Biodistribution PET/CT Study of Hemoglobin-DFO-89Zr Complex in Healthy and Lung Tumor-Bearing Mice
Abstract
1. Introduction
2. Results
2.1. Formation of the Human Hb-DFO Conjugate Is Selective, Fast and Quantitative
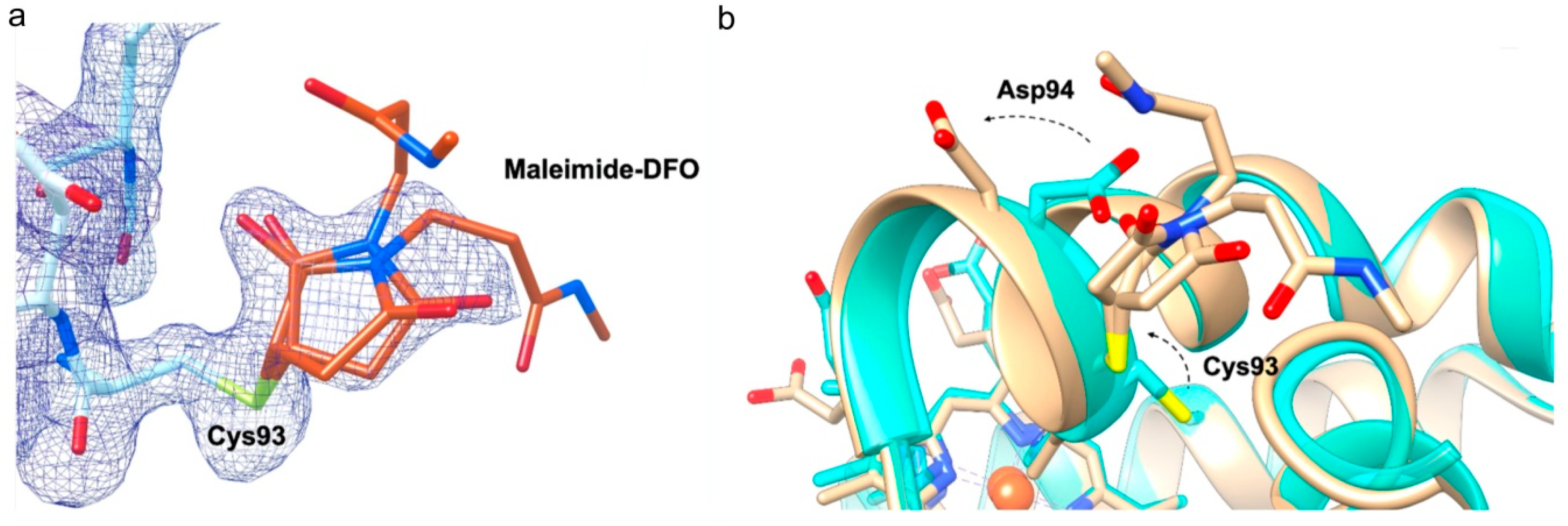
2.2. DFO Slightly Affects the Orientation of Two Side Chains
2.3. 89Zr Uptake by Hb-DFO Is Fast and Efficient
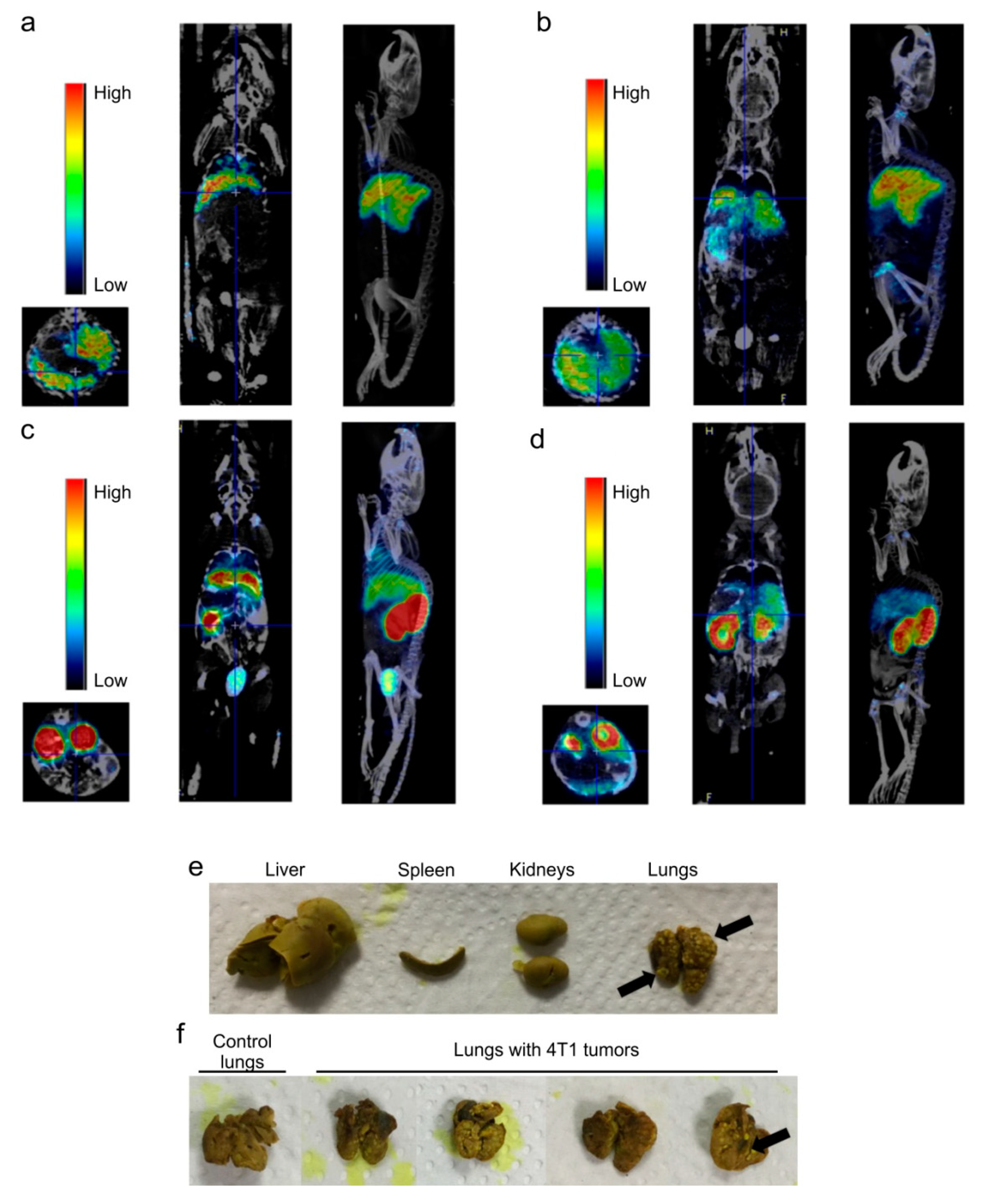
2.4. Dose-Dependent Biodistribution and Elimination of Hb-DFO-89Zr in Mice: No Preferential Internalization Observed by Damaged Tissues
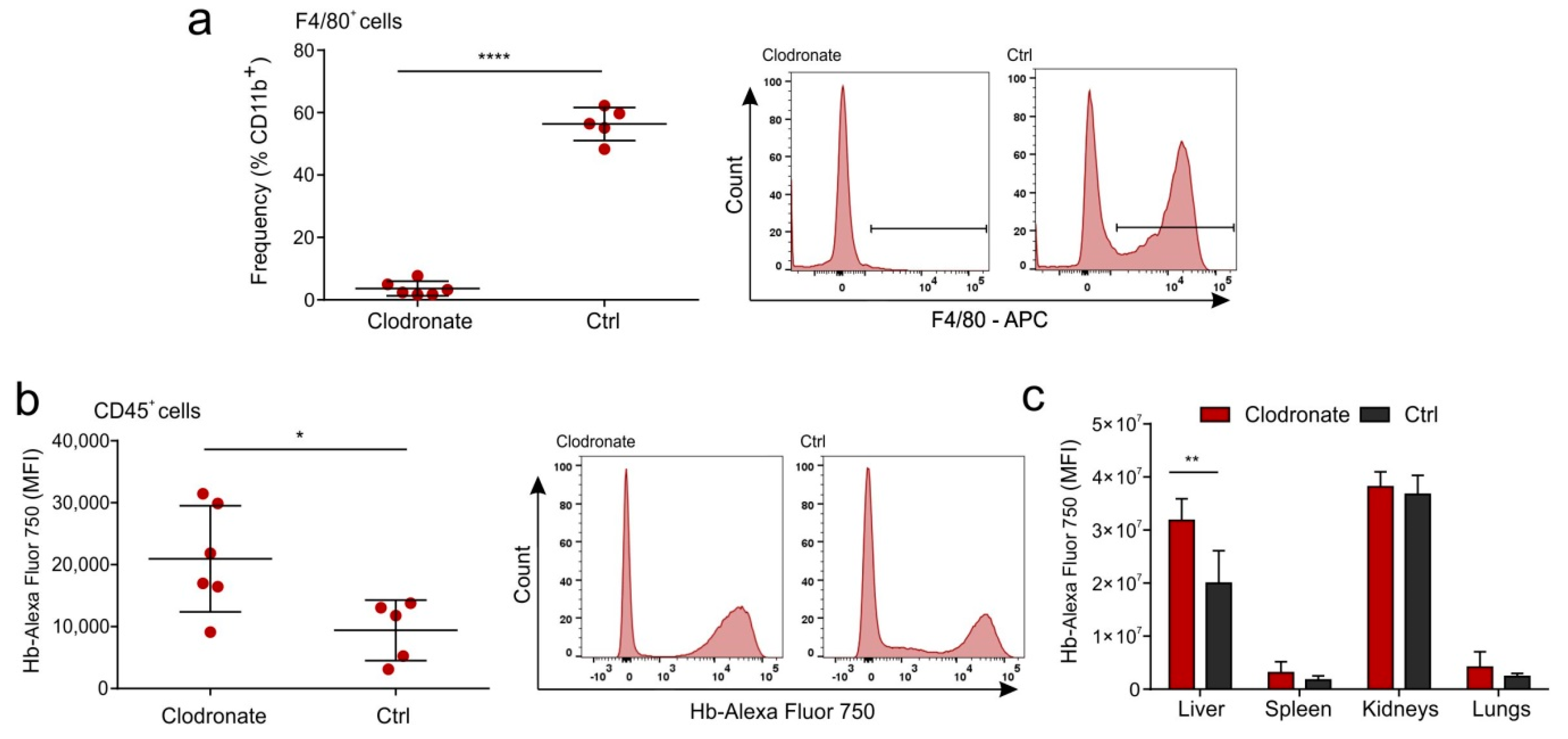
2.5. Hb Is Not Scavenged by the Liver Macrophages in a Mouse Model
3. Discussion
4. Materials and Methods
4.1. Synthesis of Hb-DFO
4.2. MALDI-TOF Analysis of Hb-DFO
4.3. Crystallization of Hb-DFO
4.4. X-ray Data Collection, Structure Determination and Refinement
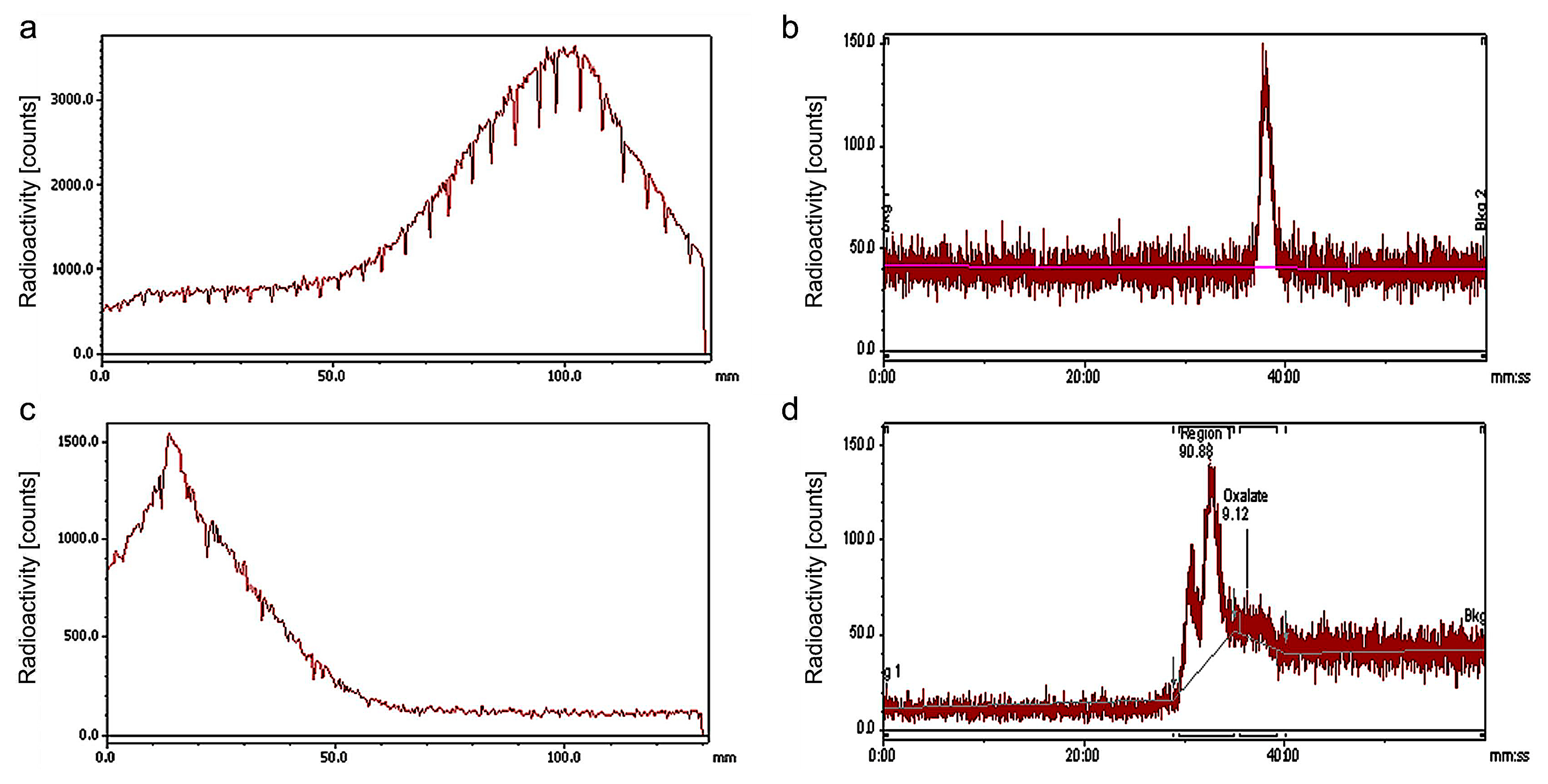
4.5. Radiosynthesis of 89Zr Oxalate
4.6. Radiolabelling of Hb-DFO
4.7. Radio-TLC
4.8. Radio-HPLC
4.9. Transplantation of 4T1 Cells to BALB/c Mice
4.10. Hb-DFO-89Zr PET/CT Imaging of BALB/c Mice
4.11. In-Vivo MS FX PRO Imaging and Flow Cytometry
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Hb | Hemoglobin |
| Hp | Haptoglobin |
| PET/CT | positron emission tomography/computed tomography |
| DFO | deferoxamine |
| MALDI-TOF | matrix-assisted laser desorption/ionization-time of flight |
References
- Patil, S.; Pancholi, S.; Agrawal, S.; Agrawal, G.P. Surface-Modified Mesoporous Ceramics as Delivery Vehicle for Haemoglobin. Drug Deliv. 2008, 11, 193–199. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamasaki, K.; Maruyama, T.; Otagiri, M. Comparison of the Pharmacokinetic Properties of Hemoglobin-Based Oxygen Carriers. J. Funct. Biomater. 