Abstract
The Retinoblastoma protein (pRb) is a key cell cycle regulator conserved in a wide variety of organisms. Experimental analysis of pRb’s functions in animals and plants has revealed that this protein participates in cell proliferation and differentiation processes. In addition, pRb in animals and its orthologs in plants (RBR), are part of highly conserved protein complexes which suggest the possibility that analogies exist not only between functions carried out by pRb orthologs themselves, but also in the structure and roles of the protein networks where these proteins are involved. Here, we present examples of pRb/RBR participation in cell cycle control, cell differentiation, and in the regulation of epigenetic changes and chromatin remodeling machinery, highlighting the similarities that exist between the composition of such networks in plants and animals.
1. Introduction
Eukaryotic organisms evolved from a common unicellular ancestor, from which they diverged 1500 million years ago [1,2,3]. Testimonies of this ancient kinship are some processes and genetic components that have been preserved throughout evolution, along with many others which subsequently emerged exclusively in plants or animals [4,5,6]. Interestingly, it has been shown that plants and animals evolved multicellularity independently, but unlike animals, plants have an extensive post-embryonic growth and development, that is highly influenced by the environment [7,8]. However, in both multicellular organisms, morphogenesis depends on a delicate balance between cell division and differentiation rates. In animals, when this balance is lost, it can cause tumors and cancer, not seen during most of plant development. Nonetheless, Arabidopsis thaliana (hereafter Arabidopsis), the most studied model plant, has been shown to be an important model system to understand basic regulatory mechanisms involved in human diseases [9,10,11]. For example, Arabidopsis has homologous genes for 70% of those involved in human cancer. Interestingly, a higher percentage than that found in the genome of Drosophila melanogaster or of Saccharomyces cerevisiae [9,10]. Hence, Arabidopsis has already been used as a screening tool to evaluate the action and efficacy of some drugs to treat human cancer and other diseases [11,12,13].
Retinoblastoma (RB1) is a highly conserved gene, that encodes the so-called tumor suppressor protein, that regulates different developmental processes of phylogenetically distant organisms. The name of this gene comes from the retinoblastoma illness, a rare intraocular malignant tumor that has its onset during early childhood. As determined by karyotyping, this disease is associated with losses in chromosome 13, which contains the RB1 gene [14,15,16]. RB1 was identified by positional cloning and after subsequent molecular analysis, it became known as the first tumor suppressor gene, giving robust evidence for the genetic predisposition of cancer development in some cases [17,18]. After its discovery, alterations in this gene were described in other malignant tumors such as osteosarcomas, cervical cancer, prostate carcinoma, small cell lung cancer, and some forms of leukemia [19,20]. RB1 is an essential gene whose best studied function is the regulation of the cell cycle transition from G1 to S phase through formation of a protein complex with transcription factors of the E2F-family; that are regulated by the Retinoblastoma protein (pRb) multiple phosphorylation states. In many cancer types, an altered regulation of pRb, like permanent hyperphosphorylation that promotes pRb dissociation from the complex with E2F, leads to an unregulated cell proliferation [21,22]. Moreover, altered regulation of the pRb pathway is considered one of the most common traits in different types of cancer [23,24], and several studies have proposed targeting pRb regulation pathway as alternative treatments [25,26,27]. In fact, cyclin dependent kinases (CDKs), the kinases that phosphorylate pRb are commonly deregulated in many malignant tumors. From the therapeutic standpoint, pRb cannot be a target of drugs, however, CDKs are therapeutic targets, and several generations of non-specific cell cycle CDKs inhibitors have been under clinical evaluation as cancer treatments with mixed results. More recently specific cell cycle CDK4/6 and transcriptional CDKs inhibitors may become alternative therapeutic strategies under current clinical evaluation [28,29,30]. In summary, a more thorough understanding of pRb’s developmental functions could help find new efficient treatments for different cancer types.
In this review, we will focus on how the protein encoded by the RB1 gene, and its plant ortholog RETINOBLASTOMA-RELATED (RBR), participates in important developmental processes such as cell cycle control, cell differentiation, as well as in the homeostasis of stem cell/pluripotency, that are cellular processes shared by plants and animals. First, we analyze the structure of the RB/RBR proteins, secondly, we describe their interaction with proteins that participate in different developmental processes and regulatory networks. Finally, we analyze their participation in the epigenetic and chromatin structure regulation, which is important for different developmental decisions. Interestingly, many of the protein interactions involving RETINOBLASTOMA, as well as the overall structures of the regulatory networks in which this protein participates are similar between plants and animals. This suggests that once such regulatory networks were assembled during evolution, the key role of this protein as an integrator of internal developmental cues remained functionally constrained among eukaryotic organisms’ evolution.
2. Structure of the Retinoblastoma Protein
In humans, Retinoblastoma susceptibility gene is a member of a small gene family that includes RB1 (p105/pRb), RBl1 (p107/pRBL1), and RBl2 (p130/pRBL2), whose protein structure are very similar, and that share some overlapping functions [31,32,33]. From the three family members, RB1 has been the most studied gene since it participates in tumor onset and progression, while RBl1 and RBl2 rarely display mutations in human retinal cancer [34,35].
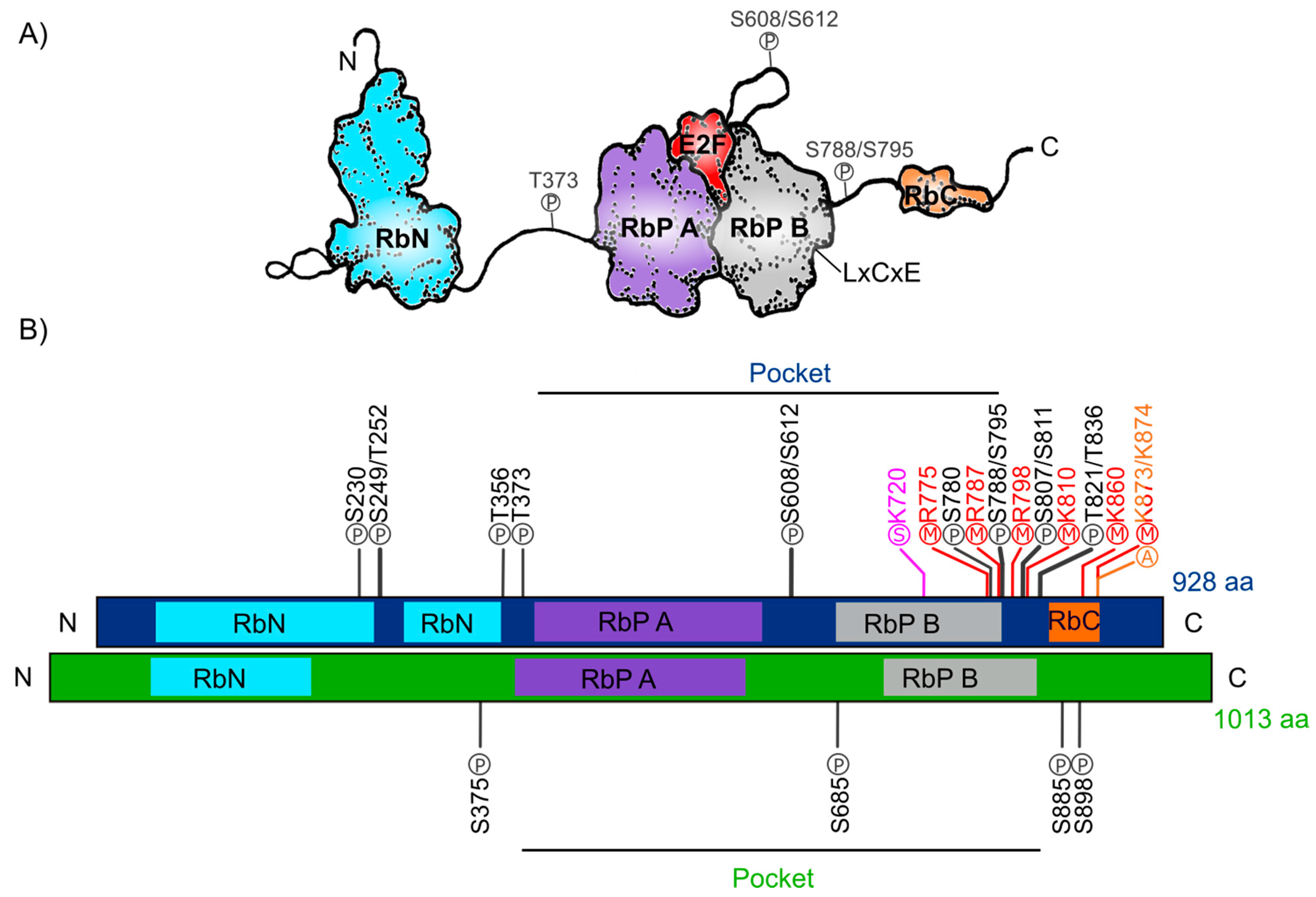
The human Retinoblastoma protein (pRb) consists of 928 amino acids and includes three distinctive domains: the N-terminal structural domain (RbN), the so-called “pocket” (RbP) domain, the C-terminal domain (RbC), and the non-structured regions between them (Figure 1A). The pocket domain includes two highly conserved subdomains (A and B) called cyclin folds, which are formed by two structural nuclei, each conformed by three helix bundles with two additional helices packing at the sides in each one. These subdomains are required to mediate interactions with other proteins like several oncoproteins and transcription factors (TFs) [34,36,37,38]. According to current interaction databases 322 proteins interact with human pRb, the E2F TFs being the best characterized ones (Figure 1A) [39,40]. The interaction of pRb with many other proteins depends on the pRb structure and its post-translational modifications, which determine this protein’s function in different developmental processes [40,41]. Many of the pRb-interacting proteins contain the motif ‘LxCxE’ (Leu-X-Cys-X-Glu where X stands for any amino acid), essential to bind with the Pocket B subdomain of pRb (Figure 1A). Examples of such proteins are D-type cyclins that are cell cycle regulators, the histone deacetylases 1 and 2 (HDAC1/2); several viral proteins like SV40 large T antigen (SV40 T-ag) and two viral proteins that stimulate the cell cycle progression in infected cells through pRb inactivation: Human Papillomavirus E7 (HPV E7) and Adenovirus early region 1A (Ad5 E1A) [38,42,43,44]. The RbN domain is also composed of two cyclin folds very similar to those found in the “pocket” domain. The RbN domain can physically interact with the RbP one and deletion of this domain abrogates the regulation of the pRb/E2F complex [45]. Finally, the RbC region, that includes approximately 150 residues, is intrinsically disordered and has been shown to be required for the interaction between pRb and the E2F/DP complex [36,46]. The three pRb domains are connected by sequences that confer flexibility to the protein and contain sites that can be subjected to post-translational modifications, that play important roles in the regulation of pRb activity [47].
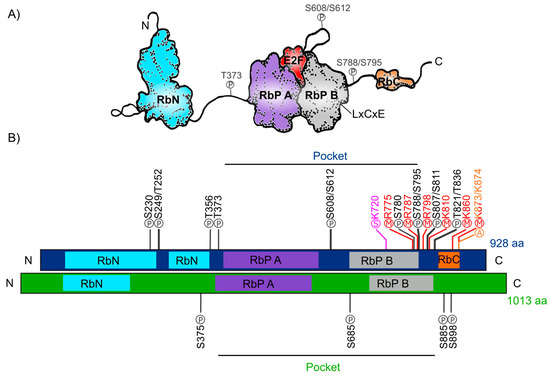
Figure 1.
Retinoblastoma protein structure. (A) Representation of the human Rb protein structure with the domains RbN (blue), Pocket (RbP), with the RbP A (purple) and B (grey) subdomains interacting with an E2F TF (red), the RbC domain (orange) and the inter-domains (black lines) are also shown. The “P” inside a circle represents three examples of phosphorylation sites that change the structure of the protein. The position of the LxCxE cleft that allows Retinoblastoma protein (pRb) to interact with different proteins is also shown. (B) Comparison of the domains of human Rb protein (blue foreground) and Arabidopsis RBR protein (green foreground) and their reported post-translational modifications. Phosphorylation sites (P) are shown in black, methylation sites (M) in red, acetylation sites (A) in orange, and sumoylation sites (S) in pink.
The post-translational modifications can assist or avoid occurrence of other modifications that, together, can modulate pRb protein interactions and function and create a diversity of biological activities, making this protein a key node in several regulatory networks. In total, human pRb has 14 phosphorylation sites [37,40], two acetylation sites [43,48], six methylation sites [49,50,51], and it can also be modified through ubiquitination or sumoylation [52,53,54] (Figure 1B). Studies on the structure of pRb have revealed that phosphorylation changes pRb structure and promotes new interactions with other proteins. For example, phosphorylation of residues S608/S612, localized between subdomains A and B of the Pocket domain, induces a new conformation of the pocket domain and its interdomain, that partially prevents E2F binding to pRb. Phosphorylation of residue T373 promotes the binding of the RbN domain to the Pocket domain. The result is a globular structure of pRb that can no longer interact with E2F. In addition, phosphorylation of the S788/S795 and T821/T826 residues, affects the interactions of the RbC domain with the E2F/DP complex [45,46,47,55,56]. Furthermore, acetylation and methylation sites are close to the carboxyl-terminal of the protein. Introduction of these post-translational modifications depend upon different signals such as pRb localization, DNA damage, and cell differentiation [48,51,57].
The RB ortholog in plants was identified approximately a decade after the animal gene was discovered: RB1 gene was first described in humans between 1986 and 1989 [17,18,58] and the first plant orthologous RB1 cDNA (RBR), was first identified and cloned from maize in 1994 (ZmRBR) [59,60,61], then in tobacco (NtRBR) [62] and then in Arabidopsis (AtRBR) [63]. Afterwards, there have been numerous reports characterizing RBR orthologs in different plant species. Intriguingly, monocotyledons seem to have various RBR paralogs while dicotyledons have only one [64]. As a dicot plant, Arabidopsis carries a single copy of the RBR gene displaying ≈35% sequence similarity within the pocket domain with respect to the human pRb. Interestingly, and even though the function of the N-terminal domain has not yet been characterized, this region is highly conserved between pRb from mammals and the RBR from Zea mays and Arabidopsis [65,66,67], suggesting that this region could also be involved in a conserved function in plants and animals. The Arabidopsis RBR (AtRBR) protein contains 1013 amino acids with the same modular structure of human pRb with putative phosphorylation residues. The role of four of them, located in the protein’s inter-domains, have been experimentally tested (Figure 1B) [68,69,70]. Interestingly, we observe that the S685 phosphorylation site in AtRBR is conserved in the same interdomain, between RbP A and RbP B subdomains, found in pRb (S608/S612), suggesting a conserved function in mediating the interaction with E2F TFs in animals and plants (Figure 1B). Additionally, human antibodies for human phospho-pRb protein in S807/811 can bind to RBR of Medicago sativa and Arabidopsis [71,72]. Even though the specific regulatory functions of these sites are still unknown, it has been shown in plants that cyclin-dependent kinases also phosphorylate RBR to regulate cell cycle through its inactivation and release of E2F, similar to what it has been described in humans [73,74].
Like in humans, in plants there are viral proteins (e.g., RepA from geminivirus) that also have the ability to interact with the pocket domain of RBR [61,75,76]. This suggests that the protein–protein interaction between pRb/RBR with specific viral proteins is a viral mechanism that controls cell cycle progression both in plants and animals. Such viral–eukaryotic cell interaction could have been established before plant and animal divergence, or it has evolved independently.
pRb is also found in unicellular organisms such as the algae Chlamydomonas reinhardtii, the choanoflagellate Monosiga brevicollis, and the amoeba Dictyostelium discoideum, suggesting that it was already present in the common eukaryotic ancestor before the separation of animal and plant lineages, before they diverged. In addition, at least one copy of RB has been identified in species from each of the eukaryotic supergroups [68,77].
3. Cell Cycle Control through Retinoblastoma
The cell cycle is a well-studied process essential for the growth and reproduction of all eukaryotic organisms. It assures the faithful duplication of the genetic material and its distribution between two daughter cells [78,79]. The cell cycle has two major phases: Interphase and Mitosis phase. Additionally, in the interphase other three stages can be distinguished: G1, S, and G2. Many of the components that regulate these phases and the transition between them, are conserved among different organisms [80,81]. The availability of growth factors, nutrients, and intrinsic developmental signals determine whether a cell remains in a quiescent state, when the cell does not divide (G0), or transits from phase G1 to S, during which the genetic material is duplicated to later divide. In humans, this transition is under the control of the pRb/E2F pathway, that regulates the transcription of genes encoding proteins involved in DNA synthesis [22].
