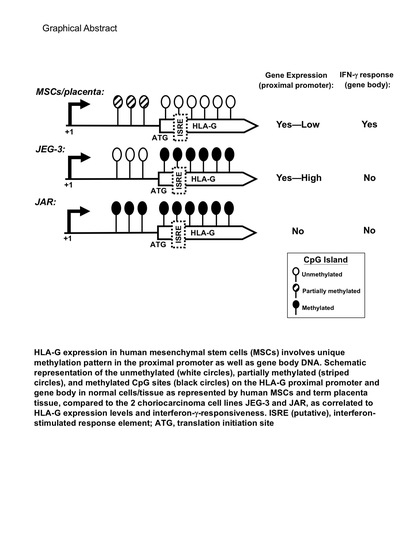
HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA
Abstract
1. Introduction
2. Results
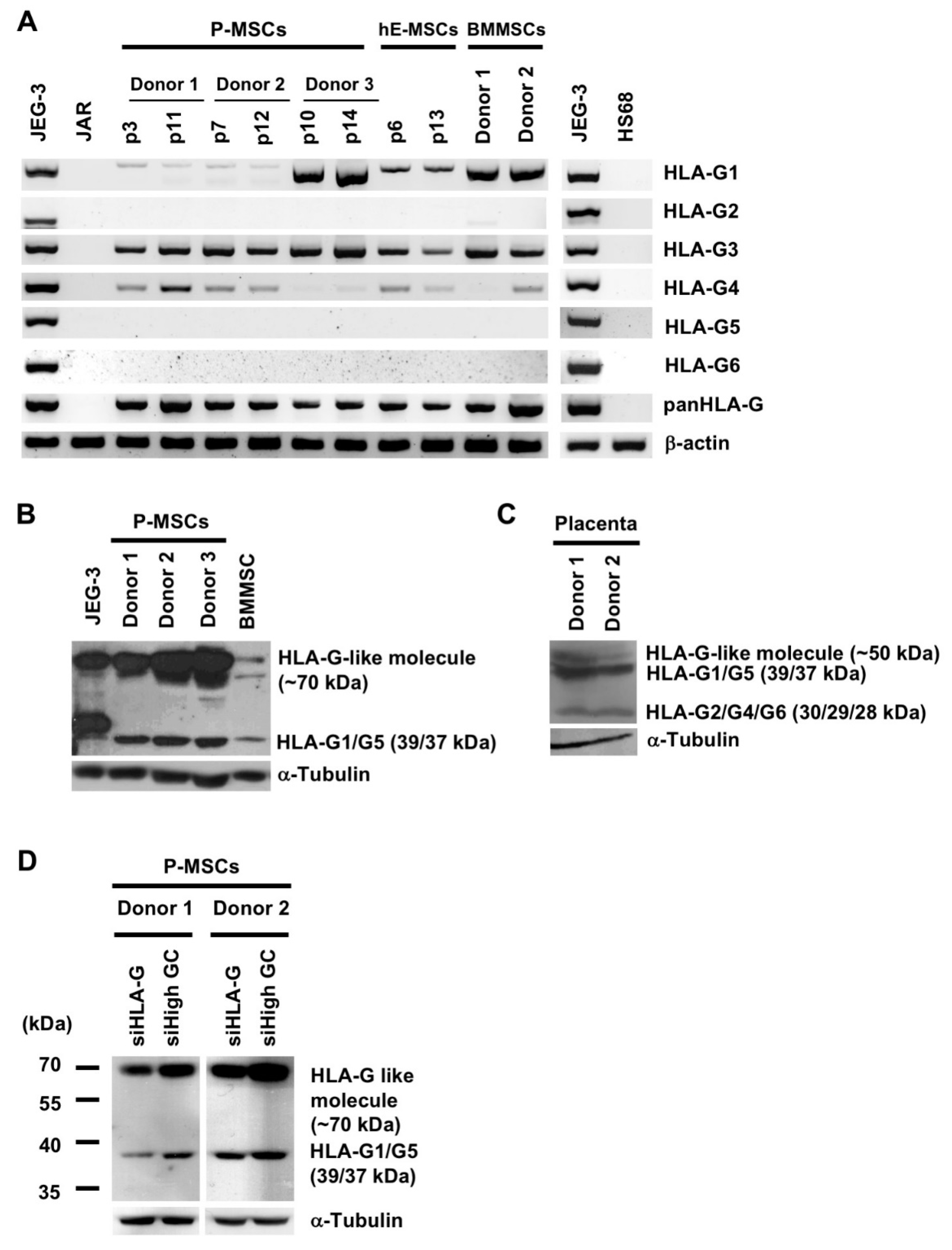
2.1. Diverse Sources of Human MSCs but Not Fibroblasts Express Multiple HLA-G mRNA and Protein Isoforms
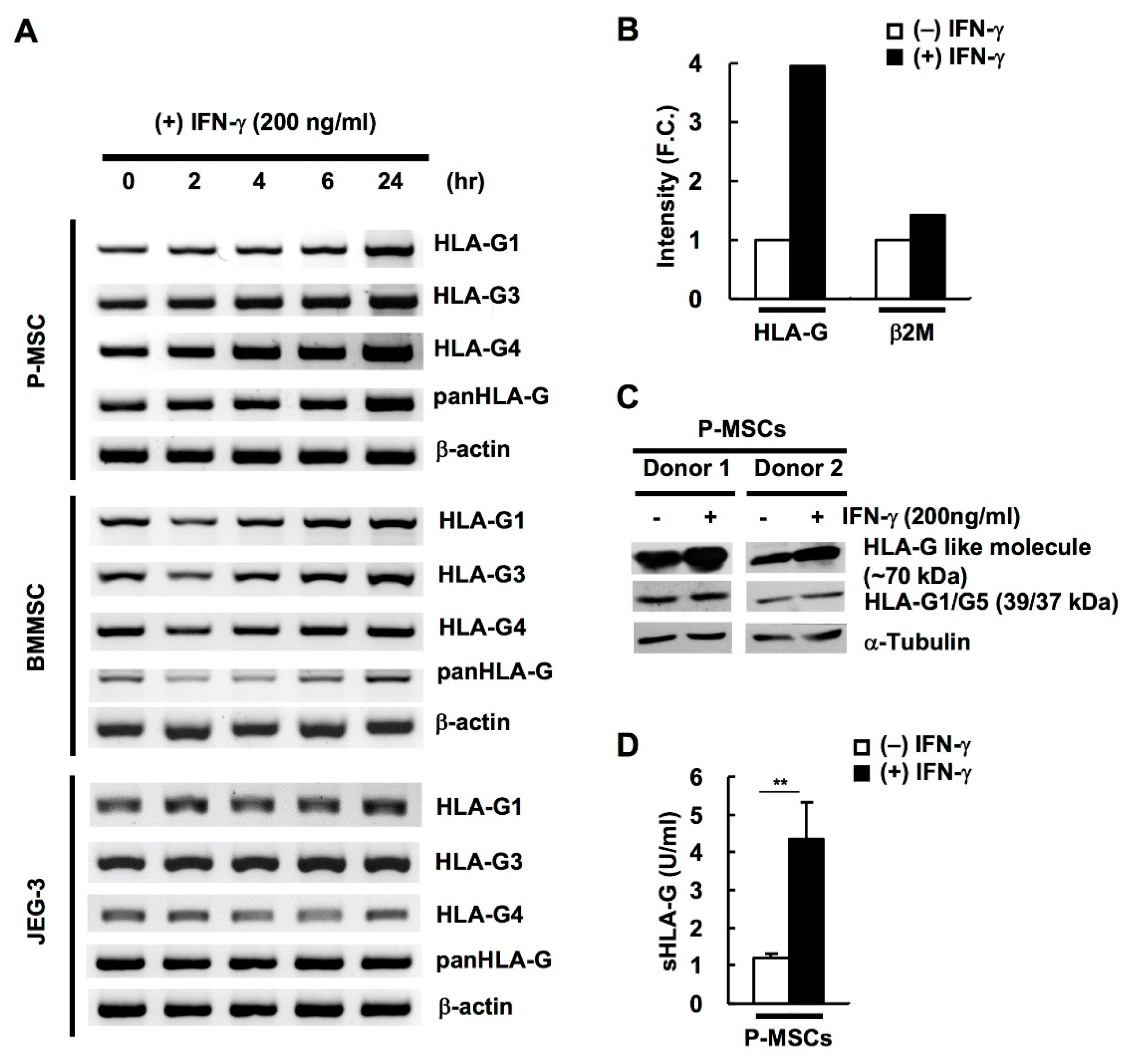
2.2. HLA-G Expression in BM & P-MSCs but Not JEG-3 Cells Is Responsive to IFN-γ Stimulation
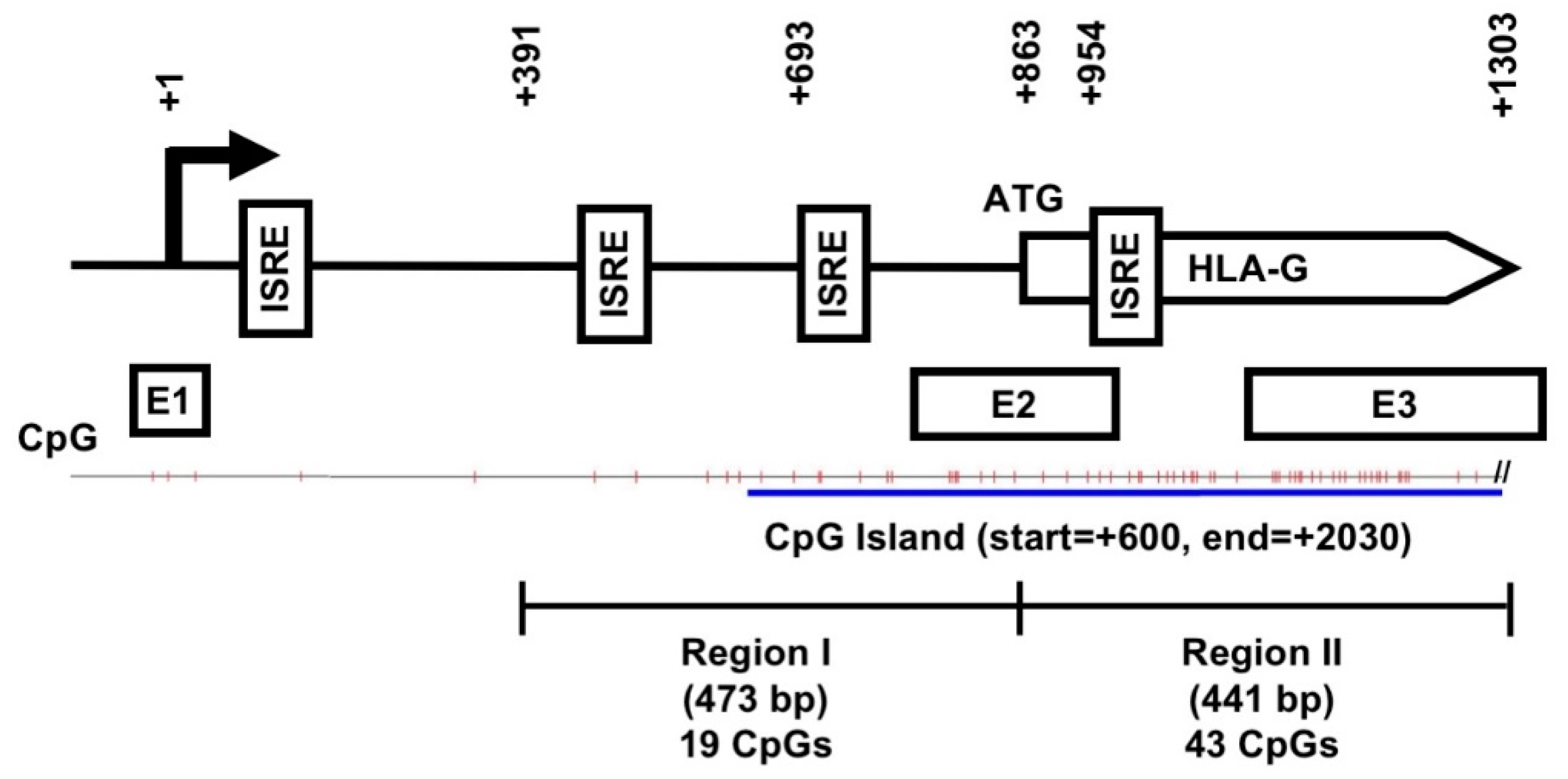
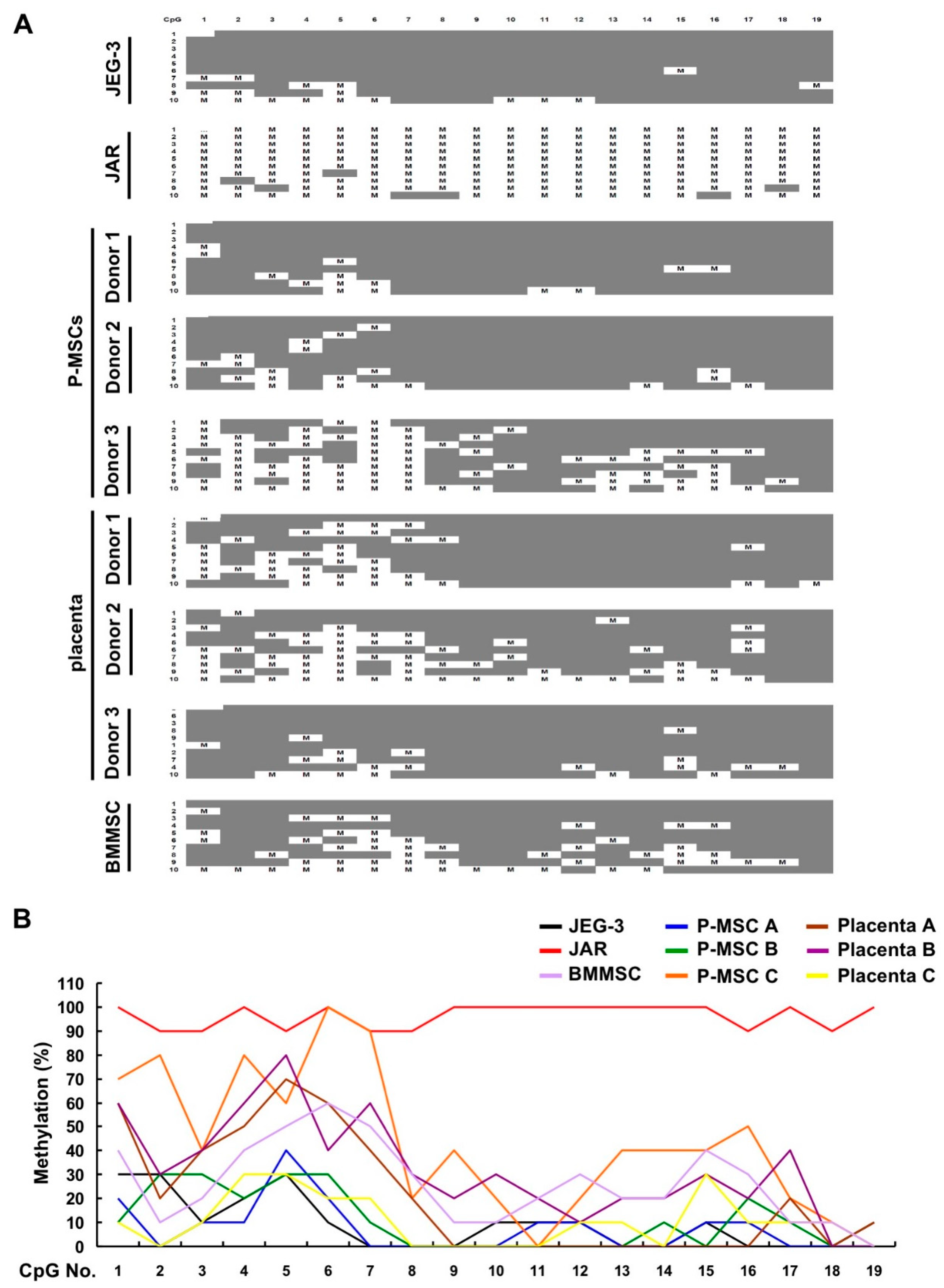
2.3. An Overall Hypomethylation Pattern Is Seen in the HLA-G Proximal Promoter CpG Sites of Human MSCs and Placental Tissue Extracts
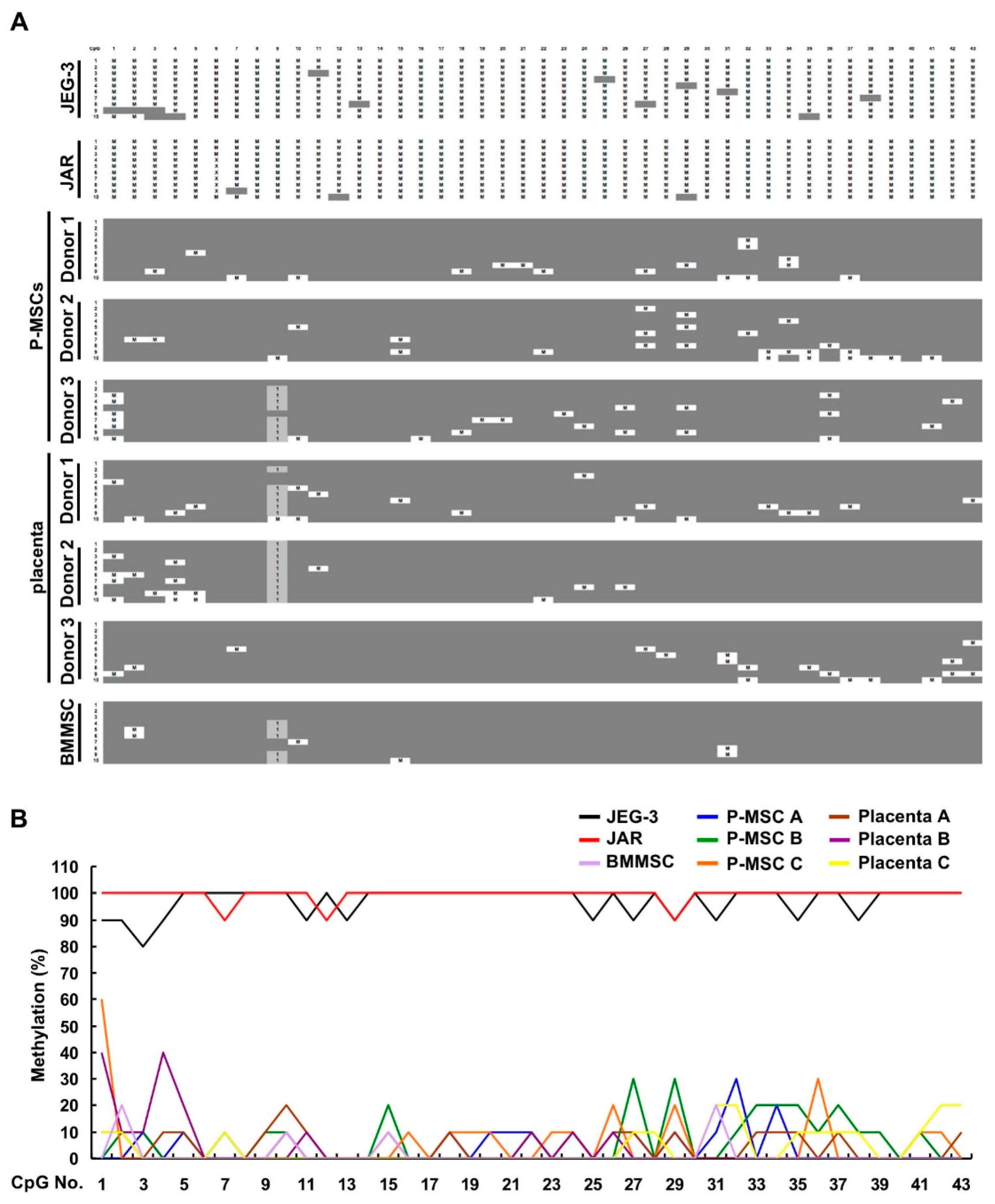
2.4. CpG Sites within the Cell Body of HLA-G Are Strongly Hypomethylated in All Human MSCs and Placental Tissue but Nearly 100% Methylated in Both Choriocarcinoma Cell Lines
2.5. Demethylation with 5-Azacytidine Results in Increased HLA-G Expression Patterns in MSCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.3. RNA Silencing
4.4. Tandem Mass Spectrometric Analysis (MS/MS)
4.5. ELISA
4.6. Western Blot Analysis
4.7. Genomic DNA Extraction and Bisulfite Modification
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, P.M.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Yen, B.L. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J. Biomed. Sci. 2011, 18, 49. [Google Scholar] [CrossRef]
- Abdelrazik, H.; Giordano, E.; Barbanti Brodano, G.; Griffoni, C.; De Falco, E.; Pelagalli, A. Substantial Overview on Mesenchymal Stem Cell Biological and Physical Properties as an Opportunity in Translational Medicine. Int. J. Mol. Sci. 2019, 20, 5386. [Google Scholar] [CrossRef] [PubMed]