2017, 8, 11. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Meng, Z.; Gong, G.; Zhao, W.; Wang, K.; Liu, T. A novel nanoparticle drug delivery system based on PEGylated hemoglobin for cancer therapy. Drug Deliv. 2019, 26, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.; Biessels, P.; Ng, N.F.; Woods, C.; Bell, D.N.; Adamson, G. Synthesis and characterization of a hemoglobin-ribavirin conjugate for targeted drug delivery. Bioconjug. Chem. 2006, 17, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.F.; Stødkilde, K.; Sæderup, K.L.; Kuhlee, A.; Raunser, S.; Graversen, J.H.; Moestrup, S.K. Haptoglobin. Antioxid. Redox Signal. 2017, 26, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.; Torvund-Jensen, M.; Nielsen, M.J.; de Oliveira, C.L.; Hersleth, H.P.; Andersen, N.H.; Pedersen, J.S.; Andersen, G.R.; Moestrup, S.K. Structure of the haptoglobin-haemoglobin complex. Nature 2012, 489, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.K.; Katta, V.; Beavis, R.C.; Chait, B.T. Origin and removal of adducts (molecular mass = 98 u) attached to peptide and protein ions in electrospray ionization mass spectra. J. Am. Soc. Mass Spectrom. 1990, 1, 382–388. [Google Scholar] [CrossRef]
- Yi, J.; Thomas, L.M.; Richter-Addo, G.B. Structure of human R-state aquomethemoglobin at 2.0 Å resolution. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 2011, 67, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Liddington, R.; Derewenda, Z.; Dodson, E.; Hubbard, R.; Dodson, G. High resolution crystal structures and comparisons of T-state deoxyhaemoglobin and two liganded T-state haemoglobins: T(alpha-oxy)haemoglobin and T(met)haemoglobin. J. Mol. Biol. 1992, 228, 551–579. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2256–2268. [Google Scholar] [CrossRef]
