Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats
Abstract
1. Introduction
2. Results
2.1. Estimated Doses of Internal Irradiation
2.2. Body and Testes Weight and Serum Testosterone Level
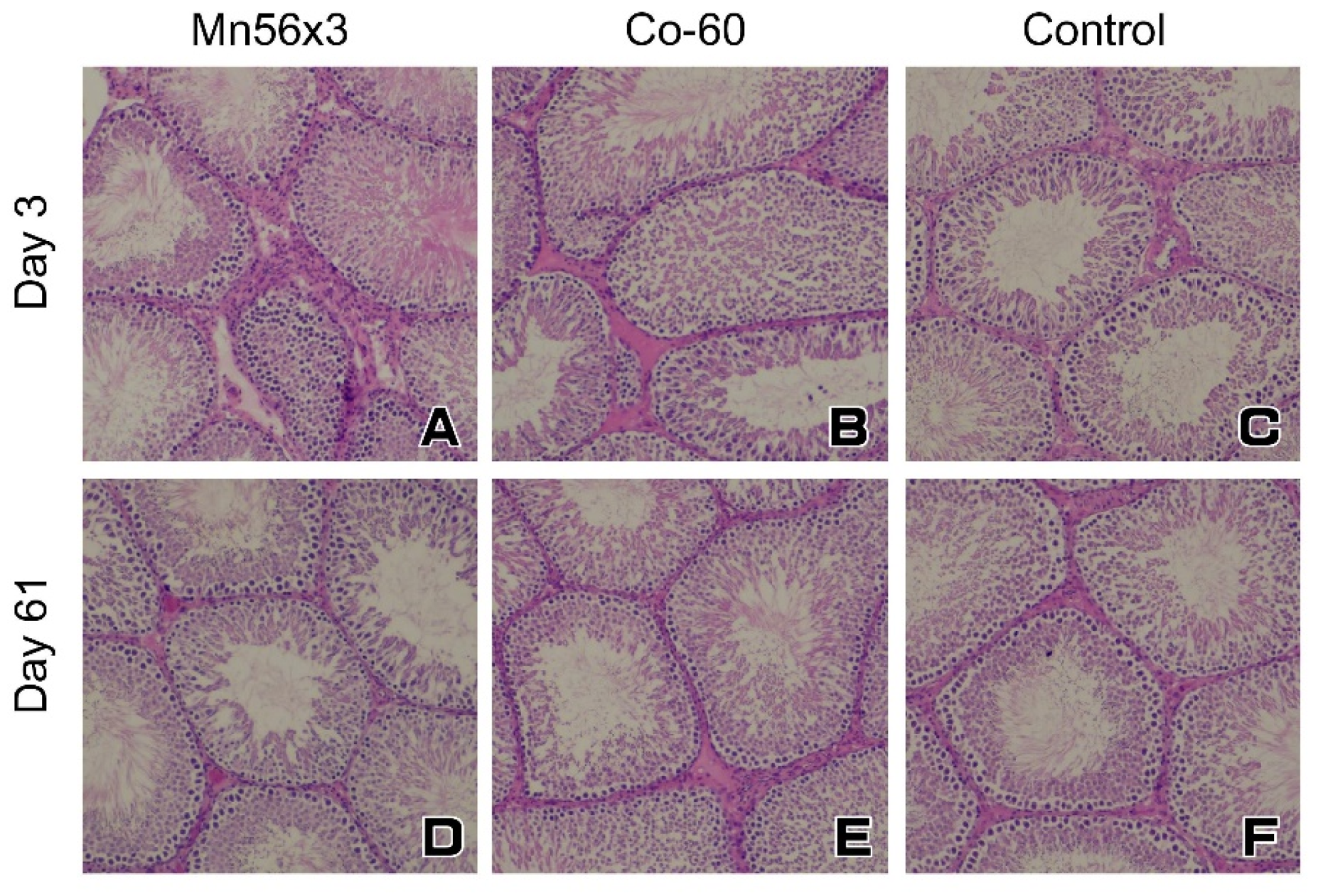
2.3. Histology of the Testes
2.4. Effects on mRNA Expression Levels of Leydig Cell-Specific Steroidogenesis Related Genes
2.5. Effects on mRNA Expression Levels of Sertoli Cell and Germ Cell-Specific Genes
2.6. Prostatic Secretory Protein mRNA Expressions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irradiation and Dosimetry
4.3. Measurement of mRNA Levels by Quantitative RT-PCR
4.4. Measurement of Serum Testosterone
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imanaka, T.; Endo, S.; Tanaka, K.; Shizuma, K. Gamma-ray exposure from neutron-induced radionuclides in soil in Hiroshima and Nagasaki based on DS02 calculations. Radiat. Environ. Biophys. 2008, 47, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Endo, S.; Imanaka, T.; Shizuma, K.; Hasai, H.; Hoshi, M. Skin dose from neutron-activated soil for early entrants following the A-bomb detonation in Hiroshima: Contribution from β and γ rays. Radiat. Environ. Biophys. 2008, 47, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Baurzhan, A.; Chaizhunusova, N.; Amantayeva, G.; Kairkhanova, Y.; Shabdarbaeva, D.; Zhunussov, Y.; Zhumadilov, K.; Stepanenko, V.; Gnyrya, V.; et al. Effects of Internal Exposure to 56MnO2 Powder on Blood Parameters in Rats. Eurasian J. Med. 2020, 52, 52–56. [Google Scholar] [CrossRef]
- Shichijo, K.; Fujimoto, N.; Uzbekov, D.; Kairkhanova, Y.; Saimova, A.; Chaizhunusova, N.; Sayakenov, N.; Shabdarbaeva, D.; Aukenov, N.; Azimkhanov, A.; et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—Part 2: Pathological effects. Radiat. Environ. Biophys. 2017, 56, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, V.; Rakhypbekov, T.; Otani, K.; Endo, S.; Satoh, K.; Kawano, N.; Shichijo, K.; Nakashima, M.; Takatsuji, T.; Sakaguchi, A.; et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats: Part 1: Dosimetry. Radiat. Environ. Biophys. 2017, 56, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Delic, J.I.; Schlappack, O.K.; Harwood, J.R.; Stanley, J.A. Comparative effects of X irradiation on the testes of adult Sprague-Dawley and Wistar rats. Radiat. Res. 1987, 112, 99–104. [Google Scholar] [CrossRef]
- Gunn, S.A.; Gould, T.C.; Anderson, W.A. The effect of x-irradiation on the morphology and function of the rat testis. Am. J. Pathol. 1960, 37, 203–213. [Google Scholar] [PubMed]
- Huckins, C. Behavior of Stem Cell Spermatogonia in the Adult Rat Irradiated Testis. Biol. Reprod. 1978, 19, 747–760. [Google Scholar] [CrossRef]