- Brooke, G.; Cook, M.; Blair, C.; Han, R.; Heazlewood, C.; Jones, B.; Kambouris, M.; Kollar, K.; McTaggart, S.; Pelekanos, R.; et al. Therapeutic applications of mesenchymal stromal cells. Semin. Cell Dev. Biol. 2007, 18, 846–858. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Prockop, S.E.; Bertoncello, I. Are clinical trials with mesenchymal stem/progenitor cells too far ahead of the science? Lessons from experimental hematology. Stem Cells 2014, 32, 3055–3061. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef]
- Carosella, E.D.; Rouas-Freiss, N.; Tronik-Le Roux, D.; Moreau, P.; LeMaoult, J. HLA-G: An Immune Checkpoint Molecule. Adv. Immunol. 2015, 127, 33–144. [Google Scholar]
- Rouas-Freiss, N.; Goncalves, R.M.; Menier, C.; Dausset, J.; Carosella, E.D. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc. Natl. Acad. Sci. USA 1997, 94, 11520–11525. [Google Scholar] [CrossRef]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Kirszenbaum, M.; Djoulah, S.; Hors, J.; Prost, S.; Dausset, J.; Carosella, E.D. Polymorphism of HLA-G gene and protein. J. Reprod. Immunol. 1999, 43, 105–109. [Google Scholar] [CrossRef]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Blaschitz, A.; Crisa, L.; Schmitt, C.; Fournel, S.; King, A.; Loke, Y.W.; Dohr, G.; Le Bouteiller, P. HLA-G in the human thymus: A subpopulation of medullary epithelial but not CD83(+) dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int. Immunol. 1999, 11, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, A.; Geraghty, D.E. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 3947–3951. [Google Scholar] [CrossRef]