- Knight, J.C.; Paisey, S.J.; Dabkowski, A.M.; Marculescu, C.; Williams, A.S.; Marshall, C.; Cornelissen, B. Scalling-down antibody radiolabelling reactions with zirconium-89. Dalton Trans. 2016, 45, 6343–6347. [Google Scholar] [CrossRef]
- Schaer, D.J.; Schaer, C.A.; Buehler, P.W.; Boykins, R.A.; Schoedon, G.; Alayash, A.I.; Schaffner, A. CD163 is the macrophage scavenger receptor for the native and chemically modified hemoglobins in the absence of haptoglobin. Blood 2006, 107, 373–380. [Google Scholar] [CrossRef]
- Weisser, S.B.; van Rooijen, N.; Sly, L.M. Depletion and Reconstitution of Macrophages in Mice. J. Vis. Exp. 2012, 66, 4105. [Google Scholar] [CrossRef]
- Gupta, N.; Price, P.M.; Aboagye, E.O. PET for in vivo pharmacokinetic and pharmacodynamic measurements. Eur. J. Cancer 2002, 38, 2094–2107. [Google Scholar] [CrossRef]
- Williams, S.P. Tissue Distribution Studies of Protein Therapeutics Using Molecular Probes: Molecular Imaging. AAPS J. 2012, 14, 389–399. [Google Scholar] [CrossRef]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Kosterink, J.G.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.; Bart, J.; de Jong, J.R.; de Vries, E.G.; Lub-de Hooge, M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef]
- Heuveling, D.A.; Karagozoglu, K.H.; van Lingen, A.; Hoekstra, O.S.; van Dongen, G.A.M.S.; De Bree, R. Feasibility of intraoperative detection of sentinel lymph nodes with 89-zirconium-labelled nanocolloidal albumin PET-CT and a handheld high-energy gamma probe. EJNMMI Res. 2018, 8, 15. [Google Scholar] [CrossRef]
