Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit
Abstract
1. Introduction
- if Kaolin had an effect on ABA biosynthetic pathway,
- if the eventual difference in ABA accumulation was related to possible bottlenecks on the carotenoid biosynthetic pathway that leads to ABA biosynthesis in leaves.
2. Results
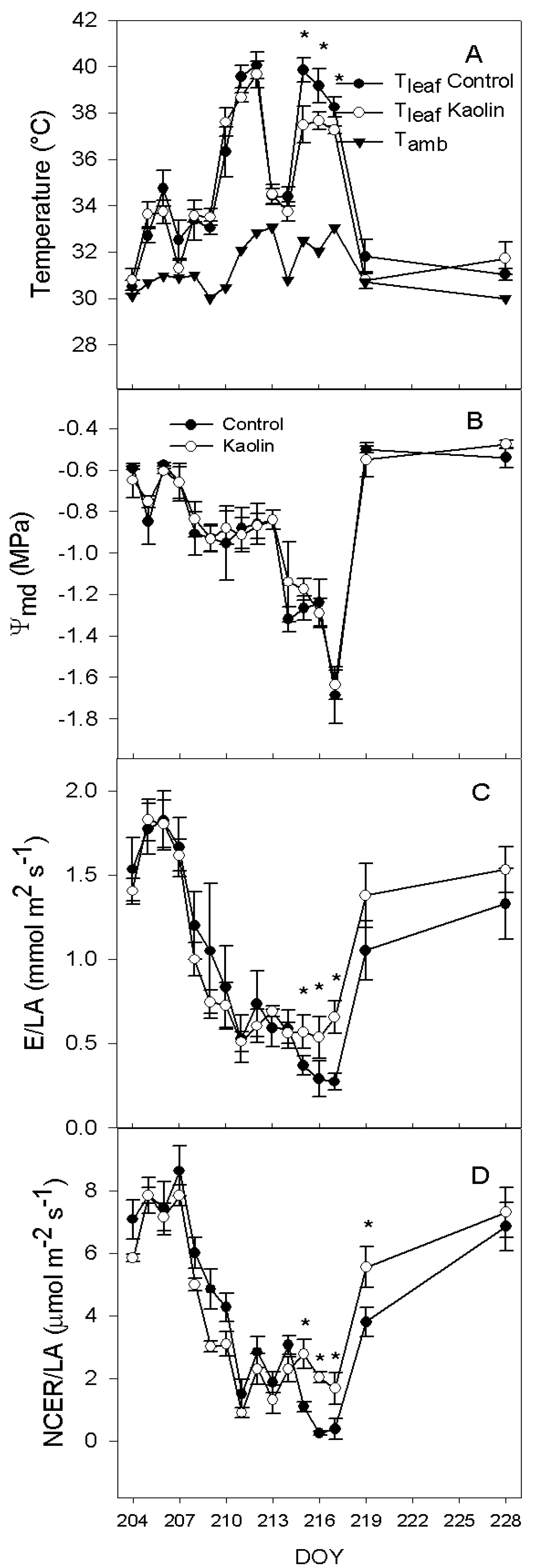
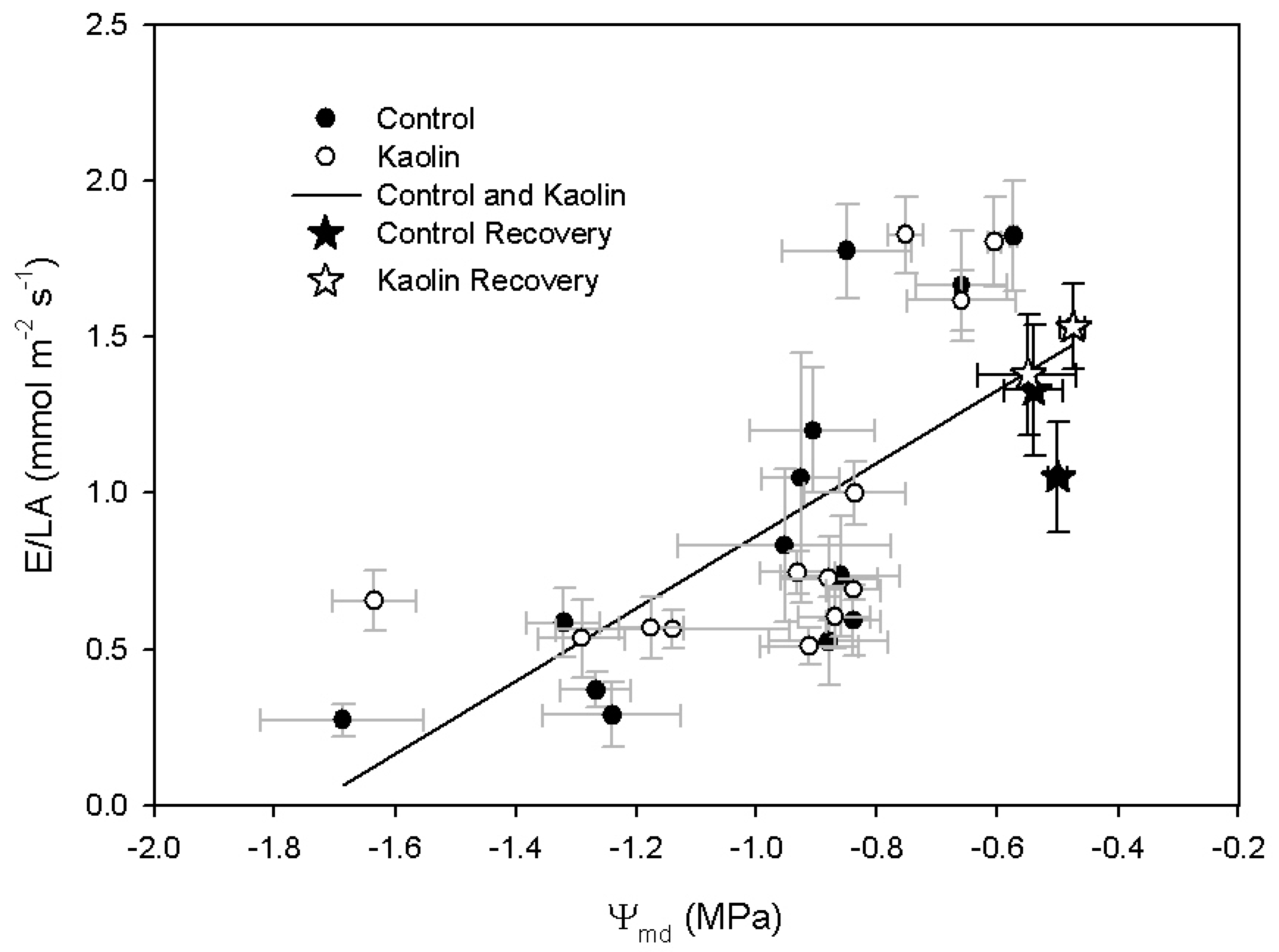
2.1. Leaf Physical Properties and Vine Physiology
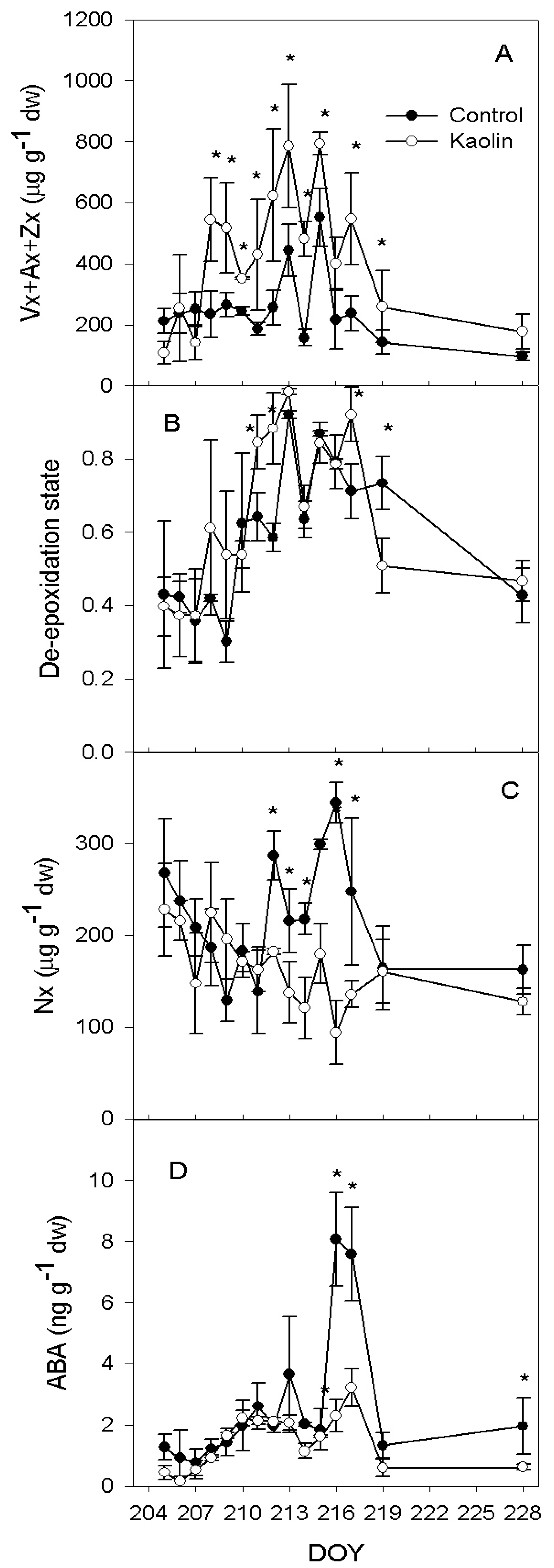
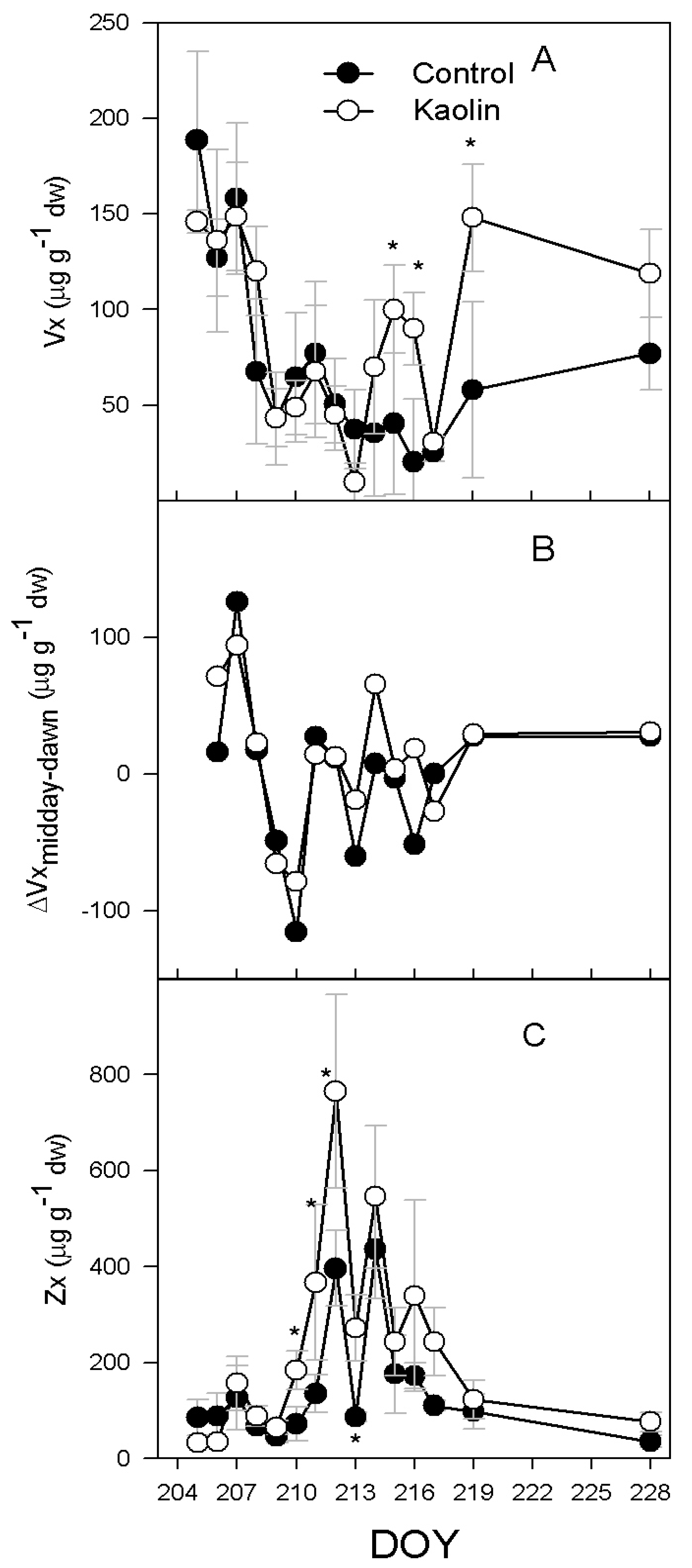
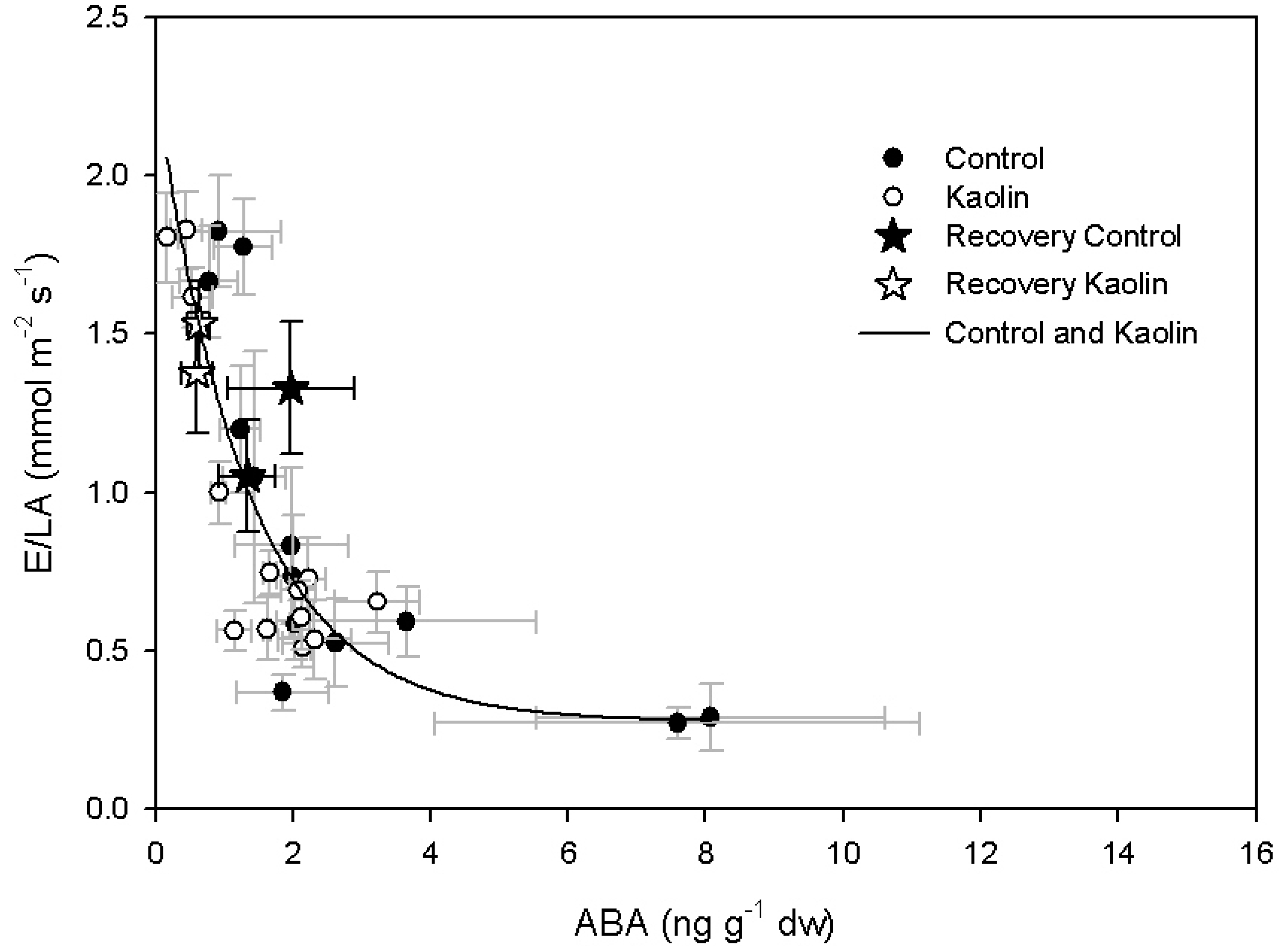
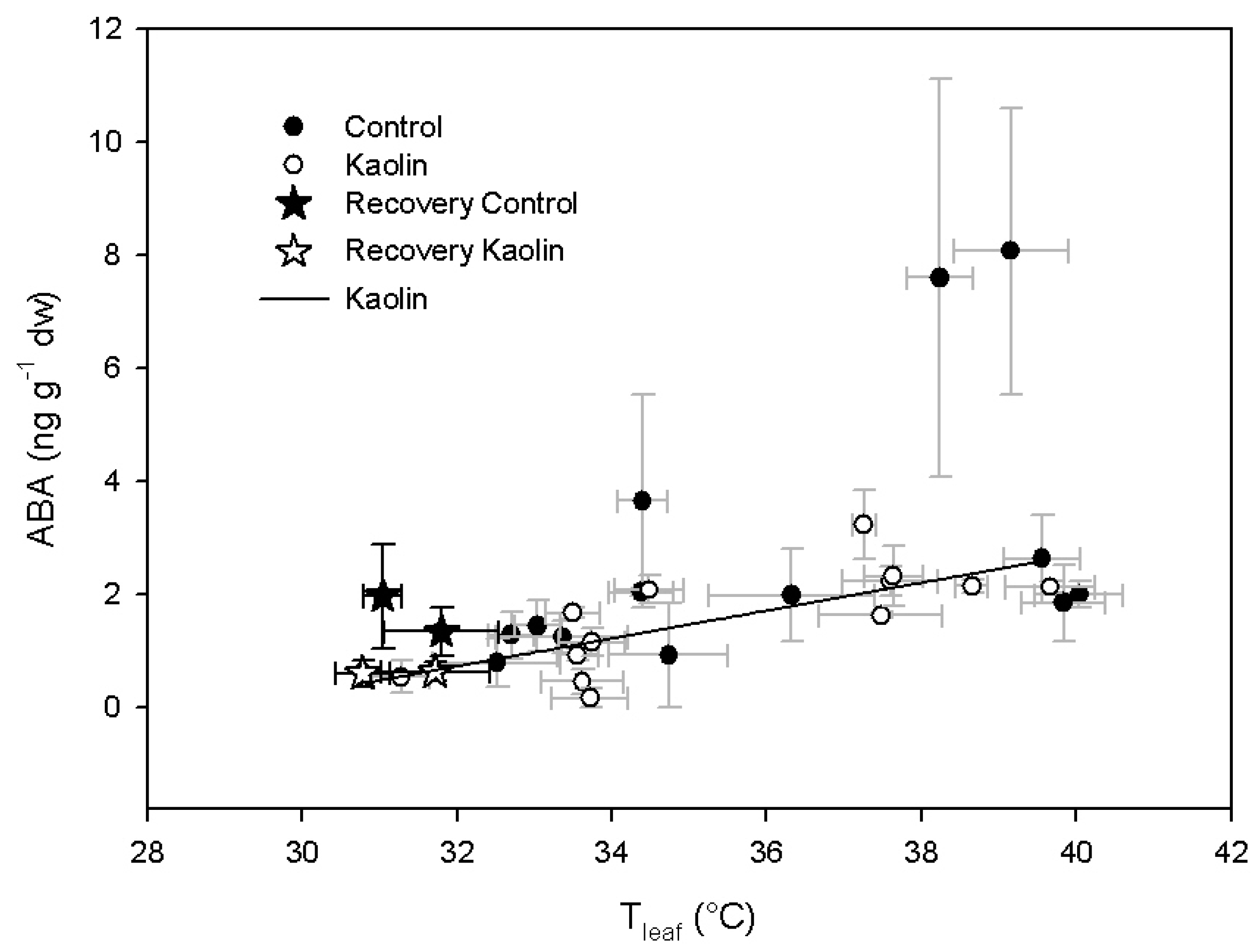
2.2. Xanthophylls and ABA Concentration
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatment Layout
4.2. Whole-Canopy Gas Exchange
4.3. Leaf Water Status, Temperature and Light Reflectance
4.4. Xanthophylls and Abscisic Acid Determination
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, R.M.; Hurd, B.H.; Lenhart, S.; Leary, N. Effects of global climate change on agriculture: An interpretative review. Clim. Res. 1998, 11, 19–30. [Google Scholar] [CrossRef]
- Olesen, J.E.; Bindi, M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002, 16, 239–262. [Google Scholar] [CrossRef]