- Paul, P.; Cabestre, F.A.; Ibrahim, E.C.; Lefebvre, S.; Khalil-Daher, I.; Vazeux, G.; Quiles, R.M.; Bermond, F.; Dausset, J.; Carosella, E.D. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum. Immunol. 2000, 61, 1138–1149. [Google Scholar] [CrossRef]
- Nasef, A.; Mathieu, N.; Chapel, A.; Frick, J.; Francois, S.; Mazurier, C.; Boutarfa, A.; Bouchet, S.; Gorin, N.C.; Thierry, D.; et al. Immunosuppressive effects of mesenchymal stem cells: Involvement of HLA-G. Transplantation 2007, 84, 231–237. [Google Scholar] [CrossRef]
- Rizzo, R.; Campioni, D.; Stignani, M.; Melchiorri, L.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Moretti, S.; Cuneo, A.; et al. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy 2008, 10, 364–375. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Gaiffe, E.; Obert, L.; Tiberghien, P.; Rouas-Freiss, N.; Carosella, E.D.; Deschaseaux, F. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation 2009, 87 (Suppl. 9), S62–S66. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Cao, M.; Yie, S.M.; Liu, J.; Ye, S.R.; Xia, D.; Gao, E. Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens 2011, 78, 120–128. [Google Scholar] [CrossRef]
- Yen, B.L.; Chang, C.J.; Liu, K.J.; Chen, Y.C.; Hu, H.I.; Bai, C.H.; Yen, M.L. Human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 2009, 27, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Boucraut, J.; Guillaudeux, T.; Alizadeh, M.; Boretto, J.; Chimini, G.; Malecaze, F.; Semana, G.; Fauchet, R.; Pontarotti, P.; Le