- Nagengast, W.B.; Lub-de Hooge, M.N.; Oosting, S.F.; den Dunnen, W.F.; Warnders, F.J.; Brouwers, A.H.; de Jong, J.R.; Price, P.M.; Hollema, H.; Hospers, G.A.; et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res. 2011, 71, 143–153. [Google Scholar] [CrossRef]
- Advancing Treatment of Disease with Innovative Protein Therapeutics and Technologies; Targeted Treatment Against Liver Cancer. Available online: https://www.therapurebio.com/innovations/tbi-302 (accessed on 7 May 2020).
- Xu, Y.; Xu, J.; Hu, X.; Xia, X.; Dong, Q.; Liu, Z.; Chen, Z.; Tan, W. Zinc-substituted hemoglobin with specific drug binding sites and fatty acid resistance ability for enhanced photodynamic therapy. Nano Res. 2019, 12, 1880–1887. [Google Scholar] [CrossRef]
- Zhang, N.; Palmer, A.F. Development of a dichloroacetic acid-hemoglobin conjugate as a potential targeted anti-cancer therapeutic. Biotechnol. Bioeng. 2011, 108, 1413–1420. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Hu, W.; Zhang, Y.; Zhi, F.; Zhou, Z.; Jinhui, W.; Yiqiao, H. Acid Denaturation Inducing Self-Assembly of Curcumin-Loaded Hemoglobin Nanoparticles. Materials 2015, 8, 8701–8713. [Google Scholar] [CrossRef]
- Bunn, H.F.; Jandl, J.H. The renal handling of hemoglobin. II. Catabolism. J. Exp. Med. 1969, 129, 925–934. [Google Scholar] [CrossRef]
- Meng, Z.; Yang, X.; Hu, D.; Wang, K.; Zhi, F.; Chen, X.; Gong, G.; Wu, J.; Hu, Y. Replacing heme with paclitaxel to prepare drug-loaded globin nanoassembles for CD163 targeting. J. Pharm. Sci. 2015, 104, 1045–1055. [Google Scholar] [CrossRef]
- Etzerodt, A.; Kjolby, M.; Nielsen, M.J.; Maniecki, M.; Svendsen, P.; Moestrup, S.K. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption. Antioxid. Redox Signal. 2013, 18, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-Based Therapeutics and Theranostics Drug Delivery Systems for Next Generation of Liver Cancer Nanodrug Modalities. Int. J. Nanomed. 2020, 15, 1437–1456. [Google Scholar] [CrossRef]