The G1/S transition is one of the main regulation checkpoints of the cell cycle, being the “commitment point”, also known as the “restriction point” in animals, the one that determines the cell’s commitment to engage proliferation in a way independent from environmental signals [82,83]. Therefore, at this point the cell integrates environmental and intrinsic signals to prepare its nucleus to start cell division. A deregulation of this transition in humans can lead to the generation of tumors and cancer [22,84,85]. pRb hypophosphorylated acts as a negative regulator of cell cycle progression through its interaction with the E2F proteins. The heterodimer keeps the chromatin in a closed conformation in the regulatory regions of E2F-regulated genes [22,86]. The E2F family includes the transcription factors E2F1, E2F2, and E2F3a, which typically promote transcription and E2F3b, E2F4-E2F8 that are associated with transcriptional repression. However, it has been reported that, depending on the developmental stage, some E2Fs, like E2F1-4, can function both as activators or as repressors of transcription [87,88,89]. E2F1-6 members can heterodimerize with the Dimerization Partner (DP) proteins, although this interaction is not always required for transcriptional activation [90]. E2F7 and E2F8 are independent of DP and these TFs are also different to other E2Fs because they possess two DNA-binding domains [87,91,92]. The hypophosphorylated pRb form binds and inhibits activating E2F1-3, whereas E2F4 and E2F5 bind to pRBL1 (p107) and pRBL2 (p130) at the promoters of target genes, to repress transcription [22,93]. In addition, E2F1-3 carry a nuclear localization signal, whereas E2F4 and E2F5 lack it, and apparently rely on pRBL1 and pRBL2 for their nuclear translocation. On the other hand, E2F6-8 lacks the sequences required to bind with pRbs [94,95,96]. At the same time, the expression of different E2Fs is itself subjected to spatiotemporal regulation during cell cycle progression and different stages of development [97]. Moreover, when the pRb/E2F interaction is disrupted by loss or reduction of pRb, a high rate of cell proliferation is observed, and this generally triggers cancer [98].
pRb phosphorylation by cyclin/CDKs (cyclin-dependent kinases) complexes changes the pRb protein structure and its interactions with other proteins, inducing the release of E2F. For instance, this occurs in human cells when cyclin type D or E (CYCD/E) associate with cyclin kinases 4 or 2 (CDK4/2), respectively (Figure 2A) [22,99,100]. When pRb phosphorylation is altered, for example by overexpression of CYCDs, or by a miss regulation of CDKs, or by a disruption of the LxCxE-binding function the cell cycle is altered [101,102,103].
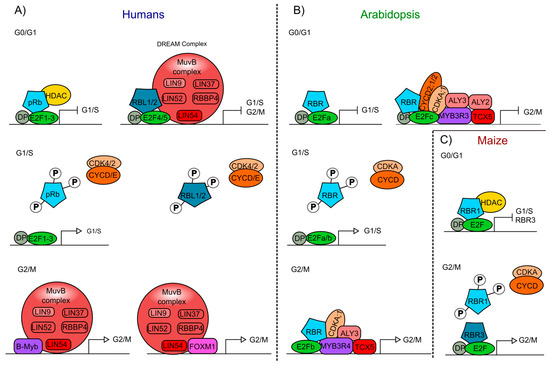
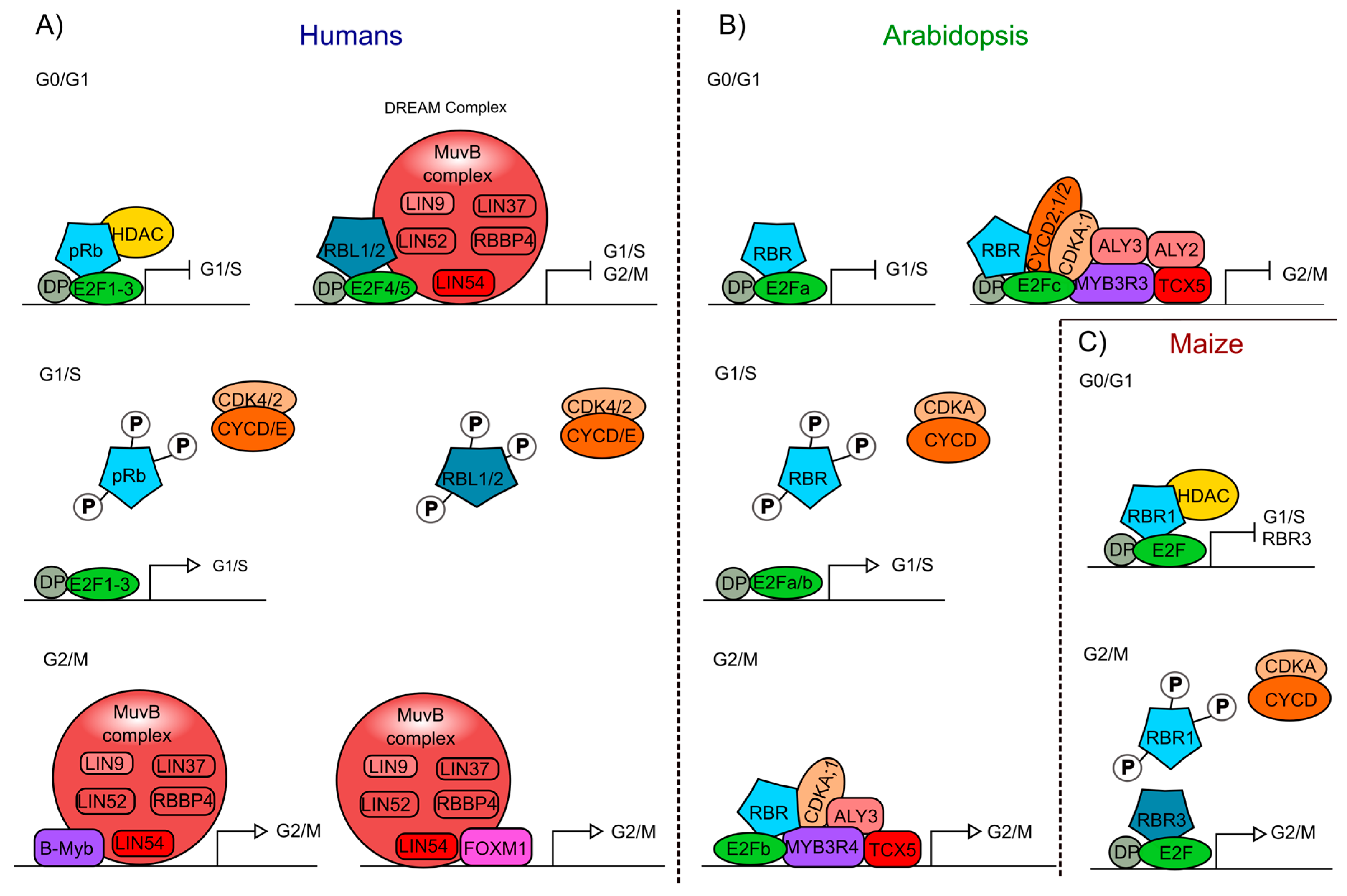
Figure 2.
Interaction of the Retinoblastoma protein involved in cell cycle regulation of humans and plants. (A) pRb- and pRBL1/2-containing protein complexes from humans, formed at different stages of the cell cycle (G0/G1; G1/S; G2/M). (B) Arabidopsis protein complexes formed at different stages of the cell cycle (G0/G1; G1/S; G2/M), including AtRBR as a component. (C) Maize protein complexes formed at different stages of the cell cycle (G0/G1; G2/M), involving the ZmRBR proteins (RBR1 and RBR3) as components. Similar components in humans, Arabidopsis, and maize are displayed using the same colors: Rb proteins (blue), E2F transcription factors (TFs) (green), DP (grey), cyclins (CYC), and cyclin-dependent kinases (CDK) (orange), Muv complex proteins (red and pink), Myb TFs (purple), FOXM1 TF (pink).
In humans, pRBL1 and pRBL2 participate in the repression of genes when cells are in the G0 quiescent state, through a complex called DREAM (DP-Rb-E2F-MuvB), that coordinates the repression of genes during quiescence and also the periodic gene expression during the G1/S and G2/M transitions [104,105]. DREAM is a multimeric protein complex that in humans is composed of DP (DP1-DP3), pRBL1 or pRBL2, E2F proteins (E2F4 and E2F5), and the subcomplex MuvB (Multi vulval class B). The MuvB subcomplex acts as a repressor when it is part of the DREAM complex, and is composed of LIN (LIN9, LIN37, LIN52, LIN54) and RBBP4 proteins. When pRb is phosphorylated, the DREAM complex is disassembled and the MuvB subcomplex can associate with TFs such as B-Myb (Myb type) and Fox-M1 (Forkhead box protein M 1), to promote the regulation of gene expression and the transition from quiescence to proliferation (Figure 2A) [104,106,107]. This complex is also conserved in phylogenetically distant organisms, but some of its components can vary among species, as in Arabidopsis compared to mammals (see below).
At about the same time that the pRb ortholog in plants was discovered, homologs of other components of the animal’s cell cycle regulatory machinery were identified and characterized in corn, as well as in Arabidopsis and Medicago sativa (alfalfa) [61,108,109,110,111]. In these plants, it was shown that the phosphorylation of RBR by the CDKA/CYCD protein complex regulates cell cycle progression, as it occurs in humans (Figure 2B) [73,74].
In Arabidopsis, the null mutant plants of AtRBR are gametophytic lethal because of supernumerary nuclei alterations in the divisions at late stages of female gametogenesis; whereas the male gametophyte (pollen) contains multiple sperm cells [112,113]. Therefore, in order to study the function of RBR function of Arabidopsis and maize, either its transcript accumulation has been reduced by RNAi or conditional repression has been employed. In addition, protein competence for RBR binding, has also been used to address the role of RBR in cell proliferation [114,115,116,117].
In monocots, like rice, wheat, barley, and sugarcane, at least two different RBR types are present, RBR1 and RBR3 [64], although maize carries four ZmRBR genes: ZmRBR1, ZmRBR3, and two paralogs, ZmRBR2 and ZmRBR4 [118,119]. ZmRBR1 is constitutively expressed, and its protein interacts with E2F and with a HDAC (ZmRpd3I) that lacks the characteristic LxCxE motif. Besides, the two paralogs, ZmRBR1/2, negatively regulate cell cycle as it occurs in humans and Arabidopsis (Figure 2C) [119,120,121,122]. In addition, ZmRBR3/4 transcripts accumulate in mitotic tissues from the endosperm. Recently, high levels of ZmRBR3/4 were found in tumor-like formations (induced by the fungus Ustilago maydis) from maize leaves [118,123]. Interestingly, the protein complex ZmRBR3/4/E2F has a unique role not observed in other plants or animals: high levels of ZmRBR3/4 in complex with E2F promotes the expression of genes involved in DNA replication and cell cycle progression; contrary to what has been reported for overexpression of pRb and AtRBR (Figure 2C) [118,119,123,124]. Besides, ZmRBR3/4 is negatively regulated by the ZmRBR1-E2F complex; as in lines expressing RepA, which inhibits ZmRBR1, ZmRBR3 is upregulated, suggesting a compensatory mechanism to ensure cell cycle progression [118,125]. This mechanism has also been observed in undifferentiated germline cells in which the absence of pRb is compensated by pRBL1 (p107) to maintain the cell’s quiescent state [126].
The conservation in plants and animals of the “restrictions point” and of key cell cycle regulators like Cyclin-CDKs and RB/E2F, suggests that the common ancestor of these two groups of organisms already had these components. Interestingly, there is a different number of genes encoding for each cell cycle protein suggesting the emergence of different and novel lineage-dependent proteins between plants and animals [78]. For example, it is known that Arabidopsis has 10 genes that code for cyclin-D (CYCD) classified into seven subtypes [127,128], while in humans there are only three [129]. In contrast, nine E2F are found in humans (three activators and six repressors), and only six in Arabidopsis. Of these, AtE2Fa functions as activator, AtE2Fb functions either as an activator or repressor, depending on the plant developmental stages; E2Fc is a repressor; and the other three members of this family (E2Fd/e/f) are atypical since they have a duplicated DNA-binding domain, do not heterodimerize with DP and lack the trans-activating and Rb pocket-binding domains, and thus resemble E2F7/8 in animals [130,131,132,133]. Finally, some components of the cell cycle regulatory machinery are plant-specific such as type B cyclin dependent kinases (CDKB1/2) [134,135], while others are animal-specific, such as cyclin E in humans [136,137].
The DREAM complex also appears to be conserved in plants [138,139]. In addition to the presence of AtRBR, E2Fs/DPs, a MuvB-like complex has also been found that contains ALY2/3 (orthologs of LIN9) and TCX5 that is part of the TSO1-like family members (orthologs of LIN54) [140,141]. Furthermore, MYB3R, a transcription factor of the Myb family, has a protein structure resembling the DNA binding domain of B-Myb, which in humans forms part of the MuvB complex. These plant’s MYB3Rs also participate in the regulation of the G2/M transition as follows: MYB3R3 associates with E2Fc and AtRBR to repress G2/M genes, while MYB3R4 associates with E2Fb and AtRBR to activate G2/M genes (Figure 2B) [142,143,144]. CDKA;1 and cyclins could as well be involved in the plant DREAM complex since MYB3R3 and MYB3R4 interact with CDKA;1 and, in tobacco, CDKA;1 regulates MYB3R phosphorylation [139,144,145]. Moreover, E2Fc can physically interact with CDKA;1, CYCD2;1, and CYCD2;2 in vitro (Figure 2B) [146]. Interestingly, the function of the activation complex MYB3R4/AtRBR1/E2Fb is similar to the function of ZmRBR3 in maize that positively regulates transcript accumulation of genes involved in DNA replication and cell cycle progression in the transition G2/M (Figure 2B,C) [124]. However, in contrast to what has been reported in animals, RBR presence in the DREAM complex is able to control cell cycle in different stages of cell cycle in plants (Figure 2B,C).
4. The Roles of Retinoblastoma in Cell Differentiation
The formation of any organ relies on two different but interlinked cellular processes: cell proliferation and cell differentiation. Proliferation produces all the cells that later will acquire fates, specialized functions, and morphologies through differential gene expression during cell differentiation [147,148]. pRb has been widely studied in proliferation and, recently, its participation in many different animal differentiation processes in the eye, brain, peripheral nervous system, muscle, liver, placenta, lung, cerebellum, pituitary gland, and heart has been elucidated (Figure 3A) [149,150,151,152,153,154].
