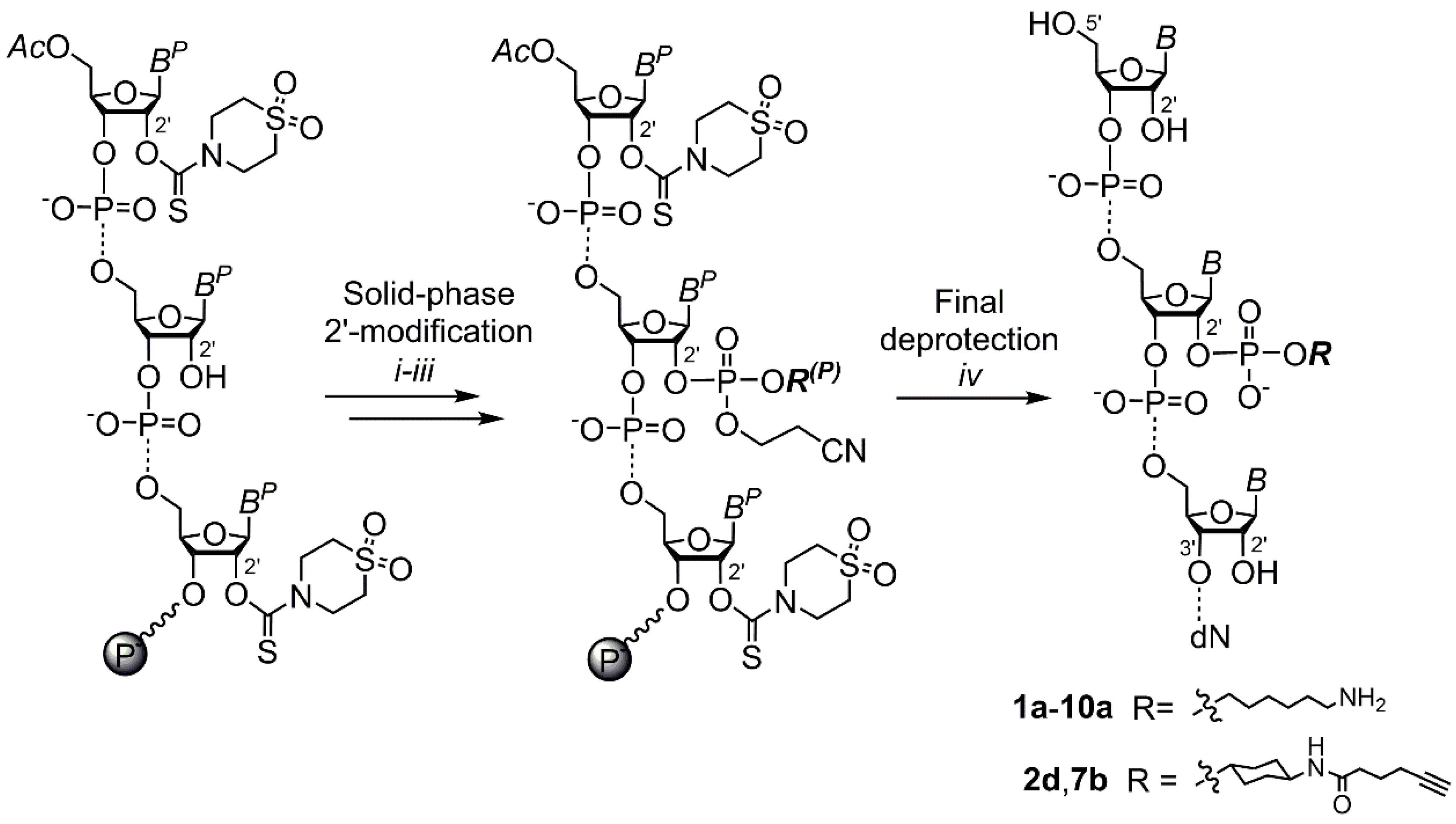
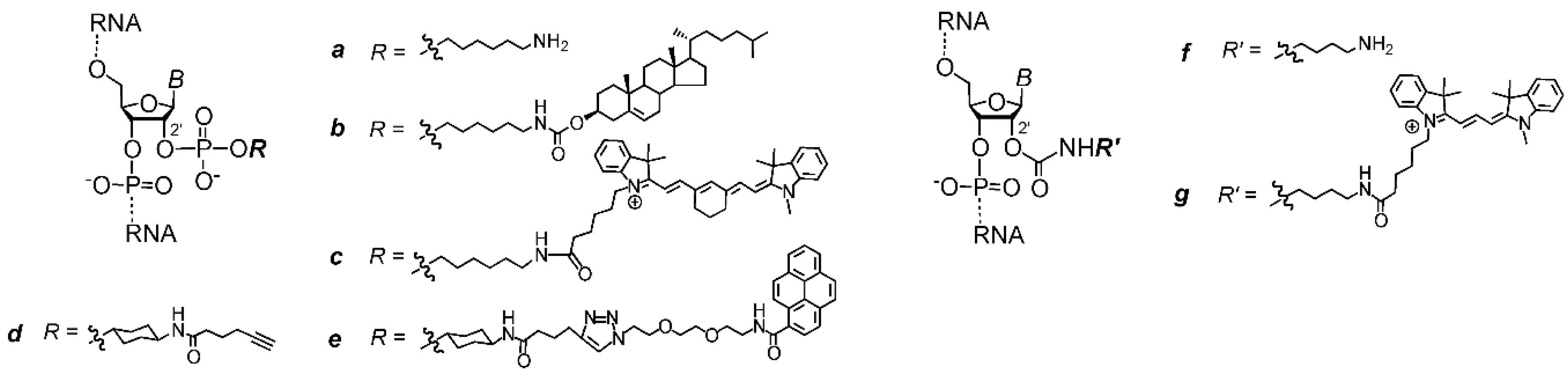
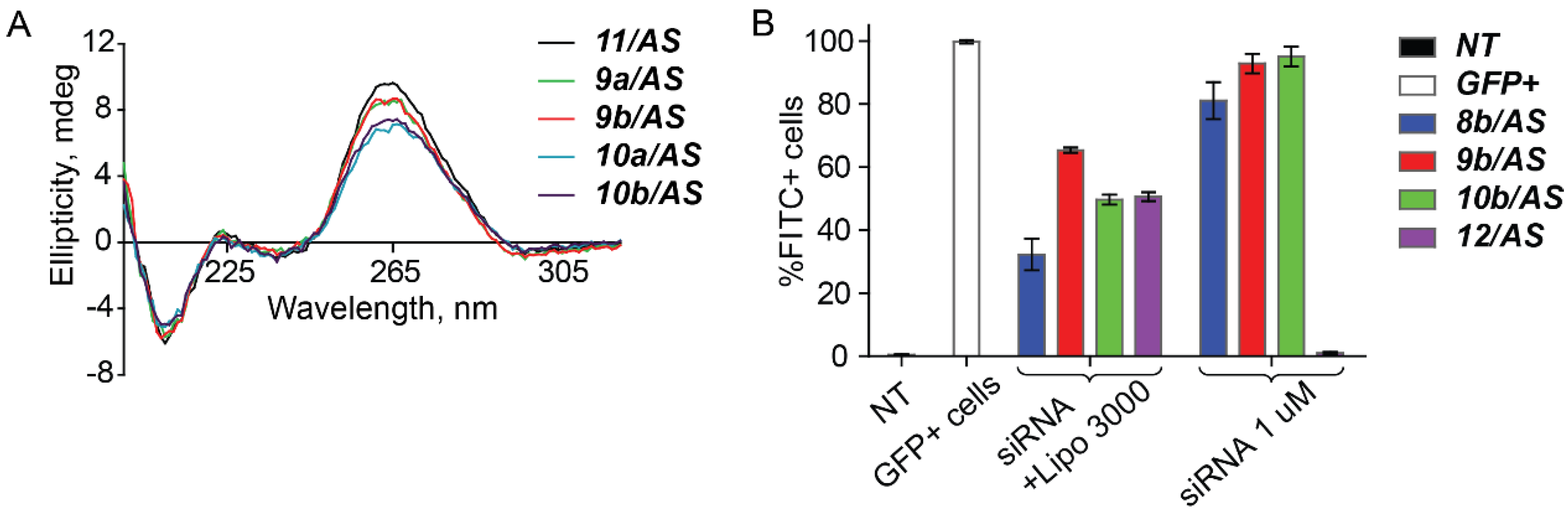
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Oligoribonucleotide Synthesis and Selective Partial Deprotection
3.3. Solid-Phase 2′ Modification by Modifying Phosphoramidite
3.4. Final Deprotection and Purification of Oligoribonucleotides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RNA | Ribonucleic acid |
| siRNAs | Small interfering RNAs |
| miRNAs | MicroRNA |
| sgRNAs | Single guide RNA |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| MALDI-TOF | Matrix-assisted laser desorption/ionization |
| ESI | Electrospray ionization |
| PAGE | Polyacrylamide gel electrophoresis |
| RP-HPLC | Reversed phase high performance liquid chromatography |
| ON | Oligonucleotide |
References
- Fauster, K.; Hartl, M.; Santner, T.; Aigner, M.; Kreutz, C.; Bister, K.; Ennifar, E.; Micura, R. 2′-Azido RNA, a Versatile Tool for Chemical Biology: Synthesis, X-ray Structure, siRNA Applications, Click Labeling. ACS Chem. Biol. 2012, 7, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pei, F.; Zhang, J.; Wu, J.; Feng, M.; Wang, Y.; Jin, H.; Zhang, L.; Tang, X. Synthesis of Site-Specifically Phosphate-Caged siRNAs and Evaluation of Their RNAi Activity and Stability. Chem. Eur. J. 2014, 20, 12114–12122. [Google Scholar] [CrossRef]
- Meade, B.R.; Gogoi, K.; Hamil, A.S.; Palm-Apergi, C.; van den Berg, A.; Hagopian, J.C.; Springer, A.D.; Eguchi, A.; Kacsinta, A.D.; Dowdy, C.F.; et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 2014, 32, 1256–1261. [Google Scholar] [CrossRef]
- Selvam, C.; Mutisya, D.; Prakash, S.; Ranganna, K.; Thilagavathi, R. Therapeutic potential of chemically modified siRNA: Recent trends. Chem. Biol. Drug Des. 2017, 90, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Dovydenko, I.; Tarassov, I.; Venyaminova, A.; Entelis, N. Method of Carrier-Free Delivery of Therapeutic RNA Importable Into Human Mitochondria: Lipophilic Conjugates With Cleavable Bonds. Biomaterials 2016, 76, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Granqvist, L.; Virta, P. 2′-O-[(4-CF3-triazol-1-yl)methyl] Uridine—A Sensitive 19F NMR Sensor for the Detection of RNA Secondary Structures. J. Org. Chem. 2015, 80, 7961–7970. [Google Scholar] [CrossRef]
- Neuner, S.; Santner, T.; Kreutz, C.; Micura, R. The “Speedy” Synthesis of Atom-Specific 15N Imino/Amido-Labeled RNA. Chem. Eur. J. 2015, 21, 11634–11643. [Google Scholar] [CrossRef]
- Krasheninina, O.A.; Novopashina, D.S.; Lomzov, A.A.; Venyaminova, A.G. 2′-Bispyrene-Modified 2′-O -Methyl RNA Probes as Useful Tools for the Detection of RNA: Synthesis, Fluorescent Properties, and Duplex Stability. ChemBioChem 2014, 15, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, T.S.; Romanova, E.A.; Oretskaya, T.S. Nucleosides and oligonucleotides containing 2′-reactive groups: Synthesis and applications. Russ. Chem. Rev. 2004, 73, 701–733. [Google Scholar] [CrossRef]
