A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats
Abstract
1. Introduction
2. Results
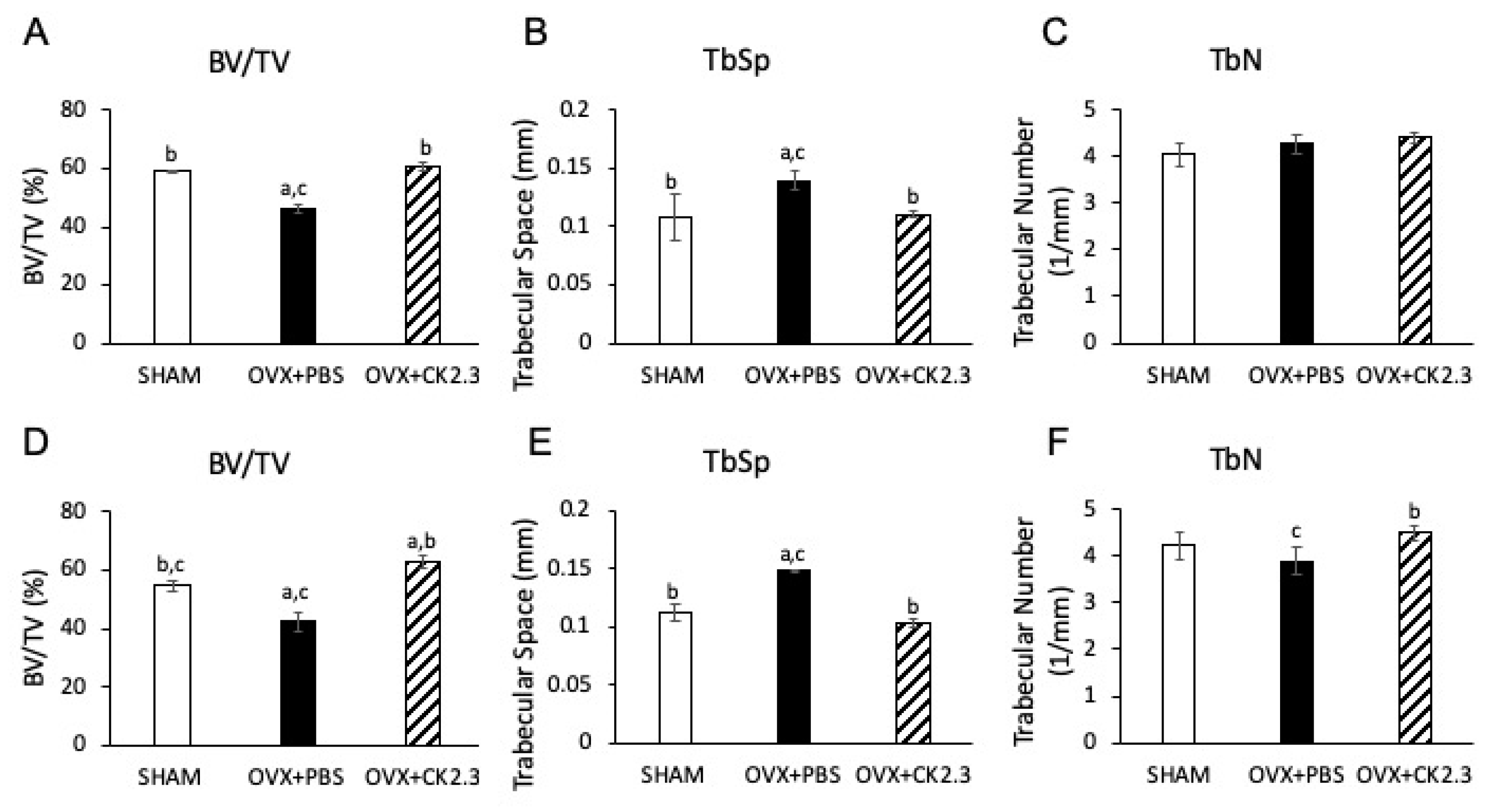
2.1. CK2.3 Increases Trabecular Bone Architecture of the Femoral Head of OVX Rats
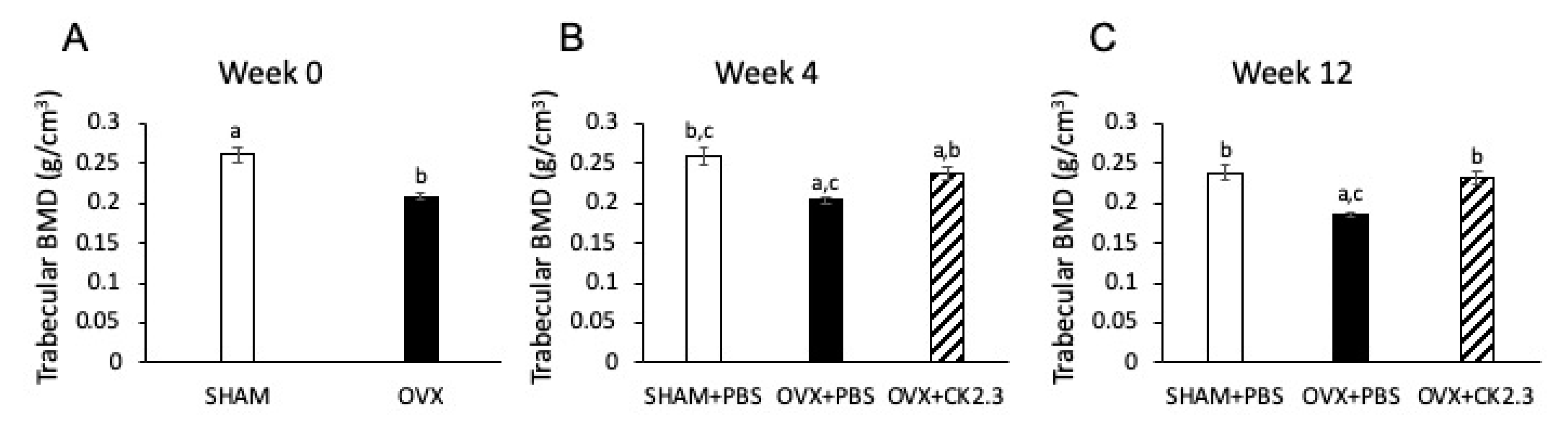
2.2. CK2.3 Recovered Trabecular BMD in the Femoral Head of OVX Rats
2.3. CK2.3 Increased Lumbar Spine and Pelvis Trabecular BMD of OVX Rats
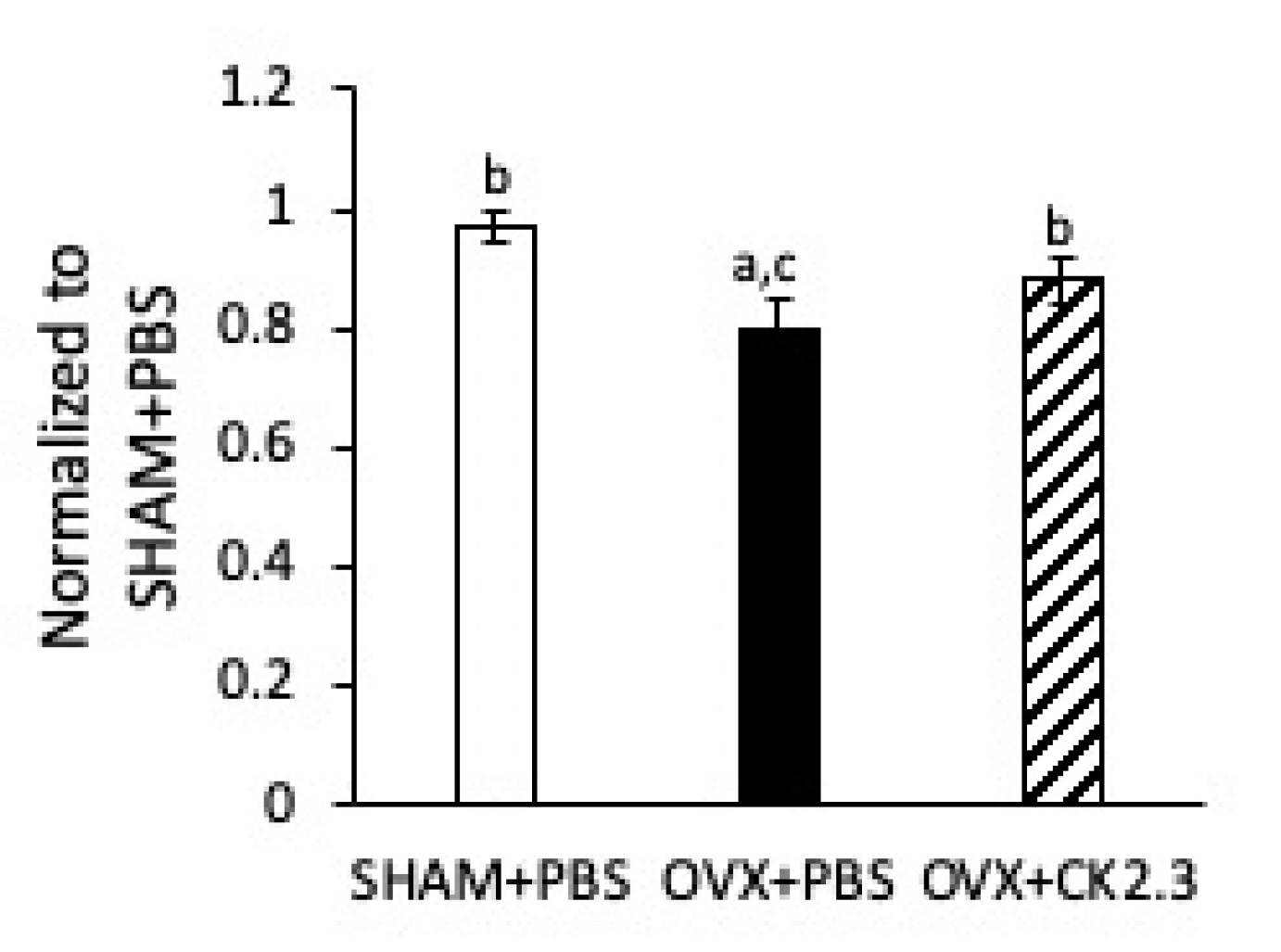
2.4. CK2.3 Increased Stiffness of Femoral Shaft of OVX Rats
2.5. CK2.3 Did Not Affect Organs of OVX Rats
3. Discussion
4. Materials and Methods
4.1. Design of Blocking Peptide CK2.3
4.2. Rat Injection and Live Micro-Computed Tomography of the Femoral Head
4.3. Micro-Computed Tomography (micro-CT)
4.4. Single Photon Absorptiometry (SPA) of Rat Pelvis and Lumbar Spine and Calculation of BMD
4.5. Three-Point Bending Test
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OP | Osteoporosis |
| RANK | Receptor activator of nuclear kappa beta |
| SERMs | Selective estrogen receptor modulators |
| PPi | Inorganic pyrophosphates |
| PTH | Parathyroid hormone |
| M-CSF | Macrophage colony stimulating factor |
| BMP2 | Bone morphogenetic protein 2 |
| BMPRIa | Bone morphogenetic protein receptor type 1a |
| CK2 | Casein kinase 2 |
| OVX | Ovariectomized |
| BMSC | Bone marrow stromal cells |
| BV/TV | Bone volume/tissue volume |
| TbSp | Trabecular space |
| TbN | Trabecular number |
| BMD | Bone mineral density |
| SPA | Single photon absorptiometry |
References
- Office of the Surgeon General (USA). Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General (USA): Rockville, MD, USA, 2004.