- Santillan, D.; Iglesias, A.; La Jeunesse, I.; Garrote, L.; Sotes, V. Vineyards in transition: A global assessment of the adaptation needs of grape producing regions under climate change. Sci. Tot. Env. 2009, 657, 830–852. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2004, 178, 43–54. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. Interactions of crop level and late season water stress on growth and physiology of field-grown Concord grapevines. Am. J. Enol. Vitic. 1994, 45, 252–258. [Google Scholar]
- Flexas, J.; Bota, J.; Escalona, J.; Sampol, B.; Medrano, H. Effects of drought on photosynthesis in grapevines under field conditions: An evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 2002, 29, 461–471. [Google Scholar] [CrossRef]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. HortScience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Effects of kaolin application on light absorption and distribution, radiation use efficiency and photosynthesis of almond and walnut canopies. Ann. Bot. 2007, 99, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Env. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing Kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Dinis, L.T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Frioni, T.; Saracino, S.; Squeri, C.; Tombesi, S.; Palliotti, A.; Sabbatini, P.; Magnanini, E.; Poni, S. Understanding kaolin effects on grapevine leaf and whole-canopy physiology during water stress and re-watering. J. Plant Physiol. 2019, 242, 153020. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Phys. Biochem. 2008, 133, 29–39. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Salleo, S.; Nardini, A.; Pitt, F.; Gullo, M.A. Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.). Plant Cell Environ. 2000, 23, 71–79. [Google Scholar] [CrossRef]
- Franks, P.J. Stomatal control and hydraulic conductance, with special reference to tall trees. Tree Physiol. 2004, 24, 865–878. [Google Scholar] [CrossRef]
- Luan, S. Signalling drought in guard cells. Plant Cell Environ. 2002, 25, 229–237. [Google Scholar] [CrossRef]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant’s response to drought. J. Plant Gro. Reg. 2005, 24, 285–295. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Passive origins of stomatal control in vascular plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Sack, L.; John, G.P.; Buckley, T.N. ABA accumulation in dehydrating leaves is associated with decline in cell volume, not turgor pressure. Plant Physiol. 2018, 176, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Arnoux, P.; Morosinotto, T.; Saga, G.; Bassi, R.; Pignol, D. A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 2009, 21, 2036–2044. [Google Scholar] [CrossRef]
- Jahns, P.; Miehe, B. Kinetic correlation of recovery from photoinhibition and zeaxanthin epoxidation. Planta 1996, 198, 202–210. [Google Scholar] [CrossRef]
- Bouvier, F.; D’Harlingue, A.; Backhaus, R.A.; Kumagai, M.H.; Camara, B. Identification of neoxanthin synthase as a carotenoid cyclase paralog. Eur. J. Biochem. 2000, 267, 6346–6352. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Sussmilch, F.C.; Brodribb, T.J. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016, 39, 485–491. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correira, C.; Moutinho-Pereira, J. Kaolin modulates ABA and IAA dynamics and physiology of grapevine under Mediterranean summer stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef]
- Steiman, S.R.; Bittenbender, H.C.; Idol, T.W. Analysis of kaolin particle film use and its application on coffee. HortScience 2007, 42, 1605–1608. [Google Scholar] [CrossRef]
- Wand, S.J.; Theron, K.I.; Ackerman, J.; Marais, S.J. Harvest and post-harvest apple fruit quality following applications of kaolin particle film in South African orchards. Sci. Hortic. 2006, 107, 271–276. [Google Scholar] [CrossRef]
- Luciani, E.; Palliotti, A.; Frioni, T.; Tombesi, S.; Villa, F.; Zadra, C.; Farinelli, D. Kaolin treatments on Tonda Giffoni hazelnut (Corylus avellana L.) for the control of heat stress damages. Sci. Hortic. 2020, 263, 109097. [Google Scholar] [CrossRef]
- Shellie, K.C.; King, B.A. Kaolin particle film and water deficit influence Malbec leaf and berry temperature, pigments, and photosynthesis. Amer. J. Enol. Vitic. 2013, 64, 223–230. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Brodribb, T.J. Mesophyll cells are the main site of abscisic acid biosynthesis in water-stressed leaves. Plant Physiol. 2018, 177, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth stages of the grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Austr. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Poni, S.; Merli, M.C.; Magnanini, E.; Galbignani, M.; Bernizzoni, F.; Vercesi, A.; Gatti, M. An improved multichamber gas exchange system for determining whole-canopy water-use efficiency in grapevine. Am. J. Enol. Vitic. 2014, 65, 268–276. [Google Scholar] [CrossRef]
- Gatti, M.; Pirez, F.J.; Frioni, T.; Squeri, C.; Poni, S. Calibrated, delayed-cane winter pruning controls yield and significantly postpones berry ripening parameters in Vitis vinifera L. cv. Pinot Noir. Aust. J. Grape Wine Res. 2018, 24, 305–316. [Google Scholar] [CrossRef]
- Taylor, A.H. The measurement of diffuse reflection factors and a new absolute reflectometer. J. Opt. Soc. Am. 1920, 4, 9–23. [Google Scholar] [CrossRef]
- Yuan, F.; Qian, M.C. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food chem. 2016, 192, 633–641. [Google Scholar] [CrossRef]
- Vilaró, F.; Canela-Xandri, A.; Canela, R. Quantification of abscisic acid in grapevine leaf (Vitis vinifera) by isotope-dilution liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 2006, 386, 306–312. [Google Scholar] [CrossRef] [PubMed]
| Reflected PAR | Transmitted PAR | |||||