- Azéma, L.; Bathany, K.; Rayner, B. 2′-O-Appended Polyamines that Increase Triple-Helix-Forming Oligonucleotide Affinity are Selected by Dynamic Combinatorial Chemistry. ChemBioChem 2010, 11, 2513–2516. [Google Scholar] [CrossRef]
- Jeong, J.H.; Mok, H.; Oh, Y.-K.; Park, T.G. siRNA Conjugate Delivery Systems. Bioconjug. Chem. 2009, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Biscans, A.; Bertrand, J.-R.; Dubois, J.; Rüger, J.; Vasseur, J.-J.; Sczakiel, G.; Dupouy, C.; Debart, F. Lipophilic 2′-O-Acetal Ester RNAs: Synthesis, Thermal Duplex Stability, Nuclease Resistance, Cellular Uptake, and siRNA Activity after Spontaneous Naked Delivery. ChemBioChem 2016, 17, 2054–2062. [Google Scholar] [CrossRef] [PubMed]
- Biscans, A.; Rouanet, S.; Vasseur, J.-J.; Dupouy, C.; Debart, F. A versatile post-synthetic method on a solid support for the synthesis of RNA containing reduction-responsive modifications. Org. Biomol. Chem. 2016, 14, 7010–7017. [Google Scholar] [CrossRef] [PubMed]
- Aigner, M.; Hartl, M.; Fauster, K.; Steger, J.; Bister, K.; Micura, R. Chemical Synthesis of Site-Specifically 2′-Azido-Modified RNA and Potential Applications for Bioconjugation and RNA Interference. ChemBioChem 2011, 12, 47–51. [Google Scholar] [CrossRef]
- Matsuda, S.; Keiser, K.; Nair, J.K.; Charisse, K.; Manoharan, R.M.; Kretschmer, P.; Peng, C.G.; Kel’in, A.V.; Kandasamy, P.; Willoughby, J.L.S.; et al. siRNA Conjugates Carrying Sequentially Assembled Trivalent N-Acetylgalactosamine Linked Through Nucleosides Elicit Robust Gene Silencing In Vivo in Hepatocytes. ACS Chem. Biol. 2015, 10, 1181–1187. [Google Scholar] [CrossRef]
- Steinmeyer, J.; Walter, H.-K.; Bichelberger, M.A.; Schneider, V.; Kubař, T.; Rönicke, F.; Olshausen, B.; Nienhaus, K.; Nienhaus, G.U.; Schepers, U. “siRNA traffic lights”: Arabino-configured 2′-anchors for fluorescent dyes are key for dual color readout in cell imaging. Org. Biomol. Chem. 2018, 16, 3726–3731. [Google Scholar] [CrossRef]
- Pradère, U.; Brunschweiger, A.; Gebert, L.F.R.; Lucic, M.; Roos, M.; Hall, J. Chemical Synthesis of Mono- and Bis-Labeled Pre-MicroRNAs. Angew. Chem. Int. Ed. Engl. 2013, 52, 12028–12032. [Google Scholar] [CrossRef]
- Pradère, U.; Hall, J. Site-Specific Difunctionalization of Structured RNAs Yields Probes for microRNA Maturation. Bioconjug. Chem. 2016, 27, 681–687. [Google Scholar] [CrossRef]
- Menzi, M.; Pradère, U.; Wang, Y.; Fischer, M.; Baumann, F.; Bigatti, M.; Hall, J. Site-Specific Labeling of MicroRNA Precursors: A Structure–Activity Relationship Study. ChemBioChem 2016, 17, 2012–2017. [Google Scholar] [CrossRef]