- Birioukov, A.; Meurer, M.; Peter, R.U.; Braun-Falco, O.; Plewig, G. Male reproductive system in patients exposed to ionizing irradiation in the chernobyl accident. Syst. Biol. Reprod. Med. 1993, 30, 99–104. [Google Scholar] [CrossRef]
- Mudie, N.Y.; Gusev, B.I.; Pivina, L.M.; Schoemaker, M.J.; Rijinkova, O.N.; Apsalikov, K.N.; Swerdlow, A.J. Sex Ratio in the Offspring of Parents with Chronic Radiation Exposure from Nuclear Testing in Kazakhstan. Radiat. Res. 2007, 168, 600–607. [Google Scholar] [CrossRef]
- Abuelhija, M.; Weng, C.C.; Shetty, G.; Meistrich, M.L. Differences in radiation sensitivity of recovery of spermatogenesis between rat strains. Toxicol. Sci. 2012, 126, 545–553. [Google Scholar] [CrossRef]
- Chen, M.; Hao, J.; Yang, Q.; Li, G. Effects of icariin on reproductive functions in male rats. Molecules 2014, 19, 9502–9514. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Karam, H.M.; Shaaban, E.A.; Safar, M.M.; El-Yamany, M.F. MitoQ ameliorates testicular damage induced by gamma irradiation in rats: Modulation of mitochondrial apoptosis and steroidogenesis. Life Sci. 2019, 232, 116655. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Lee, B.M.; Kacew, S.; Kim, H.S. Identification of differentially expressed genes in the testis of Sprague-Dawley rats treated with di(n-butyl) phthalate. Toxicology 2007, 234, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Malashenko, A.M.; Beskova, T.B.; Pomerantseva, M.D.; Ramaiya, L.K. Comparison of Three Inbred Mouse Strains 1053. Russ. J. Genet. 2003, 39, 1052–1055. [Google Scholar] [CrossRef]
- Meistrich, M.L.; Finch, M.; Lu, C.C.; de Ruiter-Bootsma, A.L.; de Rooij, D.G.; Davids, J.A.G. Strain Differences in the Response of Mouse Testicular Stem Cells to Fractionated Radiation. Radiat. Res. 1984, 97, 478. [Google Scholar] [CrossRef]
- Belling, K.C.; Tanaka, M.; Dalgaard, M.D.; Nielsen, J.E.; Nielsen, H.B.; Brunak, S.; Almstrup, K.; Leffers, H. Transcriptome profiling of mice testes following low dose irradiation. Reprod. Biol. Endocrin. 2013, 11, 1–13. [Google Scholar] [CrossRef]
- Delic, J.I.; Hendry, J.H.; Morris, I.D.; Shalet, S.M. Dose and time related responses of the irradiated prepubertal rat testis. I. Leydig cell function. Int. J. Androl. 1985, 8, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Pinon-Lataillade, G.; Viguier-Martinez, M.C.; Touzalin, A.M.; Maas, J.; Jégou, B. Effect of an acute exposure of rat testes to gamma rays on germ cells and on Sertoli and Leydig cell functions. Reprod. Nutr. Dev. 1991, 31, 617–629. [Google Scholar] [CrossRef]
- Cunha, G.R.; Donjacour, A.A.; Cooke, P.S.; Mee, S.; Bigsby, R.M.; Higgins, S.J.; Sugimura, Y. The endocrinology and developmental biology of the prostate. Endocr. Rev. 1987, 8, 338–362. [Google Scholar] [CrossRef]