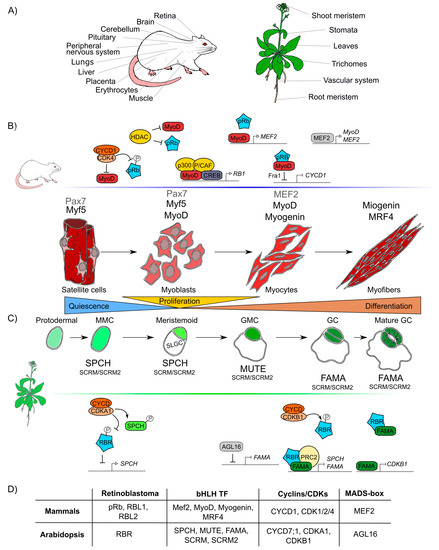
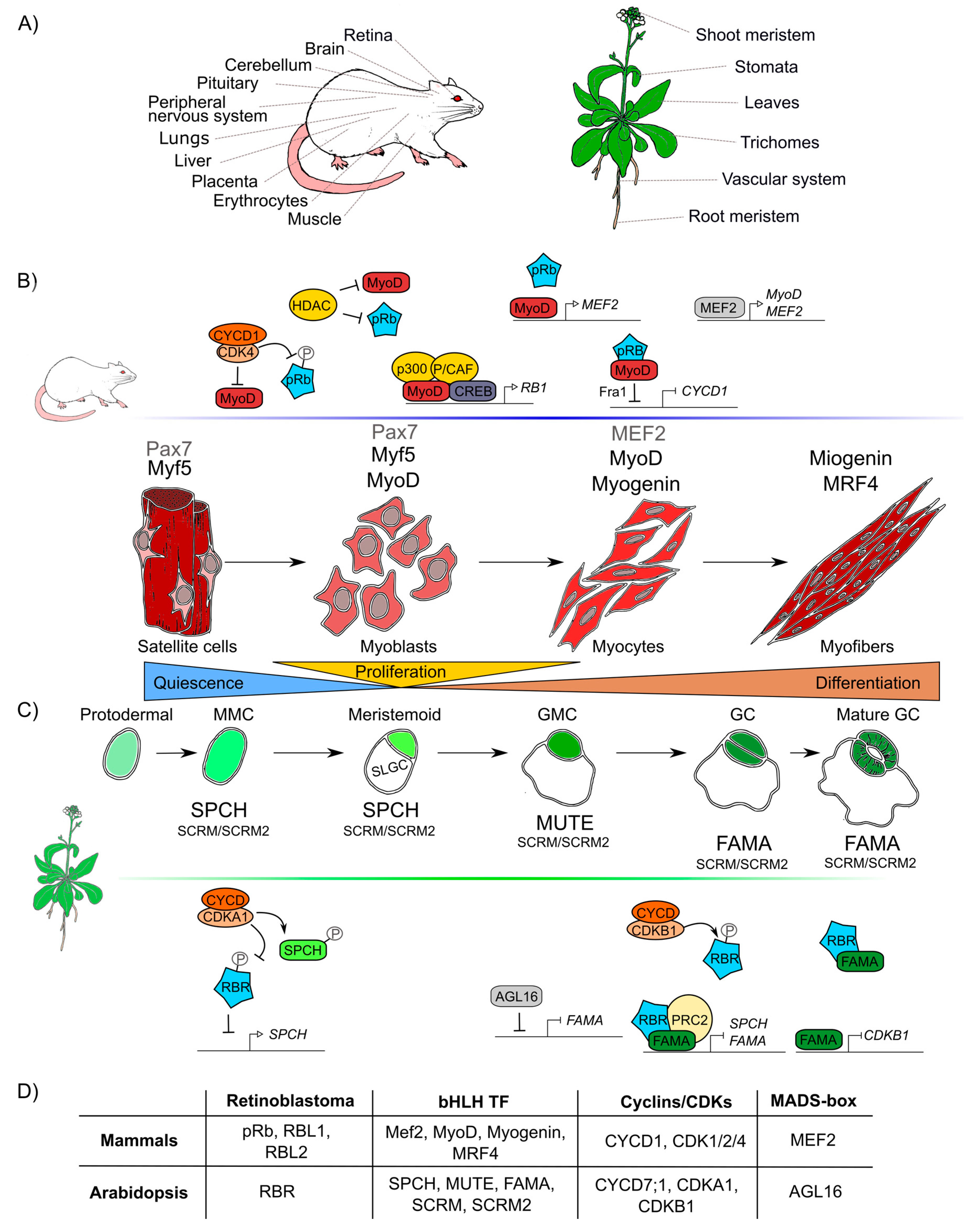
Figure 3.
Retinoblastoma proteins are involved in cell differentiation in mammals and plants. (A) Mammals’ and plants’ organs whose differentiation depends on pRb/RBR. (B) Sequential steps of skeletal muscle differentiation in mammals. Shown are pRb interactions and bHLHs-family proteins (Mef2, MyoD, Myogenin, MRF4), involved in muscle quiescence maintenance, proliferation, and differentiation. (C) Sequential steps of guard cells differentiation in Arabidopsis. Shown are RBR interactions and bHLHs-family proteins (SPCH, MUTE, FAMA, SCRM, SCRM2) involved in quiescence maintenance, proliferation, and differentiation of guard cells. (D) Correlations between components involved in muscle and guard cell development in mammals and Arabidopsis, respectively. Differentiation in both lineages involves proteins of the pRb, bHLH TF, cyclins and CDKs, MADS-box TF families.
The participation of pRb in these processes has been studied in vivo in mutant mice and/or cell cultures derived from cancer cells, characterized by alterations in their RB1 expression levels [155,156,157]. Mice with RB1 ablation are embryonic lethal, and those with low levels of the three pRb (RB1, RBl1, and RBl2) not only die in utero but also present defects in erythroid, neuronal, and muscular differentiation [158,159,160]. Additionally, in mice with conditionally-regulated levels of the three pRb, cells overproliferated inducing retinal cancer (Retinoblastoma) and also display defects in laminar organization of the retina and the crystalline [161,162,163]. E2Fs target genes are also important for differentiation of the adipose tissue, bone, nervous system, and muscles [164,165,166,167]. Interestingly, expression of pRb in mutants with altered E2F function has shown that pRb can also independently regulate tissue-specific genes in mammals, pointing to its broad roles in development [168,169].
Similar to pRb in animals, RBR participates in differentiation processes in the root and shoot meristems, the vascular system, leaves, stomas, and trichomes tissues in plants (Figure 3A) [116,117,170,171]. In Arabidopsis, AtRBR functions as a negative regulator of primary root development; its downregulation leads to longer roots with larger meristems whereas its overexpression results in shorter roots with smaller meristems [172]. In this organ, AtRBR forms a protein complex with the TF ARABIDOPSIS RESPONSE REGULATOR12 (ARR12) that activates the transcription of the AUXIN RESPONSE FACTOR19 (ARF19) TF [172]. These TFs participate in two different hormone signaling pathways: ARR12 is part of the cytokinin signaling pathway involved in root differentiation, while ARF19 is part of the auxin signaling pathway which is important for root cell proliferation [172,173]. ARF19 is activated by RBR mostly in between the meristematic and the elongation zones in roots and it is suggested to promote differentiation [172].
The role of RBR in differentiation at the shoot apical meristem (SAM) has also been analyzed. In this case, overexpression of AtRBR accelerates differentiation, increasing the expression of genes involved in metabolic pathways that are not present in the SAM and decreasing the expression of genes that are only expressed in the SAM [114,170]. AtRBR RNA interference (RNAi) in leaf primordia delays differentiation and, consequently, increments two to four-fold the cell number in both the adaxial and abaxial sides of the leaves [114,170]. In addition, also in leaves, AtRBR participates in the endoreplication and differentiation of trichomes, in which the AtRBR transcription is regulated by the TFs GLABRA1 (GL1) and GLABRA3 (GL3) [174,175]. In the vascular system, AtRBR associates with XYLEM NAC DOMAIN1 (XND1), a negative regulator of differentiation, thus controlling processes related to the differentiation of tracheary elements [176,177].
It is difficult to compare the roles of pRb and RBR during differentiation due to lack of information and because the components involved in cell differentiation are more species-specific and less conserved than those involved in cell cycle regulation. However, taking as examples skeletal muscle differentiation in humans and stomatal guard cells differentiation in Arabidopsis, conserved factors that coordinate cell differentiation can be identified, namely Rb, MADS-box TFs, and TFs of the bHLH family, as well as the cyclins/CDKs protein complexes (Figure 3D) [178,179].
At post-embryonic stages, myocyte differentiation in mammals can be triggered in response to muscle damage or a specific growth-inducing stimulus. This last provokes massive proliferation of myoblasts through the hierarchical activation of several MRF (Myogenic Regulatory Factors): Myogenic Factor 5 (Myf5), myoblast determination protein 1 (MyoD), Myogenin (MyoG), and Myogenic Regulatory Factor 4B (MRF4), which are TFs that belong to the bHLH family. These MRF are required together with pRb for the muscle regeneration process [180,181,182]. In resting state conditions, muscle stem cells or satellite cells express only the box protein Pax7 [183]. When the muscle is injured, satellite cells get activated and become myoblasts that massively proliferate to generate the myogenic progenitors, which express Myf5 and/or MyoD TFs [184]. Later, when myoblasts start differentiation into myocytes, Myf5 and Pax7 are repressed and MRF4 and myogenin are expressed promoting cell cycle exit. Finally, when the cells fuse to become myofibrils, myogenin and MRF4 are expressed as part of the final steps of differentiation (Figure 3B) [180,182]. In this process, the protein complex of MyoD with pRb, is thought to initiate differentiation, as it promotes cell cycle arrest [185,186,187,188]. Despite immunoprecipitation experiments that proved that pRb and MyoD can interact [189], later experiments using nuclear magnetic resonance allowed to determine that there is no direct protein–protein interaction between MyoD and pRb [190]. Still, MyoD does activate RB1 expression through its association with the cAMP response element-binding (CREB) TF and the coactivators p300 and P/CAF (Figure 3) [191,192]. Myocyte Enhancer Factor 2 (MEF2), that belongs to the MADS-box family of TFs, is also a component necessary to carry out the final stages of muscle differentiation [149,193,194].
Induction of muscle biogenesis also requires the regulation by cyclins-CDKs in association with pRb [195]. The stable repression of cyclin D1, required for cell cycle arrest during differentiation, is regulated by the joint action of MyoD and pRb through the regulation of the upstream intermediary gene Fra-1 (antigen related to FOS 1) (Figure 3B) [196,197]. Antagonistically, cyclin D1 inhibits the activity of MyoD: overexpression of cyclin D1 promotes nuclear accumulation of CDK4, that binds MyoD, preventing its interaction with DNA, and inhibiting the CDK4-dependent phosphorylation of pRb (Figure 3B) [198,199,200]. Additionally, when HDAC1 interacts with pRb, the MyoD protein can bind its target DNA regulatory sites [201,202]. In summary, there are two different protein complexes formed during different stages of muscle development: HDAC1/MyoD during proliferation and HDAC1/hypophosphorylated pRb during differentiation (Figure 3B) [201,202].
In plants, some epidermal cells undergo differentiation producing the two mature guard cells that form the stomata pores, structures that are conserved among land plants and allow them to regulate gas exchange and water loss [203,204]. Stomatal development is hierarchically regulated by the sequential activation of several TFs of the bHLH family: SPCH (SPEECHLESS), MUTE, and FAMA. These three bHLHs form heterodimers with either the bHLHs SCREAM (SCRM, also called ICE1) or SCRM2 [205,206,207], that belong to the same family of TFs that participate in muscle development in mammals (MRFs) (Figure 3D). These plant TFs orchestrate cell division events of protodermal cells, which give origin to guard cells (stomatogenesis). SPCH triggers the maturation of a protodermal cell into a meristemoid mother cell (MMC) and is also involved in the asymmetric cell division of the MMC, that results in one meristemoid cell and one larger sister cell (SLGC). The meristemoid cell exits stemness and engages in differentiation to become a guard meristemoid cell (GMC). MUTE must be expressed at this stage, to direct further differentiation of a GMC, and to ensure that this cell undergoes a single symmetric division. In addition MUTE regulates the expression of FAMA, which controls the final stages of differentiation, promoting guard cell (GC) identity acquisition and the irreversible termination of the meristematic activity of the cells (Figure 3C) [208,209,210]. AtRBR plays important roles in the regulatory network of stomata development, and its downregulation at the GMC or GC stages, induces extra divisions in differentiated GCs and the formation of aberrant stomata-in-stomatal nested structures [114,211]. In fact, AtRBR hyperphosphorylation inhibits stomatal initiation affecting the asymmetric division of protodermal cells that produces MMCs, this seems to be controlled by CDKA;1, that negatively regulates AtRBR, and regulates positively SPCH TF through phosphorylation (Figure 3C) [212,213,214]. It has also been hypothesized that AtRBR hyperphosphorylation by CDKB1;1-CYCD7;1 inhibits the AtRBR/FAMA repression complex leading to the induction of cell-cycle regulators of the GMC symmetric division event [215,216,217]. At the same time, MUTE directly upregulates FAMA and FLP; and FAMA represses cell-cycle control genes such as CDKB1;1, ensuring a single symmetric division to form GCs (Figure 3C) [208,218]. Mutation in the FAMA LxCxE sequence prevents the formation of the AtRBR/FAMA complex, making cells unable to maintain the long-term commitment to differentiate into GC, and arresting this process at the GMC stage [211,215]. A similar mechanism is present in mammals, in which downregulation of MyoD allows cells to dedifferentiate; an ability that determines the muscular capacity to regenerate [219]. Finally, it is also possible that FAMA functions at early steps of guard cell differentiation since the AtRBR/FAMA heterodimer binds to SPCH and FAMA promoters, and this complex negatively regulates the accumulation of the SPCH transcript, which is normally expressed at early stages of guard cells development (Figure 3C) [211,220].
As it can be appreciated, the differentiation processes of skeletal muscle in mammals and guard cells in Arabidopsis are both regulated by a similar set of conserved elements: pRb/RBR, bHLHs, cyclins and CDKs (Figure 3D). Moreover, in both organisms, members of the MADS-box family participate in these processes: MEF2 is involved in early stages of muscle differentiation and in cell proliferation of other tissues (Figure 3B) [221,222,223]. Similarly, a plant MADS-box gene, AGAMOUS-LIKE16 (AGL16), is expressed and participates in GC development (Figure 3D) [224,225]. AGL16 mediates the stomata development process at the MMC cell lineage level and represses FAMA as well as other genes involved in the development and differentiation of guard cells (Figure 3C) [225,226].
5. Retinoblastoma Function in Stem Cells Homeostasis
Accumulated evidence indicates that pRb loss of function in mammals results in altered progenitor cells (or stem cells) quiescence. Quiescent cells are usually in the G0 phase of the cell cycle or in a prolonged G1 phase; and have a very low proliferative activity. Through asymmetric division stem cells can give origin to a new quiescent stem cell and a new daughter cell that can proliferate several times to eventually produce one or more differentiated cell types [227,228,229]. pRb participates in pluripotency maintenance, inhibition of differentiation, and in self-renewal of stem cells [230,231,232]. Mutants with low expression levels of Rb display an increased cell division of both embryonic and post-embryonic stem cells from retina, mesenchymal, and early osteoblasts progenitors, as well as of post-embryonic stem cells of the liver, muscle, and nervous system [233,234,235,236,237,238,239]. Interestingly, in these pRb mutants somatic cells acquire stem cells features, as it occurs in some human cancers [240,241]. In human pluripotent stem cells (hPSC), the pRb/E2F pathway enhances differentiation towards all germ layers in response to a DMSO stimulus [153]. pRb together with E2F1 can bind and suppress the transcript accumulation of pluripotency promoting factors, such as SEX DETERMINING REGION Y-BOX 2 (SOX2) and OCTAMER-BINDING TRANSCRIPTION FACTOR 4 (OCT4). In addition, pRb can alter the accumulation of transcripts by directing chromatin modifiers to promoters of specific TFs, as it happens in the case of KRUPPEL-LIKE FACTOR 4 (KLF4), the homeobox NANOG and TRANSCRIPTION FACTOR 3 (TCF3), that are part of the induced stem cell pluripotency regulatory network; and also in the case of ENHANCER OF ZESTE HOMOLOG 2 (EZH2), which is a methyltransferase that participates in establishing stem cells establishment [242,243].
The plant ortholog, RBR, is also implicated in pluripotency maintenance. Lowering transcript levels of AtRBR induces disorganization of the root stem cell niche (SCN) in Arabidopsis, as well as in the shoot meristem that harbors flower and leaf stem cells [114,115,116]. The root SCN consists of a central organizer, the quiescent center (QC), which is surrounded by four type of stem cells called initial cells, that in Arabidopsis are columella initials (CI), cortex/endodermis initials (CEI), epidermis/lateral root cap initials (ELRCI), and stele initials (SI) [244]. Under normal growth conditions, proliferation rate at the QC is very low compared to adjacent zones, although the division rate increases at the QC in older seedlings [245,246]. AtRBR silencing increases the proliferation of both QC and CI cells, resulting in an overgrowth of undifferentiated cell layers. Conversely, overexpression of AtRBR causes premature differentiation of CIs [115,116,247]. Interestingly, absence of AtRBR favors the duplication of differentiated columella cells that normally do not duplicate in wild-type plants [247].