|---|---|---|---|---|---|---|
| (% of Total PAR) | (% of Total PAR) | |||||
| Control | 10.10% | ± | 0.88 b | 8.30% | ± | 0.02 a |
| Kaolin | 15.14% | ± | 0.45 a | 6.86% | ± | 0.41 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit. Int. J. Mol. Sci. 2020, 21, 4950. https://doi.org/10.3390/ijms21144950
Frioni T, Tombesi S, Sabbatini P, Squeri C, Lavado Rodas N, Palliotti A, Poni S. Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit. International Journal of Molecular Sciences. 2020; 21(14):4950. https://doi.org/10.3390/ijms21144950
Chicago/Turabian StyleFrioni, Tommaso, Sergio Tombesi, Paolo Sabbatini, Cecilia Squeri, Nieves Lavado Rodas, Alberto Palliotti, and Stefano Poni. 2020. "Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit" International Journal of Molecular Sciences 21, no. 14: 4950. https://doi.org/10.3390/ijms21144950
APA StyleFrioni, T., Tombesi, S., Sabbatini, P., Squeri, C., Lavado Rodas, N., Palliotti, A., & Poni, S. (2020). Kaolin Reduces ABA Biosynthesis through the Inhibition of Neoxanthin Synthesis in Grapevines under Water Deficit. International Journal of Molecular Sciences, 21(14), 4950. https://doi.org/10.3390/ijms21144950