- Fonvielle, M.; Mellal, D.; Patin, D.; Lecerf, M.; Blanot, D.; Bouhss, A.; Santarem, M.; Mengin-Lecreulx, D.; Sollogoub, M.; Arthur, M.; et al. Efficient Access to Peptidyl-RNA Conjugates for Picomolar Inhibition of Non-ribosomal FemXWv Aminoacyl Transferase. Chem. Eur. J. 2013, 19, 1357–1363. [Google Scholar] [CrossRef]
- Damha, M.J.; Ganeshan, K.; Hudson, R.H.; Zabarylo, S.V. Solid-phase synthesis of branched oligoribonucleotides related to messenger RNA splicing intermediates. Nucleic Acids Res. 1992, 20, 6565–6573. [Google Scholar] [CrossRef] [PubMed]
- Carriero, S.; Damha, M. Template-Mediated Synthesis of Lariat RNA and DNA. J. Org. Chem. 2003, 68, 8328–8338. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Damha, M.J. A Novel Approach to the Synthesis of DNA and RNA Lariats. J. Org. Chem. 2007, 72, 9491–9500. [Google Scholar] [CrossRef] [PubMed]
- Katolik, A.; Johnsson, R.; Montemayor, E.; Lackey, J.G.; Hart, P.J.; Damha, M.J. Regiospecific Solid-Phase Synthesis of Branched Oligoribonucleotides That Mimic Intronic Lariat RNA Intermediates. J. Org. Chem. 2014, 79, 963–975. [Google Scholar] [CrossRef]
- Braich, R.S.; Damha, M.J. Regiospecific Solid-Phase Synthesis of Branched Oligonucleotides. Effect of Vicinal 2‘,5‘- (or 2‘,3‘-) and 3‘,5‘-Phosphodiester Linkages on the Formation of Hairpin DNA. Bioconjug. Chem. 1997, 8, 370–377. [Google Scholar] [CrossRef]
- Plangger, R.; Juen, M.A.; Hoernes, T.P.; Nußbaumer, F.; Kremser, J.; Strebitzer, E.; Klingler, D.; Erharter, K.; Tollinger, M.; Erlacher, M.D.; et al. Branch site bulge conformations in domain 6 determine functional sugar puckers in group II intron splicing. Nucleic Acids Res. 2019, 47, 11430–11440. [Google Scholar] [CrossRef]
- Kadina, A.; Kietrys, A.M.; Kool, E.T. RNA Cloaking by Reversible Acylation. Angew. Chem. Int. Ed. 2018, 57, 3059–3063. [Google Scholar] [CrossRef]
- Velema, W.A.; Kietrys, A.M.; Kool, E.T. RNA Control by Photoreversible Acylation. J. Am. Chem. Soc. 2018, 140, 3491–3495. [Google Scholar] [CrossRef]
- Velema, W.A.; Kool, E.T. The chemistry and applications of RNA 2′-OH acylation. Nat. Rev. Chem. 2020, 4, 22–37. [Google Scholar] [CrossRef]
- Cieślak, J.; Ausín, C.; Grajkowski, A.; Beaucage, S.L. Convenient and Efficient Approach to the Permanent or Reversible Conjugation of RNA and DNA Sequences with Functional Groups. Curr. Protoc. Nucleic Acid Chem. 2012, 50, 4.52.1–4.52.36. [Google Scholar] [CrossRef]
- Cieslak, J.; Grajkowski, A.; Ausín, C.; Gapeev, A.; Beaucage, S.L. Permanent or Reversible Conjugation of 2′-O- Or 5′-O-aminooxymethylated Nucleosides With Functional Groups as a Convenient and Efficient Approach to the Modification of RNA and DNA Sequences. Nucleic Acids Res. 2012, 40, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Biscans, A.; Bos, M.; Martin, A.R.; Ader, N.; Sczakiel, G.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Direct Synthesis of Partially Modified 2′-O-pivaloyloxymethyl RNAs by a Base-Labile Protecting Group Strategy and Their Potential for Prodrug-Based Gene-Silencing Applications. ChemBioChem 2014, 15, 2674–2679. [Google Scholar] [CrossRef]