Within CEI cells and their progeny, the TF SCARECROW (SCR), a member of the GRAS TFs family, interacts, through its LxCxE motif, with AtRBR forming a ternary complex with SHORTROOT (SHR), which is another member of the GRAS family. This AtRBR/SCR/SHR complex inhibits the transcription of target genes of the SHR/SCR heterodimer. One of these target genes is CYCLIN D6;1 (CYCD6;1), which controls the cell cycle progression of the CEI cells progeny. Moreover, CYCD6;1 together with the kinase CDKB1;1, in turn, regulates the phosphorylation of AtRBR, liberating the SCR/SHR complex, favoring in this way CEI cells’ asymmetric division [115,248,249]. AtRBR, SCR, and CYCD6;1 are degraded by the proteasome before mitosis, which is consistent with a model where the degradation of these proteins allows CEI cells to restart the asymmetric divisions [248]. Moreover, the same AtRBR/SCR interaction is necessary to establish the QC cells and is regulated by the interaction of AtRBR with the ETHYLENE RESPONSE FACTOR115 (ERF115) TF, that belongs to the AP2/ERF family. This interaction also occurs through the LxCxE domain of ERF115, that competes with SCR for AtRBR, reducing in this way the levels of the AtRBR-SCR heterodimer [250]. Importantly, most of the AtRBR functions related to differentiation and cell cycle arrest in the SCN are cell-autonomous, highlighting the crucial role of AtRBR activity level in the QC, in the columella stem cells, and in their immediate progeny in the acquisition of niche-specific features [247].
Interestingly, it has also been observed that the TOPOISOMERASE 1α (TOP1α) together with AtRBR, is also involved in the control of stem cell maintenance during root development. TOP1α is an enzyme present in plants and animals that creates breaks in double DNA strands to relax supercoiled structures. In humans, it has been used as a target to stop the proliferation of breast cancer stem cells in cell cultures [251,252]. In Arabidopsis roots it has been shown that TOP1α is downregulated by AtRBR to maintain the undifferentiated state of cells and the number of CI cells in the SCN. In addition, TOP1α is epistatic over AtRBR and its overexpression results in an increased number of CI cells, as it happens in AtRBR loss of function mutants [253]. Besides, mutations that disrupt the activity of TOP1α, induce cell death in the initial cells of the stele (SI) which can be partially reversed by the activation of ERF115 expression, since TOP1α negatively regulates the expression of ERF115 [253], and as it was mentioned above, ERF115 also interacts with AtRBR to establish the QC [250]. Although it is not yet known whether TOP1α participates with AtRBR in the shoot meristem or not, TOP1α also regulates the establishment of stem cells through indirect transcriptional repression of WUS (WUSCHEL) [254].
In the shoot meristem, silencing of AtRBR also produces disordering of the stem cell divisions within the SAM, resulting in a significant reduction in the typical height-to-width ratio of the SAM; and also in alterations in stem cell maintenance and differentiation [114]. Overexpression of AtRBR triggers cells toward a more differentiated state; but the molecular mechanism has not yet been described to explain this phenotype [170]. In addition, AtRBR regulates proliferation and differentiation of MMC of guard cells in leaves as it has been described above.
It can be noted from the examples presented, that pRb/RBR of animals and plants, respectively, participate in the maintenance of stem cells by controlling the cell cycle and cell differentiation, as well as regulating specific genes that give identity to these cells.
6. Function of Retinoblastoma in Epigenetic Modifications, Chromatin Regulation, and DNA Damage
Epigenetic modifier proteins participate in the regulation of gene expression throughout development. This allows cells with the same genetic background to exhibit different phenotypes [255,256]. Epigenetic modifications alter DNA accessibility and chromatin structure by mechanisms such as DNA methylation and histones modifications by acetylation, methylation, ubiquitination, phosphorylation, sumoylation, etc. [257,258]. In mammals and plants, many epigenetic modifier proteins interact with protein complexes that include pRb/RBR, allowing them to regulate different developmental processes.
Human pRb has been reported to interact with over 300 proteins and many such protein interactions are epigenetic modifier proteins or interact with the latter [40,256]. pRb levels decrease leads to an incomplete chromosome condensation and segregation during mitosis, as it has been observed in cancer cells; some alterations of the chromatin structure are also induced by changes in histone methylation and acetylation [259,260,261]. pRb can interact with chromatin remodeling factors, such as histone deacetylases, DNA methyltransferases, histone methyltransferases, and with complexes like SWI/SNF; and like Polycomb group (PcG), the latter is a chromatin-modifying complex that maintains repressed gene expression states and is subdivided into two main complexes: Polycomb repressive complex 1 (PRC1) and PRC2 [259,262,263,264,265].
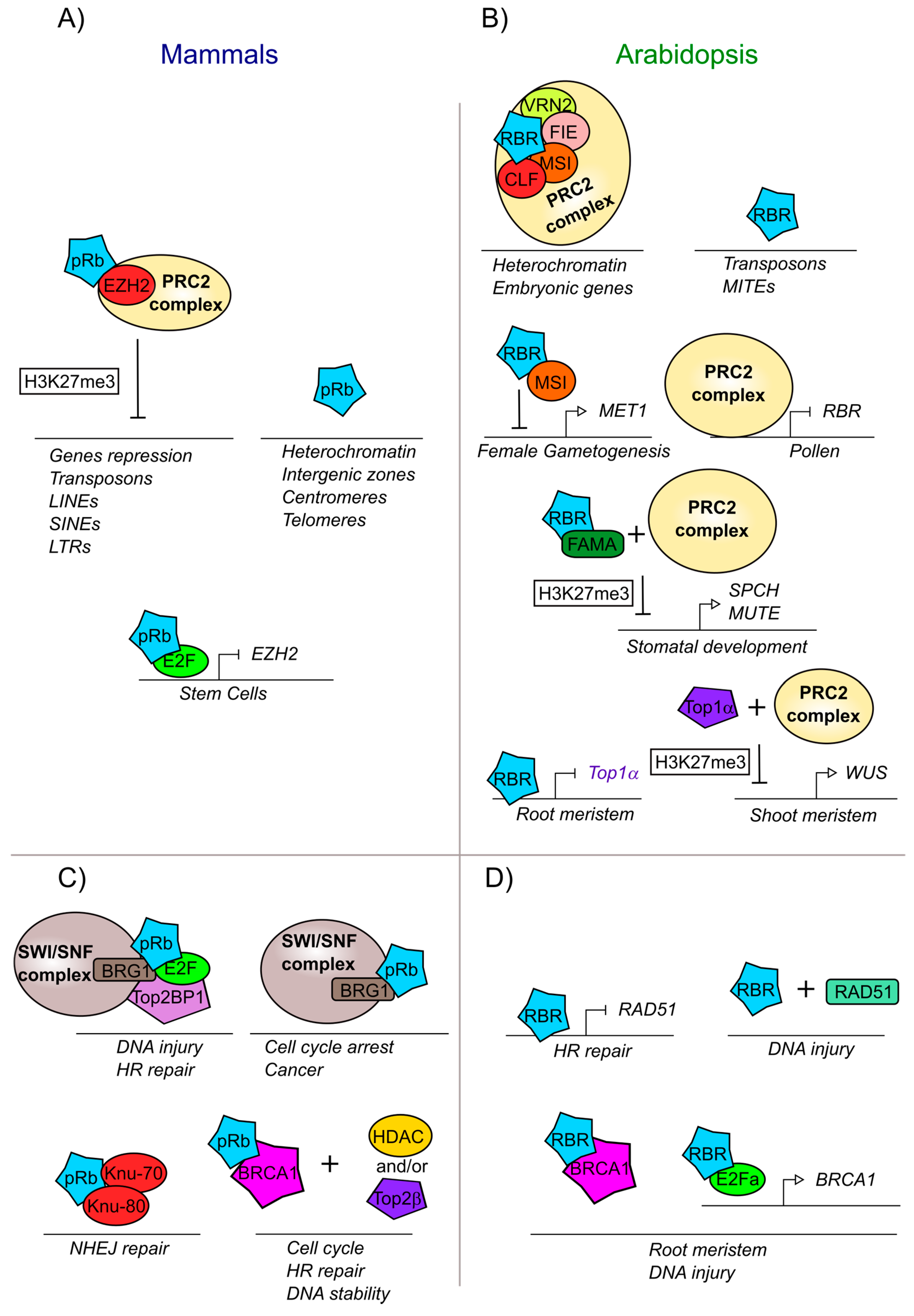
The interaction of pRb-epigenetic with modifiers complexes are also important to maintain heterochromatin in intergenic zones as well as in centromeres and telomeres (Figure 4A) [260,261]. The interaction of pRb with ENHANCER OF ZESTE HOMOLOG 2 (EZH2), a histone methyltransferase of the PRC2 complex, allows the deposition of the trimethylation of lysine 27 of histone H3 (H3K27me3), a repressive mark, on pRb target genes (Figure 4A). In turn, pRb-E2F negatively regulates EZH2 transcript accumulation and proliferation; conversely high expression of EZH2 is observed in cancer stem cells as has a critical function in regulating stem cell expansion and maintenance (Figure 4A) [242,266]. Interestingly, it has been observed that pRb can also recruit EZH2 protein into sequences within introns and intergenic regions, specifically in repeated sequences, transposons, long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), and long terminal retroviruses (LTR). The loss of the pRb-EZH2 complex provokes loss of the H3K27me3 mark at these elements, leading to dispersion or loss of heterochromatin and probably disorganized proliferation as observed in cancer cells (Figure 4A) [267].
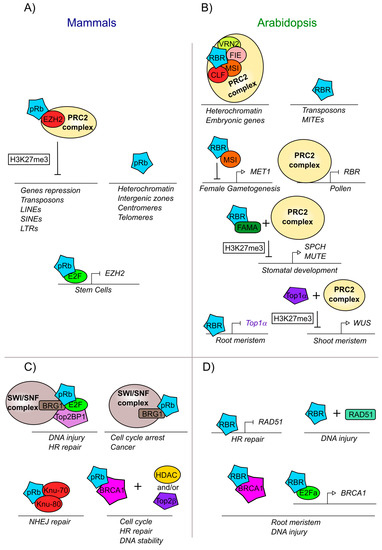
Figure 4.
pRb and RBR are involved in modifications and DNA repair mechanisms both in mammals and plants. (A) Mammalian pRb participates in developmental processes and chromatin localization together with EZH2, a component part of the PRC2 complex. (B) Arabidopsis RBR participates in development and chromatin localization together with the PRC2 complex. (C) Mammalian pRb is part of the machinery involved in DNA repair. (D) Arabidopsis RBR is part of the machinery involved in DNA repair. Proteins and complexes conserved between mammals and Arabidopsis are marked with the same colors.
DNA integrity in mammals is altered when pRb is absent and, in some cases, this can be provoked by the overexpression of E2F regulated genes that are able to introduce double-strand DNA breaks, or by stress conditions that generate aneuploidies [37]. In the case of a double strand break, pRb is necessary to form the complex of the heterodimer E2F1-pRb with TopBP1 (DNA topoisomerase 2-binding protein 1) [268]. TopBP1 is a protein that interacts with Topoisomerase 2β (Top2β) and with other proteins that participate in DNA replication and in the maintenance of the DNA integrity and genome stability [261,269]. In addition, the protein complex E2F1-pRb-TopBP1 interacts with BRG1 (also known as ATP-dependent chromatin remodeler SMARCA4) which is a member of the SWI/SNF complex that is necessary to reduce nucleosome density at injury sites, allowing DNA end resection and reparation by homologous recombination (HR) (Figure 4C) [268,270]. Another novel aspect of the pRb-BRG1 interaction is its influence in mediating cell cycle arrest, by the regulation of different genes also involved in human cancer cells (Figure 4C) [271,272,273]. pRb interacts with the tumor suppressor BRCA1 (Breast cancer 1), which is also involved in DNA repair via Homologous Recombination. The BRCA1-pRb complex interacts with histone deacetylases (HDAC1/2) and with topoisomerase 2β (Top2β) to regulate DNA stability (Figure 4C) [274,275]. Furthermore, the pRb-BRCA1 complex is involved not only in the response to DNA damage, but also in cell cycle control, as deletions in the BRCA1 binding domain with pRb, inhibits BRCA1-dependent cell cycle progression [262]. pRb also participates in another DNA repair pathway: the non-homologous end joining (NHEJ), the exact role of pRb in this mechanism is unknown but it has been reported that pRb interacts with two of the proteins that recognize the breakdown of the double chain: KU-70 and KU-80 (Figure 4C) [261,276].
In plants, there are also numerous reports of RBR interactions with epigenetic modifiers, which are important in the regulation of different developmental processes [277]. In Arabidopsis, like in animals, it has been shown that PRC2, a subcomplex of PcG, participates together with AtRBR in the establishment of the H3K27me3 mark during differentiation and development of the female and male gametophytes, in leaf development and during the establishment of stoma cell lineages. In these three processes, AtRBR associates with components of the PRC2 repressor complex such as MULTICOPYSUPPRESSOR OF IRA1 (MSI1), FERTILIZATION INDEPENDENT ENDOSPERM (FIE), VERNALIZATION 2 (VRN2), and CURLY LEAF (CLF), a gene orthologous to EZH2 from humans (Figure 4B) [66,278]. In addition, AtRBR together with MSI1 directly represses the expression of the DNA methylase METHYLTRANSFERASE 1 (MET1) (Figure 4B), that maintains DNA methylation during DNA replication and regulates gene imprinting. The repression of MET1 by this complex allows the transcriptional activation of FERTILIZATION INDEPENDENT SEED 2 (FIS2) and FLOWERING WAGENINGEN (FWA), that are important for female gametogenesis [66,113,279]. In turn, MET1 is positively expressed during male gametogenesis; and is important for maintaining the gene repression of FIS2 and FWA in the paternal allele, leading the monoparental expression of these genes during fertilization and endosperm development, [279,280]. Furthermore, AtRBR loss of function mutants present higher levels of SWINGER (SWN), MSI1, and FIE transcripts, which are components of the PRC2 complex. Interestingly, the AtRBR transcript in pollen is directly repressed by the PRC2 complex (Figure 4B) [113]. In plant embryos, the PRC2 complex with AtRBR directly binds and deposits the H2K27m3 mark on different embryonic genes, leading to their repression and subsequent seed germination (Figure 4B) [69,281]. Similarly, in stomatal development, AtRBR/FAMA heterodimer is required to recruit PRC2 to H3K27me3 deposition into SPCH and MUTE regulatory regions, and repress its transcript accumulation, necessary to control differentiation and stomatal development correctly (Figure 4B) [211,215,282].
AtRBR also appears to regulate DNA repair in several conditions. First, AtRBR binds and represses genes involved in homologous recombination such as RADIATION SENSITIVE 51 (RAD51) and helps to locate RAD51 to the right place at DNA lesions (Figure 4D) [283]. Additionally, TOP1α is critical to ensure genome integrity and survival of root stele stem cells, as the loss of function of TOP1α triggers DNA double-strand breaks and cell death in these cells; in the root, TOP1α is downregulated by AtRBR (Figure 4D) [253]. Although the participation of AtRBR and Top1α in the shoot meristem have not yet been studied, TOP1α participates with the PRC2 complex in the repression of the WUS locus (Figure 4D) [252,253,254].
AtRBR also is recruited to damaged DNA sites, along with E2Fa and AtBRCA1 and helps to maintain the integrity of the root meristem (Figure 4D). Furthermore, similar to what is observed for animals for BRCA1 and pRb (Figure 4C) [262,284], AtRBR and AtBRCA1 have been shown to physically interact when cells are damaged [285]. In addition, E2Fa is required for AtBRCA1 expression, when genotoxic stress is induced (Figure 4D) [285]. Thus, it would be interesting to analyze if the AtBRCA1-AtRBR complex participates in the regulation of the cell cycle, as it occurs in humans. Finally, analysis of chromosome sites to which AtRBR physically binds, show that this protein not only targets gene regulatory sequences, but also transposons, especially Miniature Inverted-repeat Transposable Elements (MITEs) (Figure 4B) [142].
7. Conclusions
Development is a process where proliferation and differentiation cellular rates must be finely regulated. As we can appreciate from the examples presented throughout the text, pRb/RBR are multifunctional and highly connected proteins that control cell fate determination and differentiation through interactions with different proteins. The pRb/RBR structures and diverse post-translational modifications allow the proteins to differentially interact with an exceptionally high number of proteins, making them a key node in several regulatory networks. Interestingly, many of the protein partners are conserved between animals and plants, and in both lineages are involved in equivalent cellular processes such as cell cycle regulation, stem cell homeostasis, and cell differentiation. In addition, interaction with epigenetic and DNA topology regulators suggests that the protein–protein networks that involve RETINOBLASTOMA are also similar in plants and animals. Thus, important aspects of the regulatory networks underlying cell proliferation and differentiation in which this protein is involved, seem to be shared by plants and animals, despite the fact that these two lineages have unique cellular and structural characteristics.
Author Contributions
E.Z.-M. and A.G.-A. conceived and wrote the review. V.P.-K., M.V.P.-C., S.M.-H., B.G.-P., M.d.l.P.S. and E.R.Á.-B. wrote the review. All authors have read and agreed to the published version of the manuscript.