Bouteiller, P. HLA-E is the only class I gene that escapes CpG methylation and is transcriptionally active in the trophoblast-derived human cell line JAR. Immunogenetics 1993, 38, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, G.; Marcou, C.; Rousseau, P.; Rouas-Freiss, N.; Carosella, E.D.; Moreau, P. HLA-G gene activation in tumor cells involves cis-acting epigenetic changes. Int. J. Cancer 2005, 113, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.C.; Veiga-Castelli, L.C.; Yaghi, L.; Moreau, P.; Donadi, E.A. Transcriptional and posttranscriptional regulations of the HLA-G gene. J. Immunol. Res. 2014, 2014, 734068. [Google Scholar] [CrossRef]
- Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 2002, 3, 662–673. [Google Scholar] [CrossRef]
- Cedar, H. DNA methylation and gene activity. Cell 1988, 53, 3–4. [Google Scholar] [CrossRef]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef]
- Wang, L.T.; Jiang, S.S.; Ting, C.H.; Hsu, P.J.; Chang, C.C.; Sytwu, H.K.; Liu, K.J.; Yen, B.L. Differentiation of mesenchymal stem cells (MSCs) from human induced pluripotent stem cells (iPSCs) results in downregulation of c-Myc and DNA replication pathways with immunomodulation toward CD4 and CD8 cells. Stem Cells 2018, 36, 903–914. [Google Scholar] [CrossRef]
- Wang, W.B.; Yen, M.L.; Liu, K.J.; Hsu, P.J.; Lin, M.H.; Chen, P.M.; Sudhir, P.R.; Chen, C.H.; Chen, C.H.; Sytwu, H.K.; et al. Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Rep. 2015, 5, 392–404. [Google Scholar] [CrossRef]