- Lavergne, T.; Baraguey, C.; Dupouy, C.; Parey, N.; Wuensche, W.; Sczakiel, G.; Vasseur, J.-J.; Debart, F. Synthesis and Preliminary Evaluation of pro-RNA 2′-O-Masked with Biolabile Pivaloyloxymethyl Groups in an RNA Interference Assay. J. Org. Chem. 2011, 76, 5719–5731. [Google Scholar] [CrossRef]
- Biscans, A.; Rouanet, S.; Bertrand, J.-R.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Synthesis, binding, nuclease resistance and cellular uptake properties of 2′-O-acetalester-modified oligonucleotides containing cationic groups. Bioorg. Med. Chem. 2015, 23, 5360–5368. [Google Scholar] [CrossRef]
- Gauthier, F.; Claveau, S.; Bertrand, J.-R.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Gymnotic Delivery and Gene Silencing Activity of Reduction-Responsive siRNAs Bearing Lipophilic Disulfide-Containing Modifications at 2′-position. Bioorg. Med. Chem. 2018, 26, 4635–4643. [Google Scholar] [CrossRef]
- Gauthier, F.; Bertrand, J.-R.; Vasseur, J.-J.; Dupouy, C.; Debart, F. Conjugation of Doxorubicin to siRNA Through Disulfide-based Self-immolative Linkers. Molecules 2020, 25, 2714. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, D.J.; Timár, Z.; Myerson, J.; Sierzchala, A.B.; Turner, J.; Ferreira, F.; Kupihár, Z.; Dellinger, G.; Hill, K.W.; Powell, J.A.; et al. Streamlined Process for the Chemical Synthesis of RNA Using 2′-O-Thionocarbamate-Protected Nucleoside Phosphoramidites in the Solid Phase. J. Am. Chem. Soc. 2011, 133, 11540–11556. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.; Ogilvie, K.K.; Jiang, M.Y.; Cedergren, R.J. The automated chemical synthesis of long oligoribuncleotides using 2′-O-silylated ribonucleoside 3′-O-phosphoramidites on a controlled-pore glass support: Synthesis of a 43-nucleotide sequence similar to the 3′-half molecule of an Escherichia coli formylmethionine tRNA. J. Am. Chem. Soc. 1987, 109, 7845–7854. [Google Scholar] [CrossRef]
- Wu, X.; Pitsch, S. Synthesis and Pairing Properties of Oligoribonucleotide Analogues Containing a Metal-Binding Site Attached to beta-D-allofuranosyl Cytosine. Nucleic Acids Res. 1998, 26, 4315–4323. [Google Scholar] [CrossRef]
- Pitsch, S.; Weiss, P.A.; Jenny, L.; Stutz, A.; Wu, X. Reliable Chemical Synthesis of Oligoribonucleotides (RNA) with 2′-O-[(Triisopropylsilyl)oxy]methyl(2′-O-tom)-Protected Phosphoramidites. Helv. Chim. Acta 2001, 84, 3773–3795. [Google Scholar] [CrossRef]
- Ohgi, T.; Masutomi, Y.; Ishiyama, K.; Kitagawa, H.; Shiba, Y.; Yano, J. A New RNA Synthetic Method with a 2‘-O-(2-Cyanoethoxymethyl) Protecting Group. Org. Lett. 2005, 7, 3477–3480. [Google Scholar] [CrossRef]