Funding
A.G.-A., M.d.l.P.S., B.G.-P. and E.R.Á.-B. received funding from UNAM-DGAPA-PAPIIT (IN200920, IN203220, IN206220, IN208517). E.Z.-M. was financially supported by CONACYT through a Ph.D. scholarship.
Acknowledgments
We would like to thank Diana Belén Sánchez Rodríguez for logistical and technical support and to Crisanto Gutiérrez (Universidad Autónoma de Madrid, Spain) for comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest and the research was conducted in the absence of any commercial or financial relationships.
References
- Koonin, E.V. The Incredible Expanding Ancestor of Eukaryotes. Cell 2010, 140, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 2010, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.A.; Leger, M.M.; Wideman, J.G.; Ruiz-Trillo, I. Concepts of the last eukaryotic common ancestor. Nat. Ecol. Evol. 2019, 3, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Krinsky, B.H.; Long, M. New genes as drivers of phenotypic evolution. Nat. Rev. Genet. 2013, 14, 645–660. [Google Scholar] [CrossRef]
- Meyerowitz, E.M. Plants, animals and the logic of development. Trends Cell Biol. 1999, 9, M65–M68. [Google Scholar] [CrossRef]
- Meyerowitz, E.M. Comparative genomics. Plants compared to animals: The broadest comparative study of development. Science 2002, 295, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Grosberg, R.K.; Strathmann, R.R. The Evolution of Multicellularity: A Minor Major Transition? Annu. Rev. Ecol. Evol. Syst. 2007, 38, 621–654. [Google Scholar] [CrossRef]
- West, M.; Harada, J.J. Embryogenesis in Higher Plants: An Overview. Plant Cell 1993, 5, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Chory, J.; Dangl, J.L.; Estelle, M.; Jacobsen, S.E.; Meyerowitz, E.M.; Nordborg, M.; Weigel, D. The Impact of Arabidopsis on Human Health: Diversifying Our Portfolio. Cell 2008, 133, 939–943. [Google Scholar] [CrossRef]
- Xu, X.M.; Møller, S.G. The value of Arabidopsis research in understanding human disease states. Curr. Opin. Biotechnol. 2011, 22, 300–307. [Google Scholar] [CrossRef]
- Spampinato, C.P.; Gomez-Casati, D.F. Research on Plants for the Understanding of Diseases of Nuclear and Mitochondrial Origin. J. Biomed. Biotechnol. 2012, 2012, 836196. [Google Scholar] [CrossRef]
- Vergara, D.; de Domenico, S.; Maffia, M.; Piro, G.; Di Sansebastiano, G. Pietro Transgenic plants as low-cost platform for chemotherapeutic drugs screening. Int. J. Mol. Sci. 2015, 16, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Papadia, P.; Barozzi, F.; Hoeschele, J.D.; Piro, G.; Margiotta, N.; Di Sansebastiano, G. Pietro Cisplatin, oxaliplatin, and kiteplatin subcellular effects compared in a plant model. Int. J. Mol. Sci. 2017, 18, 306. [Google Scholar] [CrossRef]
- Francke, U.; Kung, F. Sporadic bilateral retinoblastoma and 13q-chromosomal deletion. Med. Pediatric Oncol. 1976, 2, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G.; Meadows, A.T.; Nichols, W.W.; Hill, R. Chromosomal Deletion and Retinoblastoma. N. Engl. J. Med. 1976, 295, 1120–1123. [Google Scholar] [CrossRef]
- Friend, S.H.; Bernards, R.; Rogelj, S.; Weinberg, R.A.; Rapaport, J.M.; Albert, D.M.; Dryja, T.P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 1986, 323, 643–646. [Google Scholar] [CrossRef]
- McGee, T.L.; Yandell, D.W.; Dryja, T.P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene 1989, 80, 119–128. [Google Scholar] [CrossRef]
- Sellers, W.R.; Kaelin, W.G. Role of the retinoblastoma protein in the pathogenesis of human cancer. J. Clin. Oncol. 1997, 15, 3301–3312. [Google Scholar] [CrossRef]
- Burkhart, D.L.; Sage, J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat. Rev. Cancer 2008, 8, 671–682. [Google Scholar] [CrossRef]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.D.; Cook, J.G. The temporal regulation of s phase proteins during G1. Adv. Exp. Med. Biol. 2017, 1042, 335–369. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Wang, J.Y.J. Targeting the RB-pathway in cancer therapy. Clin. Cancer Res. 2010, 16, 1094–1099. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Pruitt, S.C.; Hershberger, P.A.; Witkiewicz, A.K.; Goodrich, D.W. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, U.M.; O’Brien, J.M. Understanding pRb: Toward the necessary development of targeted treatments for retinoblastoma. J. Clin. Investig. 2012, 122, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Tarang, S.; Pyakurel, U.; Weston, M.D.; Vijayakumar, S.; Jones, T.; Wagner, K.U.; Rocha-Sanchez, S.M. Spatiotemporally controlled overexpression of cyclin D1 triggers generation of supernumerary cells in the postnatal mouse inner ear. Hear. Res. 2020, 390. [Google Scholar] [CrossRef]
- Henry, D.; Brumaire, S.; Hu, X. Involvement of pRb-E2F pathway in green tea extract-induced growth inhibition of human myeloid leukemia cells. Leuk. Res. 2019, 77, 34–41. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Y.; Wang, Q.; Chen, F.; Zeng, Y.; Yu, X.; Wang, Y.; Zhou, F.; Zhou, Y. Ribociclib (LEE011) suppresses cell proliferation and induces apoptosis of MDA-MB-231 by inhibiting CDK4/6-cyclin D-Rb-E2F pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4001–4011. [Google Scholar] [CrossRef]
- Kwapisz, D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: Palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017, 166, 41–54. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Boccia, V.; Claudio, P.P.; De Luca, A.; Giordano, A. Genomic structure of the human retinoblastoma-related Rb2/p130 gene. Proc. Natl. Acad. Sci. USA 1996, 93, 4629–4632. [Google Scholar] [CrossRef] [PubMed]
- Claudio, P.P.; Tonini, T.; Giordano, A. The retinoblastoma family: Twins or distant cousins? Genome Biol. 2002, 3, 3012. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Hanafusa, H.; Takimoto, H.; Ohgama, Y.; Akagi, T.; Shimizu, K. Structure of the human retinoblastoma-related p107 gene and its intragenic deletion in a B-cell lymphoma cell line. Gene 2000, 251, 37–43. [Google Scholar] [CrossRef]
- Chinnam, M.; Goodrich, D.W. RB1, Development, and Cancer. Curr. Top. Dev. Biol. 2011, 94, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, G.; Jacks, T. The retinoblastoma gene family: Cousins with overlapping interests. Trends Genet. 1998, 14, 223–229. [Google Scholar] [CrossRef]
- Dick, F.A. Structure-function analysis of the retinoblastoma tumor suppressor protein—Is the whole a sum of its parts? Cell Div. 2007, 2, 26. [Google Scholar] [CrossRef]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef]
- Lee, J.O.; Russo, A.A.; Pavletich, N.P. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature 1998, 391, 859–865. [Google Scholar] [CrossRef]
- Morris, E.J.; Dyson, N.J. Retinoblastoma protein partners. Adv. Cancer Res. 2001, 82, 1–54. [Google Scholar] [CrossRef]
- Sanidas, I.; Morris, R.; Fella, K.A.; Rumde, P.H.; Boukhali, M.; Tai, E.C.; Ting, D.T.; Lawrence, M.S.; Haas, W.; Dyson, N.J. A Code of Mono-phosphorylation Modulates the Function of RB. Mol. Cell 2019, 73, 985–1000.e6. [Google Scholar] [CrossRef]
- Macdonald, J.I.; Dick, F.A. Posttranslational modifications of the retinoblastoma tumor suppressor protein as determinants of function. Genes Cancer 2012, 3, 619–633. [Google Scholar] [CrossRef]
- Dyson, N.; Guida, P.; McCall, C.; Harlow, E. Adenovirus E1A makes two distinct contacts with the retinoblastoma protein. J. Virol. 1992, 66, 4606–4611. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; Krstic-Demonacos, M.; Smith, L.; Demonacos, C.; La Thangue, N.B. Acetylation control of the retinoblastoma tumour-suppressor protein. Nat. Cell Biol. 2001, 3, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.; Huang, J.; Wu, S.Q.; Hauser, P.; Reznikoff, C.A. Role of sv40 t antigen binding to prb and p53 in multistep transformation in vitro of human uroepithelial cells. Carcinogenesis 1993, 14, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.R.; Deshong, A.J.; Pelton, J.G.; Rubin, S.M. Phosphorylation-induced conformational changes in the retinoblastoma protein inhibit E2F transactivation domain binding. J. Biol. Chem. 2010, 285, 16286–16293. [Google Scholar] [CrossRef]
- Rubin, S.M.; Gall, A.L.; Zheng, N.; Pavletich, N.P. Structure of the Rb C-terminal domain bound to E2F1-DP1: A mechanism for phosphorylation-induced E2F release. Cell 2005, 123, 1093–1106. [Google Scholar] [CrossRef]
- Rubin, S.M. Deciphering the retinoblastoma protein phosphorylation code. Trends Biochem. Sci. 2013, 38, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.; Wong, P.P.; McCance, D.J. Acetylation of Rb by PCAF is required for nuclear localization and keratinocyte differentiation. J. Cell Sci. 2010, 123, 3718–3726. [Google Scholar] [CrossRef]
- Kim, K.Y.; Wang, D.-H.; Campbell, M.; Huerta, S.B.; Shevchenko, B.; Izumiya, C.; Izumiya, Y. PRMT4-mediated arginine methylation negatively regulates retinoblastoma tumor suppressor protein and promotes E2F-1 dissociation. Mol. Cell. Biol. 2015, 35, 238–248. [Google Scholar] [CrossRef]
- Saddic, L.A.; West, L.E.; Aslanian, A.; Yates, J.R.; Rubin, S.M.; Gozani, O.; Sage, J. Methylation of the retinoblastoma tumor suppressor by SMYD2. J. Biol. Chem. 2010, 285, 37733–37740. [Google Scholar] [CrossRef]
- Munro, S.; Khaire, N.; Inche, A.; Carr, S.; La Thangue, N.B. Lysine methylation regulates the pRb tumour suppressor protein. Oncogene 2010, 29, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Ledl, A.; Schmidt, D.; Müller, S. Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 2005, 24, 3810–3818. [Google Scholar] [CrossRef]
- Meng, F.; Qian, J.; Yue, H.; Li, X.; Xue, K. SUMOylation of Rb enhances its binding with CDK2 and phosphorylation at early G1 phase. Cell Cycle 2016, 15, 1724–1732. [Google Scholar] [CrossRef]
- Miwa, S.; Uchida, C.; Kitagawa, K.; Hattori, T.; Oda, T.; Sugimura, H.; Yasuda, H.; Nakamura, H.; Chida, K.; Kitagawa, M. Mdm2-mediated pRB downregulation is involved in carcinogenesis in a p53-independent manner. Biochem. Biophys. Res. Commun. 2006, 340, 54–61. [Google Scholar] [CrossRef]
- Burke, J.R.; Hura, G.L.; Rubin, S.M. Structures of inactive retinoblastoma protein reveal multiple mechanisms for cell cycle control. Genes Dev. 2012, 26, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Lamber, E.P.; Beuron, F.; Morris, E.P.; Svergun, D.I.; Mittnacht, S. Structural Insights into the Mechanism of Phosphoregulation of the Retinoblastoma Protein. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Carr, S.M.; La Thangue, N.B. Diversity within the pRb pathway: Is there a code of conduct? Oncogene 2012, 31, 4343–4352. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.D.; Huang, H.J.S.; To, H.; Young, L.J.S.; Oro, A.; Bookstein, R.; Lee, E.Y.H.P.; Lee, W.H. Structure of the human retinoblastoma gene. Proc. Natl. Acad. Sci. USA 1989, 86, 5502–5506. [Google Scholar] [CrossRef]
- Shen, B.; Carneiro, N.; Torres-Jerez, I.; Stevenson, B.; McCreery, T.; Helentjaris, T.; Baysdorfer, C.; Almira, E.; Ferl, R.J.; Habben, J.E. Partial sequencing and mapping of clones from two maize cDNA libraries. Plant Mol. Biol. 1994, 26, 1085–1101. [Google Scholar] [CrossRef]
- Xie, Q.; Sanz-Burgos, A.P.; Hannon, G.J.; Gutiérrez, C. Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 1996, 15, 4900–4908. [Google Scholar] [CrossRef]
- Xie, Q.; Suárez-López, P.; Gutiérrez, C. Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant DNA virus: Requirement for efficient viral DNA replication. EMBO J. 1995, 14, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Sekine, M.; Murakami, H.; Shinmyo, A. Tobacco retinoblastoma-related protein phosphorylated by a distinct cyclin-dependent kinase complex with Cdc2/cyclin D in vitro. Plant J. Cell Mol. Biol. 1999, 18, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-J. A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 2000, 19, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, A.; Pettko-Szandtner, A.; Csordas-Toth, E.; Miskolczi, P.; Horvath, G.V.; Gyorgyey, J.; Dudits, D. Dicot and monocot plants differ in retinoblastoma-related protein subfamilies. J. Exp. Bot. 2007, 58, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Durfee, T.; Feiler, H.S.; Gruissem, W. Retinoblastoma-related proteins in plants: Homologues or orthologues of their metazoan counterparts? Plant Mol. Biol. 2000, 43, 635–642. [Google Scholar] [CrossRef]
- Kuwabara, A.; Gruissem, W. Arabidopsis Retinoblastoma-related and Polycomb group proteins: Cooperation during plant cell differentiation and development. J. Exp. Bot. 2014, 65, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- De Jager, S.M.; Murray, J.A.H. Retinoblastoma proteins in plants. Plant Mol. Biol. 1999, 41, 295–299. [Google Scholar] [CrossRef]
- Desvoyes, B.; De Mendoza, A.; Ruiz-Trillo, I.; Gutierrez, C. Novel roles of plant RETINOBLASTOMA-RELATED (RBR) protein in cell proliferation and asymmetric cell division. J. Exp. Bot. 2014, 65, 2657–2666. [Google Scholar] [CrossRef]
- Gutzat, R.; Borghi, L.; Gruissem, W. Emerging roles of RETINOBLASTOMA-RELATED proteins in evolution and plant development. Trends Plant Sci. 2012, 17, 139–148. [Google Scholar] [CrossRef]
- Reiland, S.; Messerli, G.; Baerenfaller, K.; Gerrits, B.; Endler, A.; Grossmann, J.; Gruissem, W.; Baginsky, S. Large-scale arabidopsis phosphoproteome profiling reveals novel chloroplast kinase substrates and phosphorylation networks1[w]. Plant Physiol. 2009, 150, 889–903. [Google Scholar] [CrossRef]
- Magyar, Z.; Horváth, B.; Khan, S.; Mohammed, B.; Henriques, R.; De Veylder, L.; Bakó, L.; Scheres, B.; Bögre, L. Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. EMBO J. 2012, 31, 1480–1493. [Google Scholar] [CrossRef]
- Ábrahám, E.; Miskolczi, P.; Ayaydin, F.; Yu, P.; Kotogány, E.; Bakó, L.; Ötvös, K.; Horváth, G.V.; Dudits, D. Immunodetection of retinoblastoma-related protein and its phosphorylated form in interphase and mitotic alfalfa cells. J. Exp. Bot. 2011, 62, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Boniotti, M.B.; Gutierrez, C. A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. Plant J. 2001, 28, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Kawamura, K.; Sugisaka, K.; Sekine, M.; Shinmyo, A. Phosphorylation of retinoblastoma-related protein by the cyclin D/cyclin-dependent kinase complex is activated at the G1/S-phase transition in tobacco. Plant Cell 2002, 14, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Ramirez-Parra, E.; Castellano, M.M.; Sanz-Burgos, A.P.; Luque, A.; Missich, R. Geminivirus DNA replication and cell cycle interactions. Vet. Microbiol. 2004, 98, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C. Geminiviruses and the plant cell cycle. Plant Mol. Biol. 2000, 43, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Peng, B.; Yao, L.; Zhang, X.; Sun, K.; Yang, X.; Yu, L. The ancient function of RB-E2F Pathway: Insights from its evolutionary history. Biol. Direct 2010, 5, 1–21. [Google Scholar] [CrossRef]