- Stepanenko, V.F.; Yaskova, E.K.; Petriev, V.M.; Skvortsov, V.G.; Kolyzhenkov, T.V.; Petukhov, A.D.; Dubov, D.V. The calculation of internal irradiation of nano-, micro- and macro-biostructures by electrons, beta particles and quantum radiation of different energy for the development and research of new radiopharmaceuticals in nuclear medicine. Radiat. Risk 2015, 24, 35–60. [Google Scholar]
- Harrison, J.D.; Stather, J.W. The assessment of doses and effects from intakes of radioactive particles. J. Anat. 1996, 189, 521–530. [Google Scholar] [PubMed]
- Lang, S.; Raunemaa, T. Behavior of Neutron-Activated Uranium Dioxide Dust Particles in the Gastrointestinal Tract of the Rat. Radiat. Res. 1991, 126, 273. [Google Scholar] [CrossRef] [PubMed]
- Limpanussorn, J.; Simon, L.; Dayan, A.D. Transepithelial transport of large particles in rat: A new model for the quantitative study of particle uptake. J. Pharm. Pharmacol. 1998, 50, 753–760. [Google Scholar] [CrossRef]
- Ahmad, G.; Agarwal, A. Ionizing Radiation and Male Fertility. In Male Infertility: A Clinical Approach; Gunasekaran, K., Pandiyan, N., Eds.; Springer: New Delhi, India, 2017; pp. 185–195. [Google Scholar] [CrossRef]
- Ogilvy-Stuart, A.L.; Shalet, S.M. Effect of radiation on the human reproductive system. Environ. Health Perspect. 1993, 101, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.P.; Katsiya, G.V.; Kolesnikova, G.S.; Dobracheva, G.A.D.; Todua, T.N.; Vax, V.V.; Giwercman, A.; Waites, G.M.H. Endocrine and reproductive health status of men who had experienced short-term radiation exposure at Chernobyl. Int. J. Androl. 1998, 21, 271–276. [Google Scholar] [CrossRef]
- Grosche, B. Semipalatinsk test site: Introduction. Radiat. Environ. Biophys. 2002, 41, 53–55. [Google Scholar] [CrossRef]
- Schull, W.J.; Neel, J.V. Radiation and the sex ratio in man. Science 1958, 128, 343–348. [Google Scholar] [CrossRef]
- Fujimoto, N.; Suzuki, T.; Ohta, S.; Kitamura, S. Identification of rat prostatic secreted proteins using mass spectrometric analysis and androgen-dependent mRNA expression. J. Androl. 2009, 30, 669–678. [Google Scholar] [CrossRef]


| Groups | Body Weight (g) | Testes (g/kg bw) | Testosterone (pg/mL) | |
|---|---|---|---|---|
| Day 3 | Control | 248 ± 16 | 10.5 ± 1.1 | 1.2 ± 0.30 |
| Cold Mn | 235 ± 14 | 11.4 ± 0.7 | 0.94 ± 0.13 | |
| Mn56×1 | 235 ± 11 | 11.7 ± 0.6 | 0.7 ± 0.24 | |
| Mn56×2 | 245 ± 16 | 11.3 ± 0.6 | 0.8 ± 0.17 | |
| Mn56×3 | 237 ± 12 | 12.2 ± 0.6 | not determined | |
| Co-60 | 234 ± 14 | 11.5 ± 0.8 | 1.15 ± 0.33 | |
| Day 61 | Control | 330 ± 17 | 9.3 ± 0.7 | 1.45 ± 0.35 |
| Cold Mn | 337 ± 19 | 9.8 ± 0.4 | 1.3 ± 0.14 | |
| Mn56×1 | 371 ± 21 | 9.2 ± 0.6 | 1.42 ± 0.14 | |
| Mn56×2 | 337 ± 17 | 9.1 ± 0.5 | 0.68 ± 0.26 * | |