- Shiba, Y.; Masuda, H.; Watanabe, N.; Ego, T.; Takagaki, K.; Ishiyama, K.; Ohgi, T.; Yano, J. Chemical synthesis of a very long oligoribonucleotide with 2-cyanoethoxymethyl (CEM) as the 2′-O-protecting group: Structural identification and biological activity of a synthetic 110mer precursor-microRNA candidate. Nucleic Acids Res. 2007, 35, 3287–3296. [Google Scholar] [CrossRef] [PubMed]
- Yamakage, S.; Sakatsume, O.; Furuyama, E.; Takaku, H. 1-(2-Chloroethoxy)ethyl group for the protection of 2′-hydroxyl group in the synthesis of oligoribonucleotides. Tetrahedron Lett. 1989, 30, 6361–6364. [Google Scholar] [CrossRef]
- Lavergne, T.; Bertrand, J.-R.; Vasseur, J.-J.; Debart, F. A Base-Labile Group for 2′-OH Protection of Ribonucleosides: A Major Challenge for RNA Synthesis. Chem. Eur. J. 2008, 14, 9135–9138. [Google Scholar] [CrossRef]
- Lavergne, T.; Janin, M.; Dupouy, C.; Vasseur, J.-J.; Debart, F. Chemical Synthesis of RNA With Base-Labile 2′-O-(pivaloyloxymethyl)-protected Ribonucleoside Phosphoramidites. Curr. Protoc. Nucleic Acid Chem. 2010, 43, 3.19.1–3.19.27. [Google Scholar] [CrossRef]
- Semenyuk, A.; Földesi, A.; Johansson, T.; Estmer-Nilsson, C.; Blomgren, P.; Brännvall, M.; Kirsebom, L.A.; Kwiatkowski, M. Synthesis of RNA Using 2‘-O-DTM Protection. J. Am. Chem. Soc. 2006, 128, 12356–12357. [Google Scholar] [CrossRef]
- Kierzek, R. The stability of trisubstituted internucleotide bond in the presence of vicinal 2′-hydroxyl. Chemical synthesis of the uridyl-(2′-phosphate)-3′-5′-uridine. Nucleosides Nucleotides Nucleic Acids 1994, 13, 1757–1768. [Google Scholar] [CrossRef]
- Sekine, M.; Tsuruoka, H.; Iimura, S.; Kusuoku, H.; Wada, T.; Furusawa, K. Studies on Steric and Electronic Control of 2‘−3‘ Phosphoryl Migration in 2‘-Phosphorylated Uridine Derivatives and Its Application to the Synthesis of 2‘-Phosphorylated Oligouridylates. J. Org. Chem. 1996, 61, 4087–4100. [Google Scholar] [CrossRef] [PubMed]
- Wincott, F.; DiRenzo, A.; Shaffer, C.; Grimm, S.; Tracz, D.; Workman, C.; Sweedler, D.; Gonzalez, C.; Scaringe, S.; Usman, N. Synthesis, deprotection, analysis and purification of RNA and ribozymes. Nucleic Acids Res. 1995, 23, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Prhavc, M.; Lesnik, E.A.; Mohan, V.; Manoharan, M. 2′-O-Carbamate-containing oligonucleotides: Synthesis and properties. Tetrahedron Lett. 2001, 42, 8777–8780. [Google Scholar] [CrossRef]
- Korshun, V.A.; Stetsenko, D.A.; Gait, M.J. Novel uridin-2′-yl carbamates: Synthesis, incorporation into oligodeoxyribonucleotides, and remarkable fluorescence properties of 2′-pyren-1-ylmethylcarbamate. J. Chem. Soc. Perkin Trans. 1 2002, 1092–1104. [Google Scholar] [CrossRef]
- Korshun, V.A.; Stetsenko, D.A.; Gait, M.J. Uridine 2′-carbamates: Facile Tools for Oligonucleotide 2′-functionalization. Curr. Protoc. Nucleic Acid Chem. 2004, 15, 4.21.1–4.21.26. [Google Scholar] [CrossRef] [PubMed]