- Harashima, H.; Dissmeyer, N.; Schnittger, A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013, 23, 345–356. [Google Scholar] [CrossRef]
- Budirahardja, Y.; Gönczy, P. Coupling the cell cycle to development. Development 2009, 136, 2861–2872. [Google Scholar] [CrossRef]
- Cooper, G.M. The Eukaryotic Cell Cycle. In The Cell: A Molecular Approach; Sinauer Associates: Washington, DC, USA, 2004; pp. 591–594. [Google Scholar]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V.; Pardee, A.B. The Restriction Point of the Cell Cycle. Cell Cycle 2002, 1, 102–109. [Google Scholar] [CrossRef]
- Komaki, S.; Sugimoto, K. Control of the Plant Cell Cycle by Developmental and Environmental Cues. Plant Cell Physiol. 2012, 53, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Takaki, T.; Fukasawa, K.; Suzuki-Takahashi, I.; Hirai, H. Cdk-mediated phosphorylation of pRB regulates HDAC binding in vitro. Biochem. Biophys. Res. Commun. 2004, 316, 252–255. [Google Scholar] [CrossRef]
- Schaal, C.; Pillai, S.; Chellappan, S.P. The Rb-E2F transcriptional regulatory pathway in tumor angiogenesis and metastasis. Adv. Cancer Res. 2014, 121, 147–182. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.L.; Wenzel, P.L.; Sáenz-Robles, M.T.; Nair, V.; Ferrey, A.; Hagan, J.P.; Gomez, Y.M.; Sharma, N.; Chen, H.Z.; Ouseph, M.; et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 2009, 462, 930–934. [Google Scholar] [CrossRef]
- Lee, B.-K.; Bhinge, A.A.; Iyer, V.R. Wide-ranging functions of E2F4 in transcriptional activation and repression revealed by genome-wide analysis. Nucleic Acids Res. 2011, 39, 3558–3573. [Google Scholar] [CrossRef]
- Komori, H.; Goto, Y.; Kurayoshi, K.; Ozono, E.; Iwanaga, R.; Bradford, A.P.; Araki, K.; Ohtani, K. Differential requirement for dimerization partner DP between E2F-dependent activation of tumor suppressor and growth-related genes. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Logan, N.; Graham, A.; Zhao, X.; Fisher, R.; Maiti, B.; Leone, G.; La Thangue, N.B. E2F-8: An E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene 2005, 24, 5000–5004. [Google Scholar] [CrossRef]
- Lammens, T.; Li, J.; Leone, G.; De Veylder, L. Atypical E2Fs: New players in the E2F transcription factor family. Trends Cell Biol. 2009, 19, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.H.; Han, Z.C. The transcription factor E2F: A crucial switch in the control of homeostasis and tumorigenesis. Histol. Histopathol. 2006, 21, 403–413. [Google Scholar] [PubMed]
- Magae, J.; Wu, C.L.; Illenye, S.; Harlow, E.; Heintz, N.H. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J. Cell Sci. 1996, 109, 1717–1726. [Google Scholar] [PubMed]
- Hsu, J.; Sage, J. Novel functions for the transcription factor E2F4 in development and disease. Cell Cycle 2016, 15, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Iaquinta, P.J.; Lees, J.A. Life and death decisions by the E2F transcription factors. Curr. Opin. Cell Biol. 2007, 19, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Cuitiño, M.C.; Pécot, T.; Sun, D.; Kladney, R.; Okano-Uchida, T.; Shinde, N.; Saeed, R.; Perez-Castro, A.J.; Webb, A.; Liu, T.; et al. Two Distinct E2F Transcriptional Modules Drive Cell Cycles and Differentiation. Cell Rep. 2019, 27, 3547–3560.e5. [Google Scholar] [CrossRef]
- Scott, M.C.; Sarver, A.L.; Tomiyasu, H.; Cornax, I.; Van Etten, J.; Varshney, J.; O’Sullivan, M.G.; Subramanian, S.; Modiano, J.F. Aberrant retinoblastoma (RB)-E2F transcriptional regulation defines molecular phenotypes of osteosarcoma. J. Biol. Chem. 2015, 290, 28070–28083. [Google Scholar] [CrossRef]
- Dowdy, S.F.; Hinds, P.W.; Louie, K.; Reed, S.I.; Arnold, A.; Weinberg, R.A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell 1993, 73, 499–511. [Google Scholar] [CrossRef]
- Kato, J.; Matsushime, H.; Hiebert, S.W.; Ewen, M.E.; Sherr, C.J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993, 7, 331–342. [Google Scholar] [CrossRef]
- Gillett, C.; Fantl, V.; Smith, R.; Fisher, C.; Bartek, J.; Dickson, C.; Barnes, D.; Peters, G. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994, 54, 1812–1817. [Google Scholar]
- Rizzolio, F.; Tuccinardi, T.; Caligiuri, I.; Lucchetti, C.; Giordano, A. CDK Inhibitors: From the Bench to Clinical Trials. Curr. Drug Targets 2010, 11, 279–290. [Google Scholar] [CrossRef]
- Markey, M.P.; Bergseid, J.; Bosco, E.E.; Stengel, K.; Xu, H.; Mayhew, C.N.; Schwemberger, S.J.; Braden, W.A.; Jiang, Y.; Babcock, G.F.; et al. Loss of the retinoblastoma tumor suppressor: Differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 2007, 26, 6307–6318. [Google Scholar] [CrossRef]
- Fischer, M.; Müller, G.A. Cell cycle transcription control: DREAM/MuvB and RB-E2F complexes. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 638–662. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, S.; DeCaprio, J.A. The DREAM complex: Master coordinator of cell cycle-dependent gene expression. Nat. Rev. Cancer 2013, 13, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Guiley, K.Z.; Liban, T.J.; Felthousen, J.G.; Ramanan, P.; Litovchick, L.; Rubin, S.M. Structural mechanisms of DREAM complex assembly and regulation. Genes Dev. 2015, 29, 961–974. [Google Scholar] [CrossRef]
- Sadasivam, S.; Duan, S.; DeCaprio, J.A. The MuvB complex sequentially recruits B-Myb and FoxM1 to promote mitotic gene expression. Genes Dev. 2012, 26, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Meskiene, I.; Bogre, L.; Ha, D.T.; Swoboda, I.; Hubmann, R.; Hirt, H.; Heberle-Bors, E. The D-type alfalfa cyclin gene cycMs4 complements G1 cyclin-deficient yeast and is induced in the G1 phase of the cell cycle. Plant Cell 1995, 7, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Huntley, R.; Healy, S.; Freeman, D.; Lavender, P.; de Jager, S.; Greenwood, J.; Makker, J.; Walker, E.; Jackman, M.; Xie, Q.; et al. The maize retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (CycD) proteins. Plant Mol. Biol. 1998, 37, 155–169. [Google Scholar] [CrossRef]
- Soni, R.; Carmichael, J.P.; Shah, Z.H.; Murray, J.A. A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 1995, 7, 85–103. [Google Scholar] [CrossRef]
- Ach, R.A.; Durfee, T.; Miller, A.B.; Taranto, P.; Hanley-Bowdoin, L.; Zambryski, P.C.; Gruissem, W. RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol. Cell. Biol. 1997, 17, 5077–5086. [Google Scholar] [CrossRef]
- Ebel, C.; Mariconti, L.; Gruissem, W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 2004, 429, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.J.; Matveeva, E.; Kirioukhova, O.; Grossniklaus, U.; Gruissem, W. A Dynamic Reciprocal RBR-PRC2 Regulatory Circuit Controls Arabidopsis Gametophyte Development. Curr. Biol. 2008, 18, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Gutzat, R.; Fütterer, J.; Laizet, Y.; Hennig, L.; Gruissem, W. Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production. Plant Cell 2010, 22, 1792–1811. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ramírez, A.; Díaz-Triviño, S.; Wachsman, G.; Du, Y.; Arteága-Vázquez, M.; Zhang, H.; Benjamins, R.; Blilou, I.; Neef, A.B.; Chandler, V.; et al. A SCARECROW-RETINOBLASTOMA Protein Network Controls Protective Quiescence in the Arabidopsis Root Stem Cell Organizer. PLoS Biol. 2013, 11. [Google Scholar] [CrossRef]
- Wildwater, M.; Campilho, A.; Perez-Perez, J.M.; Heidstra, R.; Blilou, I.; Korthout, H.; Chatterjee, J.; Mariconti, L.; Gruissem, W.; Scheres, B. The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. Cell 2005, 123, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Desvoyes, B.; Ramirez-Parra, E.; Xie, Q.; Chua, N.H.; Gutierrez, C. Cell type-specific role of the retinoblastoma/E2F pathway during arabidopsis leaf development. Plant Physiol. 2006, 140, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Sabelli, P.A.; Dante, R.A.; Leiva-Neto, J.T.; Jung, R.; Gordon-Kamm, W.J.; Larkins, B.A. RBR3, a member of the retinoblastoma-related family from maize, is regulated by the RBR1/E2F pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13005–13012. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Liu, Y.; Dante, R.A.; Lizarraga, L.E.; Nguyen, H.N.; Brown, S.W.; Klingler, J.P.; Yu, J.; LaBrant, E.; Layton, T.M.; et al. Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm. Proc. Natl. Acad. Sci. USA 2013, 110. [Google Scholar] [CrossRef]
- Grafi, G.; Burnett, R.J.; Helentjaris, T.; Larkins, B.A.; Decaprio, J.A.; Sellers, W.R.; Kaelin, W.G. A maize cDNA encoding a member of the retinoblastoma protein family: Involvement in endoreduplication. Proc. Natl. Acad. Sci. USA 1996, 93, 8962–8967. [Google Scholar] [CrossRef]
- Rossi, V.; Locatelli, S.; Lanzanova, C.; Boniotti, M.B.; Varotto, S.; Pipal, A.; Goralik-Schramel, M.; Lusser, A.; Gatz, C.; Gutierrez, C.; et al. A maize histone deacetylase and retinoblastoma-related protein physically interact and cooperate in repressing gene transcription. Plant Mol. Biol. 2003, 51, 401–413. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Larkins, B.A. Grasses Like Mammals? Redundancy and Compensatory Regulation within the Retinoblastoma Protein Family. Cell Cycle 2006, 5, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Villajuana-Bonequi, M.; Matei, A.; Ernst, C.; Hallab, A.; Usadel, B.; Doehlemann, G. Cell type specific transcriptional reprogramming of maize leaves during Ustilago maydis induced tumor formation. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Sabelli, P.A.; Hoerster, G.; Lizarraga, L.E.; Brown, S.W.; Gordon-Kamm, W.J.; Larkins, B.A. Positive regulation of minichromosome maintenance gene expression, DNA replication, and cell transformation by a plant retinoblastoma gene. Proc. Natl. Acad. Sci. USA 2009, 106, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Kamm, W.; Dilkes, B.P.; Lowe, K.; Hoerster, G.; Sun, X.; Ross, M.; Church, L.; Bunde, C.; Farrell, J.; Hill, P.; et al. Stimulation of the cell cycle and maize transformation by disruption of the plant retinoblastoma pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 11975–11980. [Google Scholar] [CrossRef] [PubMed]
- Sage, J.; Miller, A.L.; Pérez-Mancera, P.A.; Wysocki, J.M.; Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 2003, 424, 223–228. [Google Scholar] [CrossRef]
- Gutierrez, C. The Arabidopsis Cell Division Cycle. Arab. Book 2009, 7, e0120. [Google Scholar] [CrossRef]
- Wang, G.; Kong, H.; Sun, Y.; Zhang, X.; Zhang, W.; Altman, N.; DePamphilis, C.W.; Ma, H. Genome-wide analysis of the cyclin family in arabidopsis and comparative phylogenetic analysis of plant cyclin-like proteins. Plant Physiol. 2004, 135, 1084–1099. [Google Scholar] [CrossRef]
- Quandt, E.; Ribeiro, M.P.C.; Clotet, J. Atypical cyclins: The extended family portrait. Cell. Mol. Life Sci. 2020, 77, 231–242. [Google Scholar] [CrossRef]
- Magyar, Z.; De Veylder, L.; Atanassova, A.; Bakó, L.; Inzé, D.; Bögre, L. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 2005, 17, 2527–2541. [Google Scholar] [CrossRef]
- Ramirez-Parra, E.; López-Matas, M.A.; Fründt, C.; Gutierrez, C. Role of an atypical E2F transcription factor in the control of Arabidopsis cell growth and differentiation. Plant Cell 2004, 16, 2350–2363. [Google Scholar] [CrossRef]
- Vandepoele, K.; Vlieghe, K.; Florquin, K.; Hennig, L.; Beemster, G.T.S.; Gruissem, W.; Van De Peer, Y.; Inzé, D.; De Veylder, L. Genome-wide identification of potential plant E2F target genes. Plant Physiol. 2005, 139, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Oszi, E.; Papdi, C.; Mohammed, B.; Petkó-Szandtner, A.; Leviczky, T.; Molnár, E.; Galvan-Ampudia, C.; Khan, S.; Juez, E.L.; Horváth, B.; et al. E2FB interacts with RETINOBLASTOMA RELATED and regulates cell proliferation during leaf development. Plant Physiol. 2020, 182, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Boudolf, V.; Barrôco, R.; De Engler, J.A.; Verkest, A.; Beeckman, T.; Naudts, M.; Inzé, D.; De Veylder, L. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell 2004, 16, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Scofield, S.; Jones, A.; Murray, J.A.H. The plant cell cycle in context. J. Exp. Bot. 2014, 65, 2557–2562. [Google Scholar] [CrossRef]
- Caldon, C.E.; Musgrove, E.A. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010, 5, 2. [Google Scholar] [CrossRef]
- Teixeira, L.K.; Reed, S.I. Cyclin E deregulation and genomic instability. Adv. Exp. Med. Biol. 2017, 1042, 527–547. [Google Scholar] [CrossRef]
- Fischer, M.; DeCaprio, J.A. Does Arabidopsis thaliana DREAM of cell cycle control? EMBO J. 2015, 34, 1987–1989. [Google Scholar] [CrossRef]
- Magyar, Z.; Bögre, L.; Ito, M. DREAMs make plant cells to cycle or to become quiescent. Curr. Opin. Plant Biol. 2016, 34, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Suzuki, T.; Iwata, E.; Magyar, Z.; Bögre, L.; Ito, M. MYB3Rs, plant homologs of Myb oncoproteins, control cell cycle-regulated transcription and form DREAM-like complexes. Transcription 2015, 6, 106–111. [Google Scholar] [CrossRef]
- Wang, W.; Sijacic, P.; Xu, P.; Lian, H.; Liu, Z. Arabidopsis TSO1 and MYB3R1 form a regulatory module to coordinate cell proliferation with differentiation in shoot and root. Proc. Natl. Acad. Sci. USA 2018, 115, E3045–E3054. [Google Scholar] [CrossRef]
- Bouyer, D.; Heese, M.; Chen, P.; Harashima, H.; Roudier, F.; Grüttner, C.; Schnittger, A. Genome-wide identification of RETINOBLASTOMA RELATED 1 binding sites in Arabidopsis reveals novel DNA damage regulators. PLoS Genet. 2018, 14, 1–35. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, J.; Chopra, S.; Gu, X.; Peterson, T. Ordered origin of the typical two- and three-repeat Myb genes. Gene 2004, 326, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Suzuki, T.; Iwata, E.; Nakamichi, N.; Suzuki, T.; Chen, P.; Ohtani, M.; Ishida, T.; Hosoya, H.; Müller, S.; et al. Transcriptional repression by MYB 3R proteins regulates plant organ growth. EMBO J. 2015, 34, 1992–2007. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Ito, M.; Soyano, T.; Nishihama, R.; Machida, Y. Mitotic cyclins stimulate the activity of c-Myb-like factors for transactivation of G2/M phase-specific genes in tobacco. J. Biol. Chem. 2004, 279, 32979–32988. [Google Scholar] [CrossRef]