| Mn56×3 | 353 ± 17 | 9.1 ± 0.5 | 0.75 ± 0.23 | |
| Co-60 | 328 ± 23 | 9.4 ± 0.5 | 0.59 ± 0.11 * | |
| Groups | PrstC3 (fmol/fmol βact) | CRP1 (fmol/fmol βact) | KS3 (fmol/fmol βact) | PSP94 (fmol/fmol βact) |
|---|---|---|---|---|
| Control | 651 ± 45 | 158 ± 22 | 20 ± 2.6 | 103 ± 7.3 |
| Cold Mn | 566 ± 38 | 168 ± 26 | 13.5 ± 2.2 | 83 ± 11.2 |
| Mn56 × 1 | 716 ± 82 | 206 ± 34 | 18.3 ± 2.9 | 86 ± 33.8 |
| Mn56 × 2 | 611 ± 103 | 77 ± 14 * | 8.3 ± 1.6 ** | 75 ± 9.0 |
| Mn56 × 3 | 453 ± 24 | 91 ± 10 | 9.8 ± 2.8 * | 49 ± 10.7 * |
| Co-60 | 557 ± 79 | 79 ± 19 * | 11.8 ± 2.6 * | 71 ± 9.4 |
| Gene | GenBank Accession# | Q-PCR Primer Sequences (5′–3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Cyp11a1 | NM_017286 | TCCTCCCTGGTTACGTGCAG | GCAGAATAAGGAGCACCCCAG |
| Cyp17a1 | NM_012753 | CAGCCAGATCAGTTCATGCCT | GACAAAGAGCTCCTGACGGG |
| Hsd3b1 | NM_001007719 | AGAGAGATCTGGGCTATGTGCC | ACACCCAGAACCACATCCTTG |
| StAR | NM_031558 | ACCTGCATGGTGCTTCATCC | GCTGGCGAACTCTATCTGGGT |
| Cld11 | NM_053457 | TCCTCCCTGGTTACGTGCAG | GCAGAATAAGGAGCACCCCAG |
| Clu | NM_053021 | TGCTTCATTCCCTCCAGTCC | TGGGTTGTCACTGTGGAGACC |
| Spag4 | NM_031792 | CCAAGCTGATGATGACGAGACT | GGCCCCAGTTGCTTAAAATCT |
| Zpbp | NM_001025139 | TTCAGCAAGTGGAAGTCCTGG | ACACAGCACTCAGGACACTTGG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, N.; Amantayeva, G.; Chaizhunussova, N.; Shabdarbayeva, D.; Abishev, Z.; Ruslanova, B.; Zhunussov, Y.; Azhimkhanov, A.; Zhumadilov, K.; Petukhov, A.; et al. Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats. Int. J. Mol. Sci. 2020, 21, 4989. https://doi.org/10.3390/ijms21144989
Fujimoto N, Amantayeva G, Chaizhunussova N, Shabdarbayeva D, Abishev Z, Ruslanova B, Zhunussov Y, Azhimkhanov A, Zhumadilov K, Petukhov A, et al. Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats. International Journal of Molecular Sciences. 2020; 21(14):4989. https://doi.org/10.3390/ijms21144989
Chicago/Turabian StyleFujimoto, Nariaki, Gaukhar Amantayeva, Nailya Chaizhunussova, Dariya Shabdarbayeva, Zhaslan Abishev, Bakhyt Ruslanova, Yersin Zhunussov, Almas Azhimkhanov, Kassym Zhumadilov, Aleksey Petukhov, and et al. 2020. "Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats" International Journal of Molecular Sciences 21, no. 14: 4989. https://doi.org/10.3390/ijms21144989
APA StyleFujimoto, N., Amantayeva, G., Chaizhunussova, N., Shabdarbayeva, D., Abishev, Z., Ruslanova, B., Zhunussov, Y., Azhimkhanov, A., Zhumadilov, K., Petukhov, A., Stepanenko, V., & Hoshi, M. (2020). Low-Dose Radiation Exposure with 56MnO2 Powder Changes Gene Expressions in the Testes and the Prostate in Rats. International Journal of Molecular Sciences, 21(14), 4989. https://doi.org/10.3390/ijms21144989