- Perutz, M.F. Preparation of haemoglobin crystals. J. Cryst. Growth. 1968, 2, 54–56. [Google Scholar] [CrossRef]
- Luft, J.R.; DeTitta, G.T. A method to produce microseed stock for use in the crystallization of biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 988–993. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Vagin, A.; Teplyakov, A. A translation-function approach for heavy-atom location in macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 400–402. [Google Scholar] [CrossRef]
- Collaborative Computational Project, Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994, 50, 7690–7763. [Google Scholar] [CrossRef]
- Pannu, N.S.; Murshudov, G.N.; Dodson, E.J.; Read, R.J. Incorporation of prior phase information strengthens maximum-likelihood structure refinement. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 1285–1294. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2005, 25, 1605–1612. [Google Scholar] [CrossRef]
- Walther, M.; Gebhardt, P.; Grosse-Gehling, P.; Würbach, L.; Irmler, I.; Preusche, S.; Khalid, M.; Opfermann, T.; Kamradt, T.; Steinbach, J.; et al. Implementation of 89Zr production and in vivo imaging of B-cells in mice with 89Zr-labeled anti-B-cell antibodies by small animal PET/CT. Appl. Radiat. Isot. 2011, 69, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Dabkowski, A.M.; Paisey, S.J.; Talboys, M.; Marshall, C. Optimization of cyclotron production for radiometal of Zirconium 89. Acta Phys. Pol. A 2015, 127, 1479–1482. [Google Scholar] [CrossRef]