- Del Pozo, J.C.; Boniotti, M.B.; Gutierrez, C. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCFAtSKP2 pathway in response to light. Plant Cell 2002, 14, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.A. Cell differentiation: What have we learned in 50 years? J. Theor. Biol. 2020, 485, 110031. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Alvarado, A.; Yamanaka, S. Rethinking Differentiation: Stem Cells, Regeneration, and Plasticity. Cell 2014, 157, 110–119. [Google Scholar] [CrossRef] [PubMed]
- De Falco, G.; Comes, F.; Simone, C. pRb: Master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene 2006, 25, 5244–5249. [Google Scholar] [CrossRef]
- Goodrich, D.W. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene 2006, 25, 5233–5243. [Google Scholar] [CrossRef]
- Hatzistergos, K.E.; Williams, A.R.; Dykxhoorn, D.; Bellio, M.A.; Yu, W.; Hare, J.M. Tumor Suppressors RB1 and CDKN2a Cooperatively Regulate Cell-Cycle Progression and Differentiation During Cardiomyocyte Development and Repair. Circ. Res. 2019, 124, 1184–1197. [Google Scholar] [CrossRef]
- Khidr, L.; Chen, P.-L. RB, the conductor that orchestrates life, death and differentiation. Oncogene 2006, 25, 5210–5219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Narayanan, C.; Bian, J.; Sambo, D.; Brickler, T.; Zhang, W.; Chetty, S. A transient DMSO treatment increases the differentiation potential of human pluripotent stem cells through the Rb family. PLoS ONE 2018, 13, e0208110. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.M.; Jacks, T. The retinoblastoma gene family in differentiation and development. Oncogene 1999, 18, 7873–7882. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Philippeit, C.; Weise, A.; Dünker, N. Re-characterization of established human retinoblastoma cell lines. Histochem. Cell Biol. 2014, 143, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Bremner, R. The search for the retinoblastoma cell of origin. Nat. Rev. Cancer 2005, 5, 91–101. [Google Scholar] [CrossRef]
- Nair, R.M.; Vemuganti, G.K. Transgenic Models in Retinoblastoma Research. Ocul. Oncol. Pathol. 2015, 1, 207–213. [Google Scholar] [CrossRef]
- Clarke, A.R.; Maandag, E.R.; Van Roon, M.; Van Der Lugt, N.M.T.; Van Der Valk, M.; Hooper, M.L.; Berns, A.; Te Rielef, H. Requirement for a functional Rb-1 gene in murine development. Nature 1992, 359, 328–330. [Google Scholar] [CrossRef]
- Jacks, T.; Fazeli, A.; Schmitt, E.M.; Bronson, R.T.; Goodell, M.A.; Weinberg, R.A. Effects of an Rb mutation in the mouse. Nature 1992, 359, 295–300. [Google Scholar] [CrossRef]
- Lee, E.Y.H.P.; Chang, C.Y.; Hu, N.; Wang, Y.C.J.; Lai, C.C.; Herrup, K.; Lee, W.H.; Bradley, A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 1992, 359, 288–294. [Google Scholar] [CrossRef]
- Morgenbesser, S.D.; Williams, B.O.; Jacks, T.; Depinho, R.A. P53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 1994, 371, 72–74. [Google Scholar] [CrossRef]
- Zhang, J.; Gray, J.; Wu, L.; Leone, G.; Rowan, S.; Cepko, C.L.; Zhu, X.; Craft, C.M.; Dyer, M.A. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat. Genet. 2004, 36, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Robanus-Maandag, E.; Dekker, M.; Van Der Valk, M.; Carrozza, M.L.; Jeanny, J.C.; Dannenberg, J.H.; Berns, A.; Riele, H. Te p107 is a suppressor of retinoblastoma development in pRB-deficient mice. Genes Dev. 1998, 12, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L.; Landsberg, R.L.; Huss-Garcia, Y.; Sardet, C.; Lees, J.A.; Auwerx, J. E2Fs regulate adipocyte differentiation. Dev. Cell 2002, 3, 39–49. [Google Scholar] [CrossRef]
- Flowers, S.; Xu, F.; Moran, E. Cooperative activation of tissue-specific genes by pRB and E2F1. Cancer Res. 2013, 73, 2150–2158. [Google Scholar] [CrossRef]
- Julian, L.M.; Liu, Y.; Pakenham, C.A.; Dugal-Tessier, D.; Ruzhynsky, V.; Bae, S.; Tsai, S.Y.; Leone, G.; Slack, R.S.; Blais, A. Tissue-specific targeting of cell fate regulatory genes by E2f factors. Cell Death Differ. 2016, 23, 565–575. [Google Scholar] [CrossRef]
- Müller, H.; Bracken, A.P.; Vernell, R.; Moroni, M.C.; Christians, F.; Grassilli, E.; Prosperini, E.; Vigo, E.; Oliner, J.D.; Helin, K. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 2001, 15, 267–285. [Google Scholar] [CrossRef]
- Sellers, W.R.; Novitch, B.G.; Miyake, S.; Heith, A.; Otterson, G.A.; Kaye, F.J.; Lassar, A.B.; Kaelin, W.G. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998, 12, 95–106. [Google Scholar] [CrossRef]
- Cecchini, M.J.; Thwaites, M.J.; Talluri, S.; MacDonald, J.I.; Passos, D.T.; Chong, J.-L.; Cantalupo, P.; Stafford, P.M.; Saenz-Robles, M.T.; Francis, S.M.; et al. A Retinoblastoma Allele That Is Mutated at Its Common E2F Interaction Site Inhibits Cell Proliferation in Gene-Targeted Mice. Mol. Cell. Biol. 2014, 34, 2029–2045. [Google Scholar] [CrossRef][Green Version]
- Wyrzykowska, J.; Schorderet, M.; Pien, S.; Gruissem, W.; Fleming, A.J. Induction of differentiation in the shoot apical meristem by transient overexpression of a retinoblastoma-related protein. Plant Physiol. 2006, 141, 1338–1348. [Google Scholar] [CrossRef]
- Zhao, X.; Bramsiepe, J.; Van Durme, M.; Komaki, S.; Prusicki, M.A.; Maruyama, D.; Forner, J.; Medzihradszky, A.; Wijnker, E.; Harashima, H.; et al. RETINOBLASTOMA RELATED1 mediates germline entry in Arabidopsis. Science 2017, 356. [Google Scholar] [CrossRef]
- Perilli, S.; Perez-Perez, J.M.; Di Mambro, R.; Peris, C.L.; Díaz-Triviño, S.; Del Bianco, M.; Pierdonati, E.; Moubayidin, L.; Cruz-Ramírez, A.; Costantino, P.; et al. RETINOBLASTOMA-RELATED protein stimulates cell differentiation in the Arabidopsis root meristem by interacting with cytokinin signaling. Plant Cell 2013, 25, 4469–4478. [Google Scholar] [CrossRef] [PubMed]
- Moubayidin, L.; Perilli, S.; Dello Ioio, R.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Pugliesi, C. The Dynamic Genetic-Hormonal Regulatory Network Controlling the Trichome Development in Leaves. Plants 2019, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, K.; Grotewold, E. A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 2009, 5, e1000396. [Google Scholar] [CrossRef]
- Zhao, C.; Avci, U.; Grant, E.H.; Haigler, C.H.; Beers, E.P. XND1, a member of the NAC domain family in Arabidopsis thaliana, negatively regulates lignocellulose synthesis and programmed cell death in xylem. Plant J. 2008, 53, 425–436. [Google Scholar] [CrossRef]
- Zhao, C.; Lasses, T.; Bako, L.; Kong, D.; Zhao, B.; Chanda, B.; Bombarely, A.; Cruz-Ramírez, A.; Scheres, B.; Brunner, A.M.; et al. XYLEM NAC DOMAIN1, an angiosperm NAC transcription factor, inhibits xylem differentiation through conserved motifs that interact with RETINOBLASTOMA-RELATED. New Phytol. 2017, 1. [Google Scholar] [CrossRef]
- Putarjunan, A.; Torii, K.U. Stomagenesis versus myogenesis: Parallels in intrinsic and extrinsic regulation of transcription factor mediated specialized cell-type differentiation in plants and animals. Dev. Growth Differ. 2016, 58, 341–354. [Google Scholar] [CrossRef]
- Matos, J.L.; Bergmann, D.C. Convergence of stem cell behaviors and genetic regulation between animals and plants: Insights from the Arabidopsis thaliana stomatal lineage. F1000Prime Rep. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Le Grand, F.; Rudnicki, M.A. Skeletal muscle satellite cells and adult myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef]
- Novitch, B.G.; Mulligan, G.J.; Jacks, T.; Lassar, A.B. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J. Cell Biol. 1996, 135, 441–456. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Rigby, P.W.J. Gene Regulatory Networks and Transcriptional Mechanisms that Control Myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Dumont, N.A.; Wang, Y.X.; Rudnicki, M.A. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 2015, 142, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D.D.W.; Olwin, B.B.; Rudnicki, M.A.; Wold, B.J. MyoD(-/-) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev. Biol. 2000, 224, 122–137. [Google Scholar] [CrossRef]
- Endo, T.; Goto, S. Retinoblastoma gene product Rb accumulates during myogenic differentiation and is deinduced by the expression of SV40 large T antigen. J. Biochem. 1992, 112, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Huh, M.S.; Parker, M.H.; Scimè, A.; Parks, R.; Rudnicki, M.A. Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J. Cell Biol. 2004, 166, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Tapscott, S.J. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development 2005, 132, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Schneider, J.W.; Condorelli, G.; Kaushal, S.; Mahdavi, V.; Nadal-Ginard, B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell 1993, 72, 309–324. [Google Scholar] [CrossRef]
- Smialowski, P.; Singh, M.; Mikolajka, A.; Majumdar, S.; Joy, J.K.; Nalabothula, N.; Krajewski, M.; Degenkolbe, R.; Bernard, H.-U.; Holak, T.A. NMR and mass spectrometry studies of putative interactions of cell cycle proteins pRb and CDK6 with cell differentiation proteins MyoD and ID-2. Biochim. Biophys. Acta 2005, 1750, 48–60. [Google Scholar] [CrossRef]
- Magenta, A.; Cenciarelli, C.; De Santa, F.; Fuschi, P.; Martelli, F.; Caruso, M.; Felsani, A. MyoD Stimulates RB Promoter Activity via the CREB/p300 Nuclear Transduction Pathway. Mol. Cell. Biol. 2003, 23, 2893–2906. [Google Scholar] [CrossRef]
- Martelli, F.; Cenciarelli, C.; Santarelli, G.; Polikar, B.; Felsani, A.; Caruso, M. MyoD induces retinoblastoma gene expression during myogenic differentiation. Oncogene 1994, 9, 3579–3590. [Google Scholar]
- Puri, P.L.; Sartorelli, V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 2000, 185, 155–173. [Google Scholar] [CrossRef]
- Novitch, B.G.; Spicer, D.B.; Kim, P.S.; Cheung, W.L.; Lassar, A.B. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol. 1999, 9, 449–459. [Google Scholar] [CrossRef][Green Version]
- Knight, J.D.R.; Kothary, R. The myogenic kinome: Protein kinases critical to mammalian skeletal myogenesis. Skelet. Muscle 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.N.; Takahashi, C.; Ewen, M.E. Retinoblastoma protein and MyoD function together to effect the repression of Fra-1 and in turn cyclin D1 during terminal cell cycle arrest associated with myogenesis. J. Biol. Chem. 2014, 289, 23417–23427. [Google Scholar] [CrossRef] [PubMed]
- Skapek, S.X.; Rhee, J.; Kim, P.S.; Novitch, B.G.; Lassar, A.B. Cyclin-mediated inhibition of muscle gene expression via a mechanism that is independent of pRB hyperphosphorylation. Mol. Cell. Biol. 1996, 16, 7043–7053. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Wang, Q.; Goebl, M.G.; Harrington, M.A. Phosphorylation of Nuclear MyoD Is Required for Its Rapid Degradation. Mol. Cell. Biol. 1998, 18, 4994–4999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Wei, Q.; Zhao, X.; Paterson, B.M. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J. 1999, 18, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.L.; Iezzi, S.; Stiegler, P.; Chen, T.T.; Schiltz, R.L.; Muscat, G.E.O.; Giordano, A.; Kedes, L.; Wang, J.Y.J.; Sartorelli, V. Class I histone deacetylases sequentially interact with MyoD and pRb during skeletal myogenesis. Mol. Cell 2001, 8, 885–897. [Google Scholar] [CrossRef]
- Simone, C.; Forcales, S.V.; Hill, D.A.; Imbalzano, A.N.; Latella, L.; Puri, P.L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat. Genet. 2004, 36, 738–743. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Dong, J. Stomatal Development in Arabidopsis. Arab. Book 2013, 11, e0162. [Google Scholar] [CrossRef]
- Vatén, A.; Bergmann, D.C. Mechanisms of stomatal development: An evolutionary view. EvoDevo 2012, 3, 11. [Google Scholar] [CrossRef]
- Gray, J.E. Plant Development: Three Steps for Stomata. Curr. Biol. 2007, 17, R213–R215. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, M.M.; Pillitteri, L.J.; Fujii, H.; Yoshida, Y.; Bogenschutz, N.L.; Takabayashi, J.; Zhu, J.K.; Torii, K.U. SCREAM/ICE1 and SCREAM2 specify three cell-state transitional steps leading to Arabidopsis stomatal differentiation. Plant Cell 2008, 20, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Pillitteri, L.J.; Torii, K.U. Breaking the silence: Three bHLH proteins direct cell-fate decisions during stomatal development. BioEssays 2007, 29, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Qi, X.; Sugihara, K.; Dang, J.H.; Endo, T.A.; Miller, K.L.; Kim, E.D.; Miura, T.; Torii, K.U. MUTE Directly Orchestrates Cell-State Switch and the Single Symmetric Division to Create Stomata. Dev. Cell 2018, 45, 303–315.e5. [Google Scholar] [CrossRef]
- Ohashi-Ito, K.; Bergmann, D.C. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 2006, 18, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Zoulias, N.; Harrison, E.L.; Casson, S.A.; Gray, J.E. Molecular control of stomatal development. Biochem. J. 2018, 475, 441–454. [Google Scholar] [CrossRef]
- Matos, J.L.; Lau, O.S.; Hachez, C.; Cruz-Ramírez, A.; Scheres, B.; Bergmann, D.C. Irreversible fate commitment in the Arabidopsis stomatal lineage requires a FAMA and RETINOBLASTOMA-RELATED module. eLife 2014, 3, 1–15. [Google Scholar] [CrossRef]
- Han, S.-K.; Torii, K.U. Linking cell cycle to stomatal differentiation. Curr. Opin. Plant Biol. 2019, 51, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Z.; Jiang, M.; Wang, M.; Xue, S.; Zhu, L.L.; Wang, H.Z.; Zou, J.J.; Lee, E.K.; Sack, F.; Le, J. Phosphorylation of serine 186 of bHLH transcription factor SPEECHLESS promotes stomatal development in arabidopsis. Mol. Plant 2015, 8, 783–795. [Google Scholar] [CrossRef]
- Nowack, M.K.; Harashima, H.; Dissmeyer, N.; Zhao, X.; Bouyer, D.; Weimer, A.K.; De Winter, F.; Yang, F.; Schnittger, A. Genetic Framework of Cyclin-Dependent Kinase Function in Arabidopsis. Dev. Cell 2012, 22, 1030–1040. [Google Scholar] [CrossRef]
- Lee, E.; Lucas, J.R.; Sack, F.D. Deep functional redundancy between FAMA and FOUR LIPS in stomatal development. Plant J. 2014, 78, 555–565. [Google Scholar] [CrossRef]
- Weimer, A.K.; Matos, J.L.; Sharma, N.; Patell, F.; Murray, J.A.H.; Dewitte, W.; Bergmann, D.C. Lineage- and stage-specific expressed CYCD7;1 coordinates the single symmetric division that creates stomatal guard cells. Development (Camb. Engl.) 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Zaragoza, J.; Scheres, B. Tuning Division and Differentiation in Stomata: How to Silence a MUTE. Dev. Cell 2018, 45, 282–283. [Google Scholar] [CrossRef]