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; Leboff, M.S.; Glowacki, J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zhou, S.; Glowacki, J. Age-related decline in osteoblastogenesis and 1alpha-hydroxylase/CYP27B1 in human mesenchymal stem cells: Stimulation by parathyroid hormone. Aging Cell 2011, 10, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.L.; Zhou, S.; Eslami, B.; Shen, L.; LeBoff, M.S.; Glowacki, J. Effect of age on regulation of human osteoclast differentiation. J. Cell. Biochem. 2014, 115, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Phys. Ther. 2018, 43, 92–104. [Google Scholar]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Pazianas, M.; van der Geest, S.; Miller, P. Bisphosphonates and bone quality. Bonekey Rep. 2014, 3, 529. [Google Scholar] [CrossRef]
- Ma, S.; Goh, E.L.; Jin, A.; Bhattacharya, R.; Boughton, O.R.; Patel, B.; Karunaratne, A.; Vo, N.T.; Atwood, R.; Cobb, J.P.; et al. Long-term effects of bisphosphonate therapy: Perforations, microcracks and mechanical properties. Sci. Rep. 2017, 7, 43399. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Toulis, K.A.; Polyzos, S.A.; Anastasilakis, C.D.; Makras, P. Long-term treatment of osteoporosis: Safety and efficacy appraisal of denosumab. Ther. Clin. Risk Manag. 2012, 8, 295–306. [Google Scholar] [CrossRef]
- Toulis, K.A.; Anastasilakis, A.D. Increased risk of serious infections in women with osteopenia or osteoporosis treated with denosumab. Osteoporos. Int. 2010, 21, 1963–1964. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Catala-Lehnen, P.; Huebner, A.K.; Jeschke, A.; Heckt, T.; Lueth, A.; Krause, M.; Koehne, T.; Albers, J.; Schulze, J.; et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 2014, 5, 5215. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016—Executive Summary. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Ejersted, C.; Andreassen, T.T.; Hauge, E.M.; Melsen, F.; Oxlund, H. Parathyroid hormone (1-34) increases vertebral bone mass, compressive strength, and quality in old rats. Bone 1995, 17, 507–511. [Google Scholar] [CrossRef]
- Arita, S.; Ikeda, S.; Sakai, A.; Okimoto, N.; Akahoshi, S.; Nagashima, M.; Nishida, A.; Ito, M.; Nakamura, T. Human parathyroid hormone (1-34) increases mass and structure of the cortical shell, with resultant increase in lumbar bone strength, in ovariectomized rats. J. Bone Miner. Metab. 2004, 22, 530–540. [Google Scholar] [CrossRef]
- Aslan, D.; Andersen, M.D.; Gede, L.B.; de Franca, T.K.; Jorgensen, S.R.; Schwarz, P.; Jorgensen, N.R. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scand. J. Clin. Lab. Investig. 2012, 72, 14–22. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first-in-class osteoporosis drug. Nat. Rev. Drug. Discov. 2019, 18, 411. [Google Scholar] [CrossRef]
- Suen, P.K.; Qin, L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: A general review. J. Orthop. Transl. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Kida, K.; Yamaguchi, A.; Hata, K.; Ichida, F.; Meguro, H.; Aburatani, H.; Nishimura, R.; Yoneda, T. BMP2 Regulates Osterix through Msx2 and Runx2 during Osteoblast. J. Biol. Chem. 2008, 283, 29119–29125. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Beyer, K.; Jensen, E.D.; Rodriguez, J.S.; Davydova, J.; Yamamoto, M.; Petryk, A.; Gopalakrishnan, R.; Mansky, K.C. Bone morphogenetic protein 2 signaling in osteoclasts is negatively regulated by the BMP antagonist, twisted gastrulation. J. Cell. Biochem. 2011, 112, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. J. Orthop. Res. 2015, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Weidner, H.; Schell, L.M.; Sequeira, L.; Kabrick, R.; Dharmadhikari, S.; Coombs, H.; Duncan, R.L.; Wang, L.; Nohe, A. Synthetic Peptide CK2.3 Enhances Bone Mineral Density in Senile Mice. J. Bone Res. 2018, 6. [Google Scholar] [CrossRef]
- Moseychuk, O.; Akkiraju, H.; Dutta, J.; D’Angelo, A.; Bragdon, B.; Duncan, R.L.; Nohe, A. Inhibition of CK2 binding to BMPRIa induces C2C12 differentiation into osteoblasts and adipocytes. J. Cell Commun. Signal. 2013, 7, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.S. Animal models of osteoporosis--necessity and limitations. Eur. Cell. Mater. 2001, 1, 66–81. [Google Scholar] [CrossRef]