- Yen, M.L.; Hou, C.H.; Peng, K.Y.; Tseng, P.C.; Jiang, S.S.; Shun, C.T.; Chen, Y.C.; Kuo, M.L. Efficient derivation and concise gene expression profiling of human embryonic stem cell-derived mesenchymal progenitors (EMPs). Cell Transpl. 2011, 20, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Wang, C.J.; Chang, C.J.; Hu, H.I.; Hsu, P.J.; Wu, Y.C.; Bai, C.H.; Sytwu, H.K.; Yen, B.L. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transpl. 2011, 20, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; van Vollenstee, F.A.; Thomford, N.E.; Dandara, C.; Chopera, D.; Pepper, M.S.; et al. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. Int. J. Mol. Sci. 2016, 17, 1259. [Google Scholar] [CrossRef] [PubMed]
- Menier, C.; Saez, B.; Horejsi, V.; Martinozzi, S.; Krawice-Radanne, I.; Bruel, S.; Le Danff, C.; Reboul, M.; Hilgert, I.; Rabreau, M.; et al. Characterization of monoclonal antibodies recognizing HLA-G or HLA-E: New tools to analyze the expression of nonclassical HLA class I molecules. Hum. Immunol. 2003, 64, 315–326. [Google Scholar] [CrossRef]
- Gonzalez, A.; Alegre, E.; Arroyo, A.; LeMaoult, J.; Echeveste, J.I. Identification of circulating nonclassic human leukocyte antigen G (HLA-G)-like molecules in exudates. Clin Chem 2011, 57, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Moreau, P.; Guiard, V.; Ibrahim, E.C.; Adrian-Cabestre, F.; Menier, C.; Dausset, J.; Carosella, E.D.; Paul, P. Molecular mechanisms controlling constitutive and IFN-gamma-inducible HLA-G expression in various cell types. J. Reprod. Immunol. 1999, 43, 213–224. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, W.; Geraghty, D.E.; Hunt, J.S. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J. Immunol. 1996, 156, 4224–4231. [Google Scholar]