- Hachez, C.; Ohashi-Ito, K.; Dong, J.; Bergmann, D.C. Differentiation of arabidopsis guard cells: Analysis of the networks incorporating the basic helix-loop-helix transcription factor, FAMA. Plant Physiol. 2011, 155, 1458–1472. [Google Scholar] [CrossRef]
- Hecker, L.; Jagirdar, R.; Jin, T.; Thannickal, V.J. Reversible differentiation of myofibroblasts by MyoD. Exp. Cell Res. 2011, 317, 1914–1921. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Z.; Hou, S. SPEECHLESS Speaks Loudly in Stomatal Development. Front. Plant Sci. 2020, 11, 114. [Google Scholar] [CrossRef]
- Badodi, S.; Baruffaldi, F.; Ganassi, M.; Battini, R.; Molinari, S. Phosphorylation-dependent degradation of MEF2C contributes to regulate G2/M transition. Cell Cycle (Georget. Tex.) 2015, 14, 1517–1528. [Google Scholar] [CrossRef]
- Estrella, N.L.; Desjardins, C.A.; Nocco, S.E.; Clark, A.L.; Maksimenko, Y.; Naya, F.J. MEF2 transcription factors regulate distinct gene programs in mammalian skeletal muscle differentiation. J. Biol. Chem. 2015, 290, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Olson, E.N. MEF2: A central regulator of diverse developmental… [Development. 2007]—PubMed result. Development (Camb. Engl.) 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, E.R.; Pelaz, S.; Liljegren, S.J.; Gold, S.E.; Burgeff, C.; Ditta, G.S.; De Pouplana, L.R.; Martinez-Castilla, L.; Yanofsky, M.F. An ancestral MADS-box gene duplication occurred before the divergence of plants and animals. Proc. Natl. Acad. Sci. USA 2000, 97, 5328–5333. [Google Scholar] [CrossRef]
- Zhao, P.-X.; Miao, Z.-Q.; Zhang, J.; Liu, Q.-Q.; Xiang, C.-B. MADS-box factor AGL16 negatively regulates drought resistance via stomatal density and stomatal movement. bioRxiv 2019, 723106. [Google Scholar] [CrossRef]
- Kutter, C.; Schöb, H.; Stadler, M.; Meins, F.; Si-Ammour, A. MicroRNA-mediated regulation of stomatal development in Arabidopsis. Plant Cell 2007, 19, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, K.; Dakik, P.; Medkour, Y.; Mitrofanova, D.; Titorenko, V.I. Quiescence entry, maintenance, and exit in adult stem cells. Int. J. Mol. Sci. 2019, 20, 2158. [Google Scholar] [CrossRef]
- Santoro, A.; Vlachou, T.; Carminati, M.; Pelicci, P.G.; Mapelli, M. Molecular mechanisms of asymmetric divisions in mammary stem cells. EMBO Rep. 2016, 17, 1700–1720. [Google Scholar] [CrossRef]
- Rumman, M.; Dhawan, J.; Kassem, M. Concise Review: Quiescence in Adult Stem Cells: Biological Significance and Relevance to Tissue Regeneration. Stem Cells 2015, 33, 2903–2912. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gaza, H.V.; Kashuba, E.V. Role of the RB-Interacting Proteins in Stem Cell Biology. Adv. Cancer Res. 2016, 131, 133–157. [Google Scholar] [CrossRef]
- Kohno, S.; Kitajima, S.; Sasaki, N.; Takahashi, C. Retinoblastoma tumor suppressor functions shared by stem cell and cancer cell strategies. World J. Stem Cells 2016, 8, 170. [Google Scholar] [CrossRef]
- Sage, J. The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 2012, 26, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Livne-bar, I.; Vanderluit, J.L.; Slack, R.S.; Agochiya, M.; Bremner, R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 2004, 5, 539–551. [Google Scholar] [CrossRef]
- Gutierrez, G.M.; Kong, E.; Sabbagh, Y.; Brown, N.E.; Lee, J.S.; Demay, M.B.; Thomas, D.M.; Hinds, P.W. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1-/- mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18402–18407. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, T.; Nishijo, K.; Prajapati, S.I.; Li, G.; Keller, C. Rb1 gene inactivation expands satellite cell and postnatal myoblast pools. J. Biol. Chem. 2011, 286, 19556–19564. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, S.; Santos, M.; Segrelles, C.; Leis, H.; Jorcano, J.L.; Berns, A.; Paramio, J.M.; Vooijs, M. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development (Camb. Engl.) 2004, 131, 2737–2748. [Google Scholar] [CrossRef]
- Vanderluit, J.L.; Wylie, C.A.; McClellan, K.A.; Ghanem, N.; Fortin, A.; Callaghan, S.; MacLaurin, J.G.; Park, D.S.; Slack, R.S. The Retinoblastoma family member p107 regulates the rate of progenitor commitment to a neuronal fate. J. Cell Biol. 2007, 178, 129–139. [Google Scholar] [CrossRef]
- Viatour, P.; Somervaille, T.C.; Venkatasubrahmanyam, S.; Kogan, S.; McLaughlin, M.E.; Weissman, I.L.; Butte, A.J.; Passegué, E.; Sage, J. Hematopoietic Stem Cell Quiescence Is Maintained by Compound Contributions of the Retinoblastoma Gene Family. Cell Stem Cell 2008, 3, 416–428. [Google Scholar] [CrossRef]
- Viatour, P.; Ehmer, U.; Saddic, L.A.; Dorrell, C.; Andersen, J.B.; Lin, C.; Zmoos, A.F.; Mazur, P.K.; Schaffer, B.E.; Ostermeier, A.; et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J. Exp. Med. 2011, 208, 1963–1976. [Google Scholar] [CrossRef]
- Takahira, T.; Oda, Y.; Tamiya, S.; Yamamoto, H.; Kobayashi, C.; Izumi, T.; Ito, K.; Iwamoto, Y.; Tsuneyoshi, M. Alterations of the RB1 gene in dedifferentiated liposarcoma. Mod. Pathol. 2005, 18, 1461–1470. [Google Scholar] [CrossRef]
- Jopling, C.; Boue, S.; Belmonte, J.C.I. Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011, 12, 79–89. [Google Scholar] [CrossRef]
- Bracken, A.P.; Pasini, D.; Capra, M.; Prosperini, E.; Colli, E.; Helin, K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003, 22, 5323–5335. [Google Scholar] [CrossRef] [PubMed]
- Kareta, M.S.; Gorges, L.L.; Hafeez, S.; Benayoun, B.A.; Marro, S.; Zmoos, A.F.; Cecchini, M.J.; Spacek, D.; Batista, L.F.Z.; O’Brien, M.; et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell 2015, 16, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, W.S.; Kim, S.-H. Hormonal regulation of stem cell maintenance in roots. J. Exp. Bot. 2013, 64, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Feldman, L.J. REGULATION OF ROOT APICAL MERISTEM DEVELOPMENT. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef]
- Timilsina, R.; Kim, J.H.; Nam, H.G.; Woo, H.R. Temporal changes in cell division rate and genotoxic stress tolerance in quiescent center cells of Arabidopsis primary root apical meristem. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Wachsman, G.; Heidstra, R.; Scheres, B. Distinct cell-autonomous functions of RETINOBLASTOMA-RELATED in Arabidopsis stem cells revealed by the brother of Brainbow Clonal analysis system. Plant Cell 2011, 23, 2581–2591. [Google Scholar] [CrossRef]
- Cruz-Ramírez, A.; Díaz-Triviño, S.; Blilou, I.; Grieneisen, V.A.; Sozzani, R.; Zamioudis, C.; Miskolczi, P.; Nieuwland, J.; Benjamins, R.; Dhonukshe, P.; et al. A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell 2012, 150, 1002–1015. [Google Scholar] [CrossRef]
- Weimer, A.K.; Nowack, M.K.; Bouyer, D.; Zhao, X.; Harashima, H.; Naseer, S.; De Winter, F.; Dissmeyer, N.; Geldner, N.; Schnittger, A. RETINOBLASTOMA RELATED1 regulates asymmetric cell divisions in Arabidopsis. Plant Cell 2012, 24, 4083–4095. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e14. [Google Scholar] [CrossRef]
- Durand-Dubief, M.; Persson, J.; Norman, U.; Hartsuiker, E.; Ekwall, K. Topoisomerase I regulates open chromatin and controls gene expression in vivo. EMBO J. 2010, 29, 2126–2134. [Google Scholar] [CrossRef]
- Zhang, F.; Rothermund, K.; Gangadharan, S.B.; Pommier, Y.; Prochownik, E.V.; Lazo, J.S. Phenotypic screening reveals topoisomerase I as a breast cancer stem cell therapeutic target. Oncotarget 2012, 3, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, L.; Hong, J.H.; Gong, X.; Zhou, C.; Pérez-Pérez, J.M.; Xu, J. TOPOISOMERASE1α Acts through Two Distinct Mechanisms to Regulate Stele and Columella Stem Cell Maintenance. Plant Physiol. 2016, 171, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, L.; Dinh, T.T.; Shi, T.; Li, D.; Wang, R.; Gao, L.; Xiao, L.; Chen, X. DNA topoisomerase I affects polycomb group protein-mediated epigenetic regulation and plant development by altering nucleosome distribution in Arabidopsis. Plant Cell 2014, 26, 2803–2817. [Google Scholar] [CrossRef] [PubMed]
- Atlasi, Y.; Stunnenberg, H.G. The interplay of epigenetic marks during stem cell differentiation and development. Nat. Rev. Genet. 2017, 18, 643–658. [Google Scholar] [CrossRef]
- Fiorentino, F.P.; Marchesi, I.; Giordano, A. On the role of retinoblastoma family proteins in the establishment and maintenance of the epigenetic landscape. J. Cell. Physiol. 2013, 228, 276–284. [Google Scholar] [CrossRef]
- Kalozoumi, G.; Tzimas, C.; Sanoudou, D. The expanding role of epigenetics. Glob. Cardiol. Sci. Pract. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Uchida, C. Roles of pRB in the Regulation of Nucleosome and Chromatin Structures. Biomed Res. Int. 2016, 2016, 5959721. [Google Scholar] [CrossRef]
- Gonzalo, S.; Blasco, M.A. Role of Rb Family in the Epigenetic Definition of Chromatin. Cell Cycle 2005, 4, 752–755. [Google Scholar] [CrossRef]
- Vélez-Cruz, R.; Johnson, D. The Retinoblastoma (RB) Tumor Suppressor: Pushing Back against Genome Instability on Multiple Fronts. Int. J. Mol. Sci. 2017, 18, 1776. [Google Scholar] [CrossRef]
- Aprelikova, O.N.; Fang, B.S.; Meissner, E.G.; Cotter, S.; Campbell, M.; Kuthiala, A.; Bessho, M.; Jensen, R.A.; Liu, E.T. BRCA1-associated growth arrest is RB-dependent. Proc. Natl. Acad. Sci. USA 1999, 96, 11866–11871. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi-Jaulin, L.; Groisman, R.; Naguibneva, I.; Robin, P.; Lorain, S.; Le Villain, J.P.; Troalen, F.; Trouche, D.; Harel-Bellan, A. Retinoblastoma protein represses transcription by recruiting a histone deacetylase. Nature 1998, 391, 601–605. [Google Scholar] [CrossRef]
- Robertson, K.D.; Ait-Si-Ali, S.; Yokochi, T.; Wade, P.A.; Jones, P.L.; Wolffe, A.P. DNMT1 forms a complex with RB, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 2000, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Schuettengruber, B.; Bourbon, H.-M.; Di Croce, L.; Cavalli, G. Genome Regulation by Polycomb and Trithorax: 70 Years and Counting. Cell 2017, 171, 34–57. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Cai, J.; Hou, Y.; Huang, Z.; Wang, Z. Role of EZH2 in cancer stem cells: From biological insight to a therapeutic target. Oncotarget 2017, 8, 37974–37990. [Google Scholar] [CrossRef] [PubMed]
- Ishak, C.A.; Marshall, A.E.; Passos, D.T.; White, C.R.; Kim, S.J.; Cecchini, M.J.; Ferwati, S.; MacDonald, W.A.; Howlett, C.J.; Welch, I.D.; et al. An RB-EZH2 Complex Mediates Silencing of Repetitive DNA Sequences. Mol. Cell 2016, 64, 1074–1087. [Google Scholar] [CrossRef]
- Vélez-Cruz, R.; Manickavinayaham, S.; Biswas, A.K.; Clary, R.W.; Premkumar, T.; Cole, F.; Johnson, D.G. RB localizes to DNA double-strand breaks and promotes DNA end resection and homologous recombination through the recruitment of BRG1. Genes Dev. 2016, 30, 2500–2512. [Google Scholar] [CrossRef]
- Sokka, M.; Parkkinen, S.; Pospiech, H.; Syväoja, J.E. Function of TopBP1 in genome stability. Sub-Cell. Biochem. 2010, 50, 119–141. [Google Scholar] [CrossRef]
- Jiang, Y.; Chu, W.K. Potential Roles of the Retinoblastoma Protein in Regulating Genome Editing. Front. Cell Dev. Biol. 2018, 6. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Strober, B.E.; Guha, S.; Khavari, P.A.; Ålin, K.; Luban, J.; Begemann, M.; Crabtree, G.R.; Goff, S.P. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell 1994, 79, 119–130. [Google Scholar] [CrossRef]
- Marquez-Vilendrer, S.B.; Rai, S.K.; Gramling, S.J.; Lu, L.; Reisman, D.N. BRG1 and BRM loss selectively impacts RB and P53, respectively: BRG1 and BRM have differential functions in vivo. Oncoscience 2016, 3, 337–350. [Google Scholar] [CrossRef]
- Strobeck, M.W.; Knudsen, K.E.; Fribourg, A.F.; DeCristofaro, M.F.; Weissman, B.E.; Imbalzano, A.N.; Knudsen, E.S. BRG-1 is required for RB-mediated cell cycle arrest. Proc. Natl. Acad. Sci. USA 2000, 97, 7748–7753. [Google Scholar] [CrossRef]
- Xiao, H.; Goodrich, D.W. The retinoblastoma tumor suppressor protein is required for efficient processing and repair of trapped topoisomerase II-DNA-cleavable complexes. Oncogene 2005, 24, 8105–8113. [Google Scholar] [CrossRef] [PubMed]
- Yarden, R.I.; Brody, L.C. BRCA1 interacts with components of the histone deacetylase complex. Proc. Natl. Acad. Sci. USA 1999, 96, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.; Zoumpoulidou, G.; Luczynski, M.T.; Rieger, S.; Moquet, J.; Spanswick, V.J.; Hartley, J.A.; Rothkamm, K.; Huang, P.H.; Mittnacht, S. Direct involvement of retinoblastoma family proteins in DNA repair by non-homologous end-joining. Cell Rep. 2015, 10, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Desvoyes, B.; Fernández-Marcos, M.; Sequeira-Mendes, J.; Otero, S.; Vergara, Z.; Gutierrez, C. Looking at plant cell cycle from the chromatin window. Front. Plant Sci. 2014, 5, 369. [Google Scholar] [CrossRef] [PubMed]
- de la Paz Sanchez, M.; Aceves-García, P.; Petrone, E.; Steckenborn, S.; Vega-León, R.; Álvarez-Buylla, E.R.; Garay-Arroyo, A.; García-Ponce, B. The impact of Polycomb group (PcG) and Trithorax group (TrxG) epigenetic factors in plant plasticity. New Phytol. 2015, 208, 684–694. [Google Scholar] [CrossRef]
- Jullien, P.E.; Mosquna, A.; Ingouff, M.; Sakata, T.; Ohad, N.; Berger, F. Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol. 2008, 6, 1693–1705. [Google Scholar] [CrossRef]
- Feil, R.; Berger, F. Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 2007, 23, 192–199. [Google Scholar] [CrossRef]
- Gutzat, R.; Borghi, L.; Fütterer, J.; Bischof, S.; Laizet, Y.; Hennig, L.; Feil, R.; Lunn, J.; Gruissem, W. Retinoblastoma-related protein controls the transition to autotrophic plant development. Development 2011, 138, 2977–2986. [Google Scholar] [CrossRef]
- Lee, E.; Lucas, J.R.; Goodrich, J.; Sack, F.D. Arabidopsis guard cell integrity involves the epigenetic stabilization of the FLP and FAMA transcription factor genes. Plant J. 2014, 78, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, S.; Harashima, H.; Chen, P.; Heese, M.; Bouyer, D.; Sofroni, K.; Schnittger, A. The retinoblastoma homolog RBR 1 mediates localization of the repair protein RAD 51 to DNA lesions in Arabidopsis. EMBO J. 2017, 36, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.V.; Boehning, D. Structure-function of the tumor suppressor BRCA1. Comput. Struct. Biotechnol. J. 2012, 1, e201204005. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.M.; Kourova, H.; Nagy, S.; Nemeth, E.; Magyar, Z.; Papdi, C.; Ahmad, Z.; Sanchez-Perez, G.F.; Perilli, S.; Blilou, I.; et al. Arabidopsis RETINOBLASTOMA RELATED directly regulates DNA damage responses through functions beyond cell cycle control. EMBO J. 2017, 36, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).