- Seio, K.; Tawarada, R.; Sasami, T.; Serizawa, M.; Ise, M.; Ohkubo, A.; Sekine, M. Synthesis and hybridization of 2′-O-methyl-RNAs incorporating 2′-O-carbamoyluridine and unique participation of the carbamoyl group in U–G base pair. Bioorg. Med. Chem. 2009, 17, 7275–7280. [Google Scholar] [CrossRef] [PubMed]
- Seio, K.; Tokugawa, M.; Kanamori, T.; Tsunoda, H.; Ohkubo, A.; Sekine, M. Synthesis and properties of cationic 2′-O-[N-(4-aminobutyl)carbamoyl] modified oligonucleotides. Bioorg. Med. Chem. Lett. 2012, 22, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- El-Sagheer, A.H.; Brown, T. New Strategy for the Synthesis of Chemically Modified RNA Constructs Exemplified by Hairpin and Hammerhead Ribozymes. Proc. Natl. Acad. Sci. USA 2010, 107, 15329–15334. [Google Scholar] [CrossRef]
- Warminski, M.; Kowalska, J.; Jemielity, J. Synthesis of RNA 5′-Azides from 2′-O-Pivaloyloxymethyl-Protected RNAs and Their Reactivity in Azide–Alkyne Cycloaddition Reactions. Org. Lett. 2017, 19, 3624–3627. [Google Scholar] [CrossRef] [PubMed]
- Santner, T.; Hartl, M.; Bister, K.; Micura, R. Efficient Access to 3′-Terminal Azide-Modified RNA for Inverse Click-Labeling Patterns. Bioconjug. Chem. 2014, 25, 188–195. [Google Scholar] [CrossRef]
- Agustin, E.; Asare Okai, P.N.; Khan, I.; Miller, M.R.; Wang, R.; Sheng, J.; Royzen, M. A fast click–slow release strategy towards the HPLC-free synthesis of RNA. Chem. Commun. 2016, 52, 1405–1408. [Google Scholar] [CrossRef]
- Khan, I.; Seebald, L.M.; Robertson, N.M.; Yigit, M.V.; Royzen, M. Controlled in-cell activation of RNA therapeutics using bond-cleaving bio-orthogonal chemistry. Chem. Sci. 2017, 8, 5705–5712. [Google Scholar] [CrossRef]
- Mikkola, S.; Lönnberg, T.; Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 2018, 14, 803–837. [Google Scholar] [CrossRef]
- Tsuruoka, H.; Shohda Ki, K.; Wada, T.; Sekine, M. Kinetics and Mechanism of Facile and Selective Dephosphorylation of 2‘-Phosphorylated and 2‘-Thiophosphorylated Dinucleotides: Neighboring 3‘−5‘ Phosphodiester Promotes 2‘-Dephosphorylation. J. Org. Chem. 1997, 62, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Pyshnyi, D.V.; Lomzov, A.A.; Pyshnaya, I.A.; Ivanova, E.M. Hybridization of the Bridged Oligonucleotides with DNA: Thermodynamic and Kinetic Studies. J. Biomol. Struct. Dyn. 2006, 23, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Petersheim, M.; Turner, D.H. Base-stacking and base-pairing contributions to helix stability: Thermodynamics of double-helix formation with CCGG, CCGGp, CCGGAp, ACCGGp, CCGGUp, and ACCGGUp. Biochemistry 1983, 22, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Pattanayek, R.; Sethaphong, L.; Pan, C.; Prhavc, M.; Prakash, T.P.; Manoharan, M.; Egli, M. Structural Rationalization of a Large Difference in RNA Affinity Despite a Small Difference in Chemistry between Two 2‘-O-Modified Nucleic Acid Analogues. J. Am. Chem. Soc. 2004, 126, 15006–15007. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Peng, C.G.; Matsuda, S.; Addepalli, H.; Jayaprakash, K.N.; Alam, M.R.; Mills, K.; Maier, M.A.; Charisse, K.; Sekine, M.; et al. Versatile Site-Specific Conjugation of Small Molecules to siRNA Using Click Chemistry. J. Org. Chem. 2011, 76, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Chernikov, I.V.; Gladkikh, D.V.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. Cholesterol-Containing Nuclease-Resistant siRNA Accumulates in Tumors in a Carrier-free Mode and Silences MDR1 Gene. Mol. Ther. Nucleic Acids 2017, 6, 209–220. [Google Scholar] [CrossRef]
- Nakajima, M.; Kasuya, T.; Yokota, S.; Onishi, R.; Ikehara, T.; Kugimiya, A.; Watanabe, A. Gene Silencing Activity and Hepatic Accumulation of Antisense Oligonucleotides Bearing Cholesterol-Conjugated Thiono Triester at the Gap Region. Nucleic Acid Ther. 2017, 27, 232–237. [Google Scholar] [CrossRef]
- Osborn, M.F.; Khvorova, A. Improving siRNA Delivery In Vivo Through Lipid Conjugation. Nucleic Acid Ther. 2018, 28, 128–136. [Google Scholar] [CrossRef]
- Shmushkovich, T.; Monopoli, K.R.; Homsy, D.; Leyfer, D.; Betancur-Boissel, M.; Khvorova, A.; Wolfson, A.D. Functional features defining the efficacy of cholesterol-conjugated, self-deliverable, chemically modified siRNAs. Nucleic Acids Res. 2018, 46, 10905–10916. [Google Scholar] [CrossRef]
- Ryzhakov, G.; Randow, F. SINTBAD, a novel component of innate antiviral immunity, shares a TBK1-binding domain with NAP1 and TANK. Embo J. 2007, 26, 3180–3190. [Google Scholar] [CrossRef]
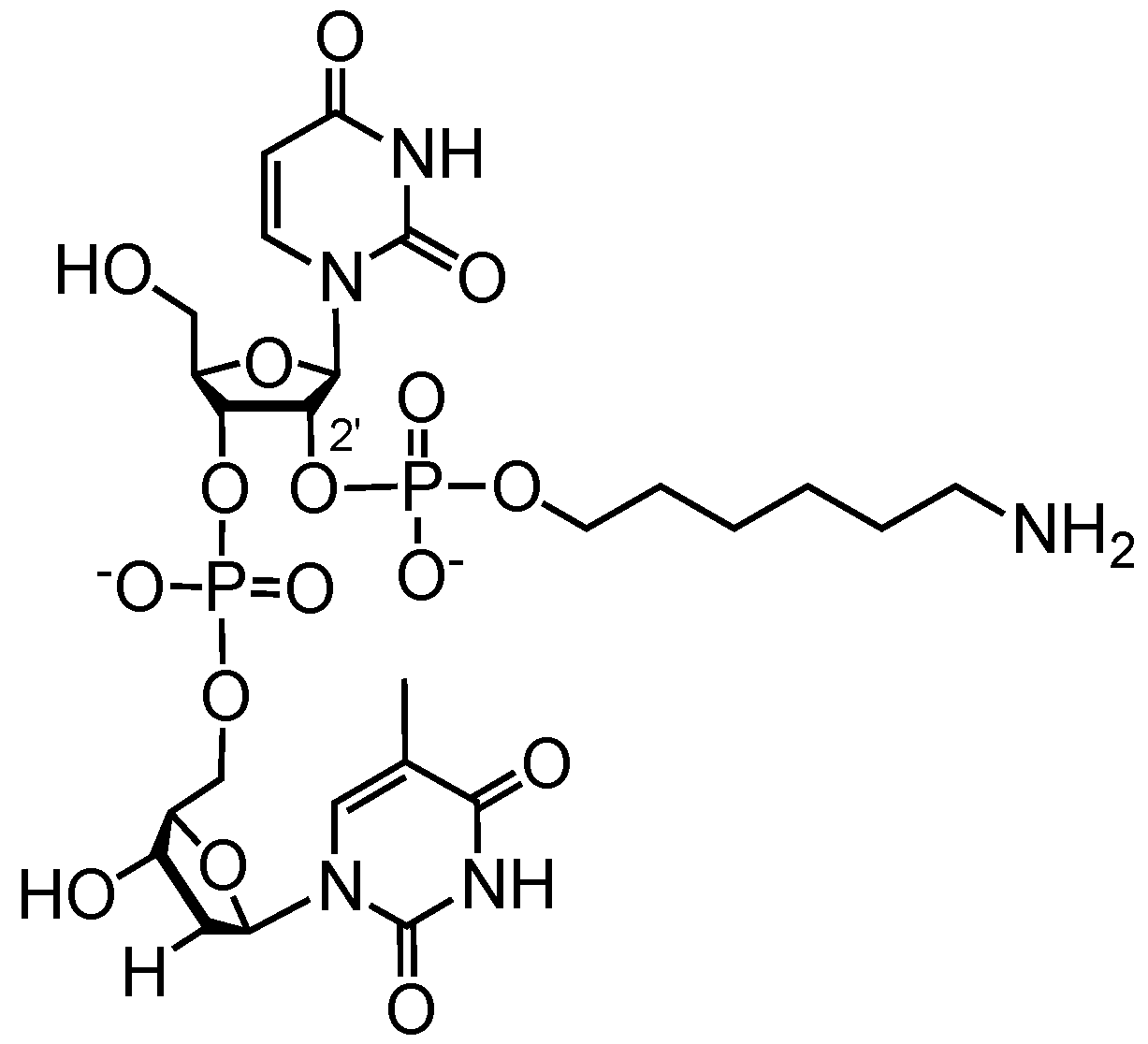
| rON | Sequence 1 | R | Isolated Yield/Conversion 2, % | RT 3, min | Molecular Weight | |
|---|---|---|---|---|---|---|
| Found 4 m/z | Calcd m/z | |||||
| 2 | 5′-r(ACGUACGU)dT | - | 12.5/85.1 | 6.3 | 2812.5 | 2813.8 |
| 2a | 5′-r(ACGU*ACGU)dT | a | 7.4/78.5 | 6.4 | 2991.9 | 2992.0 |
| 2b | 5′-r(ACGU*ACGU)dT | b | 3.1/~80 * | n/d | 3405.6 | 3404.6 |
| 2c | 5′-r(ACGU*ACGU)dT | c | 5.6/84.5 | 9.9 | 3523.6 | 3523.7 |
| 2d | 5′-r(ACGU*ACGU)dT | d | 11.2/85.2 | 6.9 | 3084.9 † | 3084.1 |
| 2e | 5′-r(ACGU*ACGU)dT | e | 10.2/81.4 | 13.4 | 3529.5 †,5 | 3486.5 |
| 2f | 5′-r(ACGU*ACGU)dT | f | 10.5/80.3 | 6.4 | 2926.2 | 2925.9 |
| 2g | 5′-r(ACGU*ACGU)dT | g | 5.4/90.9 | 12.8 | 3365.4 | 3367.6 |
| 3a | 5′-r(GUGAA*AUG)dC | a | 12.5/89.0 | 6.4 | 3041.7 | 3041.1 |
| 4a | 5′-r(GUGAU*AUG)dC | a | 18.4/84.6 | 6.4 | 3018.3 | 3018.1 |
| 5a | 5′-r(GUGAC*AUG)dC | a | 20.1/86.7 | 6.4 | 3017.9 | 3017.9 |
| 6a | 5′-r(GUGAG*AUG)dC | a | 19.5/87.4 | 6.5 | 3057.2 | 3057.9 |
| 7a | 5′-r(GCCACAACGUCUAUAUCAU*)dTdT | a | 5.6/78.9 | 7.2 | 6756.2 † | 6753.1 |