- Pillar, N.; Polsky, A.L.; Weissglas-Volkov, D.; Shomron, N. Comparison of breast cancer metastasis models reveals a possible mechanism of tumor aggressiveness. Cell Death Dis. 2018, 9, 1040. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiraga, Ł.; Cerutti, G.; Braniewska, A.; Strzemecki, D.; Sas, Z.; Boffi, A.; Savino, C.; Montemiglio, L.C.; Turnham, D.; Seaton, G.; et al. Biodistribution PET/CT Study of Hemoglobin-DFO-89Zr Complex in Healthy and Lung Tumor-Bearing Mice. Int. J. Mol. Sci. 2020, 21, 4991. https://doi.org/10.3390/ijms21144991
Kiraga Ł, Cerutti G, Braniewska A, Strzemecki D, Sas Z, Boffi A, Savino C, Montemiglio LC, Turnham D, Seaton G, et al. Biodistribution PET/CT Study of Hemoglobin-DFO-89Zr Complex in Healthy and Lung Tumor-Bearing Mice. International Journal of Molecular Sciences. 2020; 21(14):4991. https://doi.org/10.3390/ijms21144991
Chicago/Turabian StyleKiraga, Łukasz, Gabriele Cerutti, Agata Braniewska, Damian Strzemecki, Zuzanna Sas, Alberto Boffi, Carmelinda Savino, Linda Celeste Montemiglio, Daniel Turnham, Gillian Seaton, and et al. 2020. "Biodistribution PET/CT Study of Hemoglobin-DFO-89Zr Complex in Healthy and Lung Tumor-Bearing Mice" International Journal of Molecular Sciences 21, no. 14: 4991. https://doi.org/10.3390/ijms21144991
APA StyleKiraga, Ł., Cerutti, G., Braniewska, A., Strzemecki, D., Sas, Z., Boffi, A., Savino, C., Montemiglio, L. C., Turnham, D., Seaton, G., Bonamore, A., Clarkson, R., Dabkowski, A. M., Paisey, S. J., Taciak, B., Kucharzewska, P., Rygiel, T. P., & Król, M. (2020). Biodistribution PET/CT Study of Hemoglobin-DFO-89Zr Complex in Healthy and Lung Tumor-Bearing Mice. International Journal of Molecular Sciences, 21(14), 4991. https://doi.org/10.3390/ijms21144991