- Choi, J.C.; Holtz, R.; Petroff, M.G.; Alfaidy, N.; Murphy, S.P. Dampening of IFN-gamma-inducible gene expression in human choriocarcinoma cells is due to phosphatase-mediated inhibition of the JAK/STAT-1 pathway. J. Immunol. 2007, 178, 1598–1607. [Google Scholar] [CrossRef]
- Real, L.M.; Cabrera, T.; Collado, A.; Jimenez, P.; Garcia, A.; Ruiz-Cabello, F.; Garrido, F. Expression of HLA G in human tumors is not a frequent event. Int. J. Cancer 1999, 81, 512–518. [Google Scholar] [CrossRef]
- Swets, M.; Wouters, A.; Krijgsman, D.; van Vlierberghe, R.L.P.; Boot, A.; van Eendenburg, J.D.; van Wezel, T.; Gelderblom, H.; van de Velde, C.J.H.; van den Elsen, P.J.; et al. HLA-G protein expression in colorectal cancer evaluated by immunohistochemistry and western blot analysis: Its expression characteristics remain enigmatic. Clin. Immunol. 2018, 194, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Gardner, L.; Moffett, A. A critical look at HLA-G. Trends Immunol. 2008, 29, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Alegre, E.; Rebmann, V.; Lemaoult, J.; Rodriguez, C.; Horn, P.A.; Diaz-Lagares, A.; Echeveste, J.I.; Gonzalez, A. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur. J. Immunol. 2013, 43, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, P.; Abraham, B.J.; Tsai, L.; Zhou, Y.; Costa-e-Sousa, R.H.; Ye, F.; Bilban, M.; Zhao, K.; Hollenberg, A.N. Novel mechanism of positive versus negative regulation by thyroid hormone receptor beta1 (TRbeta1) identified by genome-wide profiling of