| 7b | 5′-r(GCCACAACGUCUAUAUCAU*)dTdT | d | 6.7/74.2 | 6.9 | 6847.8 † | 6844.1 |
| 8a | 5′-Alk-r(GCCACAACGUCUAUAUCAU*)dTdT | a | 6.0/69.3 | 7.3 | 7024.0 † | 7022.4 |
| 8b | 5′-Fluo-r(GCCACAACGUCUAUAUCAU*)dTdT | b | 3.6/~50 * | n/d | 7894.3 † | 7894.5 |
| 9a | 5′-Alk-r(GCCACAACGUCUAUA*UCAU)dTdT | a | 5.7/76.7 | 7.3 | 7024.0 † | 7022.4 |
| 9b | 5′-Fluo-r(GCCACAACGUCUAUA*UCAU)dTdT | b | 3.5/~45 * | n/d | 7895.0 † | 7894.5 |
| 10a | 5′-Alk-r(GCCACAACGUCU*AUAUCAU)dTdT | a | 5.7/75.6 | 7.3 | 7024.0 † | 7022.4 |
| 10b | 5′-Fluo-r(GCCACAACGUCU*AUAUCAU)dTdT | b | 3.9/~45 * | n/d | 7895.5 † | 7894.5 |
| ON | Sequence 1, 5′–3′ | Tm (Δ Tm 2), °C | |||
|---|---|---|---|---|---|
| Target | 5′-r(GCAUBUCAC) | B = U | A | G | C |
| 3 | 5′-r(GUGAAAUG)dC | 39.0 | 23 (−18.5) | 25.5 (−20.5) | 27.0 (−18.5) |
| 4 | 5′-r(GUGAUAUG)dC | 26.0 (−13.0) | 41.5 | 37.0 (−9.0) | 26.0 (−19.0) |
| 5 | 5′-r(GUGACAUG)dC | 22.5 (−16.5) | 25.5 (−16.0) | 46.0 | 24.0 (−21.0) |
| 6 | 5′-r(GUGAGAUG)dC | 35.5 (−3.5) | 25.0 (−16.5) | 25.5 (−20.5) | 47.5 |
| 3a | 5′-r(GUGAA*AUG)dC | 32.0 | 21.0 (−14.0) | 18.0 (−22.0) | 23.5 (−17.0) |
| 4a | 5′-r(GUGAU*AUG)dC | 17.7 (−14.3) | 35.0 | 28.0 (−12.0) | 21.5 (−19.0) |
| 5a | 5′-r(GUGAC*AUG)dC | 20.0 (−12.0) | 20.0 (−15.0) | 40.0 | 18.0 (−22.5) |
| 6a | 5′-r(GUGAG*AUG)dC | 29.0 (−3.0) | 21.0 (−14.0) | 27.0 (−13.0) | 40.5 |
| S/AS | Sequences | Representation |
|---|---|---|
| 8b/AS | 5′-Fluo-r(GCCACAACGUCUAUAUCAU*)dTdT3′-dTdT-r(CGGUGUUGCAGAUAUAGUA) |  |
| 9b/AS | 5′-Fluo-r(GCCACAACGUCUAUA*UCAU)dTdT 3′-dTdT-r(CGGUGUUGCAGAUAUAGUA) |  |
| 10b/AS | 5′-Fluo-r(GCCACAACGUCU*AUAUCAU)dTdT 3′-dTdT-r(CGGUGUUGCAGAUAUAGUA) |  |
| 12/AS | 5′-Fluo-r(GCCACAACGUCUAUAUCAU)dTdT 3′-dTdT-r(CGGUGUUGCAGAUAUAGUA) |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasheninina, O.A.; Fishman, V.S.; Lomzov, A.A.; Ustinov, A.V.; Venyaminova, A.G. Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method. Int. J. Mol. Sci. 2020, 21, 5127. https://doi.org/10.3390/ijms21145127
Krasheninina OA, Fishman VS, Lomzov AA, Ustinov AV, Venyaminova AG. Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method. International Journal of Molecular Sciences. 2020; 21(14):5127. https://doi.org/10.3390/ijms21145127
Chicago/Turabian StyleKrasheninina, Olga A., Veniamin S. Fishman, Alexander A. Lomzov, Alexey V. Ustinov, and Alya G. Venyaminova. 2020. "Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method" International Journal of Molecular Sciences 21, no. 14: 5127. https://doi.org/10.3390/ijms21145127
APA StyleKrasheninina, O. A., Fishman, V. S., Lomzov, A. A., Ustinov, A. V., & Venyaminova, A. G. (2020). Postsynthetic On-Column 2′ Functionalization of RNA by Convenient Versatile Method. International Journal of Molecular Sciences, 21(14), 5127. https://doi.org/10.3390/ijms21145127