- Jee, W.S.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal Interact. 2001, 1, 193–207. [Google Scholar] [PubMed]
- Lelovas, P.P.; Xanthos, T.T.; Thoma, S.E.; Lyritis, G.P.; Dontas, I.A. The laboratory rat as an animal model for osteoporosis research. Comp. Med. 2008, 58, 424–430. [Google Scholar] [PubMed]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-induced bone loss varies among inbred strains of mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Schaffler, M.B.; Wolde-Semait, H.T.; Hernandez, C.J.; Jepsen, K.J. Genetic background influences cortical bone response to ovariectomy. J. Bone Miner. Res. 2005, 20, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, U.T.; Yuan, D.; Power, R.A.; Wronski, T.J. Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J. Bone Miner. Res. 2006, 21, 1068–1074. [Google Scholar] [CrossRef]
- Wronski, T.J.; Dann, L.M.; Horner, S.L. Time course of vertebral osteopenia in ovariectomized rats. Bone 1989, 10, 295–301. [Google Scholar] [CrossRef]
- Wronski, T.J.; Lowry, P.L.; Walsh, C.C.; Ignaszewski, L.A. Skeletal alterations in ovariectomized rats. Calcif. Tissue Int. 1985, 37, 324–328. [Google Scholar] [CrossRef]
- Wronski, T.J.; Cintrón, M.; Dann, L.M. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif. Tissue Int. 1988, 43, 179–183. [Google Scholar] [CrossRef]
- Kozawa, O.; Hatakeyama, D.; Uematsu, T. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J. Biol. Chem. 2002, 277, 16452. [Google Scholar]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Borner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Bragdon, B.; Bonor, J.; Shultz, K.L.; Beamer, W.G.; Rosen, C.J.; Nohe, A. Bone morphogenetic protein receptor type Ia localization causes increased BMP2 signaling in mice exhibiting increased peak bone mass phenotype. J. Cell. Physiol. 2012, 227, 2870–2879. [Google Scholar] [CrossRef]
- Abe, E.; Yamamoto, M.; Taguchi, Y.; Lecka-Czernik, B.; O’Brien, C.A.; Economides, A.N.; Stahl, N.; Jilka, R.L.; Manolagas, S.C. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: Antagonism by noggin. J. Bone Miner. Res. 2000, 15, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kaji, H.; Kobayashi, T.; Nishiyama, K.; Fukase, M.; Kumegawa, M.; Chihara, K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J. Bone Miner. Res. 1995, 10, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef]
- Yousefzadeh, N.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Ovariectomized rat model of osteoporosis: A practical guide. EXCLI J. 2020, 19, 89–107. [Google Scholar]
- Lei, Z.; Xiaoying, Z.; Xingguo, L. Ovariectomy-associated changes in bone mineral density and bone marrow haematopoiesis in rats. Int. J. Exp. Pathol. 2009, 90, 512–519. [Google Scholar] [CrossRef]
- Chen, H.L.; Tung, Y.T.; Chuang, C.H.; Tu, M.Y.; Tsai, T.C.; Chang, S.Y.; Chen, C.M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2015, 26, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.; Saral, S.; Mercantepe, T.; İskender, H.; Tümkaya, L.; Atak, M.; Taşçı, F. White Tea Reduced Bone Loss by Suppressing the TRAP/CTX Pathway in Ovariectomy-Induced Osteoporosis Model Rats. Cells Tissues Organs 2020, 1–10. [Google Scholar] [CrossRef]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic Acid Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized Rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef]
- Zhang, L.; Takahashi, H.E.; Tanizawa, T.; Endo, N.; Yamamoto, N. Maintenance of bone mineral density of femoral cortex in ovariectomized rats after withdrawal of concurrent administration of human parathyroid hormone (1–34) and incardronate disodium (YM175). J. Bone Miner. Metab. 1997, 15, 206–212. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, T.; Chen, Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J. Cell. Biochem. 2014, 115, 625–631. [Google Scholar] [CrossRef]