binding sites in mouse liver. J. Biol. Chem. 2014, 289, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Yen, B.L.; Huang, H.I.; Chien, C.C.; Jui, H.Y.; Ko, B.S.; Yao, M.; Shun, C.T.; Yen, M.L.; Lee, M.C.; Chen, Y.C. Isolation of multipotent cells from human term placenta. Stem Cells 2005, 23, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Yen, M.L.; Chen, Y.C.; Chien, C.C.; Huang, H.I.; Bai, C.H.; Yen, B.L. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 2006, 24, 2466–2477. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Yen, B.L.; Yen, M.L.; Hsu, P.J.; Liu, K.J.; Wang, C.J.; Bai, C.H.; Sytwu, H.K. Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Rep. 2013, 1, 139–151. [Google Scholar] [CrossRef]
- Chen, P.M.; Liu, K.J.; Hsu, P.J.; Wei, C.F.; Bai, C.H.; Ho, L.J.; Sytwu, H.K.; Yen, B.L. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol. 2014, 96, 295–303. [Google Scholar] [CrossRef]
- Chen, P.M.; Lin, C.H.; Li, N.T.; Wu, Y.M.; Lin, M.T.; Hung, S.C.; Yen, M.L. c-Maf regulates pluripotency genes, proliferation/self-renewal, and lineage commitment in ROS-mediated senescence of human mesenchymal stem cells. Oncotarget 2015, 6, 35404–35418. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Barlow, D.H.; Sargent, I.L. Differential expression of alternatively spliced transcripts of HLA-G in human preimplantation embryos and inner cell masses. J. Immunol. 2005, 175, 8379–8385. [Google Scholar] [CrossRef] [PubMed]