- Drake, M.T.; Srinivasan, B.; Modder, U.I.; Peterson, J.M.; McCready, L.K.; Riggs, B.L.; Dwyer, D.; Stolina, M.; Kostenuik, P.; Khosla, S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2010, 95, 5056–5062. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.; Lee, J.W.; Hirano, K.; Isogai, Y.; Ishizuya, T.; Takao-Kawabata, R.; Iimura, T. Administration frequency as well as dosage of PTH are associated with development of cortical porosity in ovariectomized rats. Bone Res. 2017, 5, 17002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ominsky, M.S.; Warmington, K.S.; Morony, S.; Gong, J.; Cao, J.; Gao, Y.; Shalhoub, V.; Tipton, B.; Haldankar, R.; et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 2009, 24, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Buller, L.T.; Best, M.J.; Quinnan, S.M. A Nationwide Analysis of Pelvic Ring Fractures: Incidence and Trends in Treatment, Length of Stay, and Mortality. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 9–17. [Google Scholar] [CrossRef]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Jaroma, A.V.; Soininvaara, T.A.; Kröger, H. Effect of one-year post-operative alendronate treatment on periprosthetic bone after total knee arthroplasty. A seven-year randomised controlled trial of 26 patients. Bone Jt. J. 2015, 97-b, 337–345. [Google Scholar] [CrossRef]
- Turner, A.S.; Mallinckrodt, C.H.; Alvis, M.R.; Bryant, H.U. Dose-response effects of estradiol implants on bone mineral density in ovariectomized ewes. Bone 1995, 17, 421s–427s. [Google Scholar] [CrossRef]
- Iwaniec, U.T.; Samnegård, E.; Cullen, D.M.; Kimmel, D.B. Maintenance of cancellous bone in ovariectomized, human parathyroid hormone [hPTH(1-84)]-treated rats by estrogen, risedronate, or reduced hPTH. Bone 2001, 29, 352–360. [Google Scholar] [CrossRef]
- Fuse, H.; Fukumoto, S.; Sone, H.; Miyata, Y.; Saito, T.; Nakayama, K.; Takahashi, H.; Matsumoto, T.; Ogata, E. A new synthetic steroid, osaterone acetate (TZP-4238), increases cortical bone mass and strength by enhancing bone formation in ovariectomized rats. J. Bone Miner. Res. 1997, 12, 590–597. [Google Scholar] [CrossRef]
- Jordan, N.; Barry, M.; Murphy, E. Comparative effects of antiresorptive agents on bone mineral density and bone turnover in postmenopausal women. Clin. Interv. Aging 2006, 1, 377–387. [Google Scholar] [CrossRef]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K.; Pitsch, S. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Schwegler-Berry, D.; Castranova, V.; He, P. Internalization of caveolin-1 scaffolding domain facilitated by Antennapedia homeodomain attenuates PAF-induced increase in microvessel permeability. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H195–H201. [Google Scholar] [CrossRef] [PubMed]
- Bilbrey, G.L.; Weix, J.; Kaplan, G.D. Value of single photon absorptiometry in osteoporosis screening. Clin. Nucl. Med. 1988, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sogur, E.; Baksi, B.G.; Grondahl, H.G.; Sen, B.H. Pixel intensity and fractal dimension of periapical lesions visually indiscernible in radiographs. J. Endod. 2013, 39, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Lofman, O.; Larsson, L.; Toss, G. Bone mineral density in diagnosis of osteoporosis: Reference population, definition of peak bone mass, and measured site determine prevalence. J. Clin. Densitom. 2000, 3, 177–186. [Google Scholar] [CrossRef]
- Von Wowern, N.; Stoltze, K. Age differences in cortical width of mandibles determined by histoquantitation. Scand. J. Dent. Res. 1979, 87, 225–233. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeira, L.; Nguyen, J.; Wang, L.; Nohe, A. A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats. Int. J. Mol. Sci. 2020, 21, 4874. https://doi.org/10.3390/ijms21144874
Sequeira L, Nguyen J, Wang L, Nohe A. A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats. International Journal of Molecular Sciences. 2020; 21(14):4874. https://doi.org/10.3390/ijms21144874
Chicago/Turabian StyleSequeira, Linda, John Nguyen, Liyun Wang, and Anja Nohe. 2020. "A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats" International Journal of Molecular Sciences 21, no. 14: 4874. https://doi.org/10.3390/ijms21144874
APA StyleSequeira, L., Nguyen, J., Wang, L., & Nohe, A. (2020). A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats. International Journal of Molecular Sciences, 21(14), 4874. https://doi.org/10.3390/ijms21144874