- Rebmann, V.; LeMaoult, J.; Rouas-Freiss, N.; Carosella, E.D.; Grosse-Wilde, H. Quantification and identification of soluble HLA-G isoforms. Tissue Antigens 2007, 69 (Suppl. 1), 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Hou, S.M.; Chen, R.J.; Peng, H.W.; Hsieh, C.F.; Kuo, M.L.; Yen, M.L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]

| Primer | Sequence | Product Size |
|---|---|---|
| Region 1-Forward | AAGAGTATAGGAGGATAGGTAAGG | 449 bp |
| Region 1-Reverse | TGGGGAGAATGAGTTTGGGTGGG | |
| Region 2-Forward | GTTAAGGATGGTGGTTATGG | 447 bp |
| Region 2-Reverse | AAATTCATTCTATCAATCTATAC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, B.L.; Hwa, H.-L.; Hsu, P.-J.; Chen, P.-M.; Wang, L.-T.; Jiang, S.-S.; Liu, K.-J.; Sytwu, H.-K.; Yen, M.-L. HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA. Int. J. Mol. Sci. 2020, 21, 5075. https://doi.org/10.3390/ijms21145075
Yen BL, Hwa H-L, Hsu P-J, Chen P-M, Wang L-T, Jiang S-S, Liu K-J, Sytwu H-K, Yen M-L. HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA. International Journal of Molecular Sciences. 2020; 21(14):5075. https://doi.org/10.3390/ijms21145075
Chicago/Turabian StyleYen, B. Linju, Hsiao-Lin Hwa, Pei-Ju Hsu, Pei-Min Chen, Li-Tzu Wang, Shih-Sheng Jiang, Ko-Jiunn Liu, Huey-Kang Sytwu, and Men-Luh Yen. 2020. "HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA" International Journal of Molecular Sciences 21, no. 14: 5075. https://doi.org/10.3390/ijms21145075
APA StyleYen, B. L., Hwa, H.-L., Hsu, P.-J., Chen, P.-M., Wang, L.-T., Jiang, S.-S., Liu, K.-J., Sytwu, H.-K., & Yen, M.-L. (2020). HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA. International Journal of Molecular Sciences, 21(14), 5075. https://doi.org/10.3390/ijms21145075