Role of Macrophages and Microglia in Zebrafish Regeneration
Abstract
1. Introduction
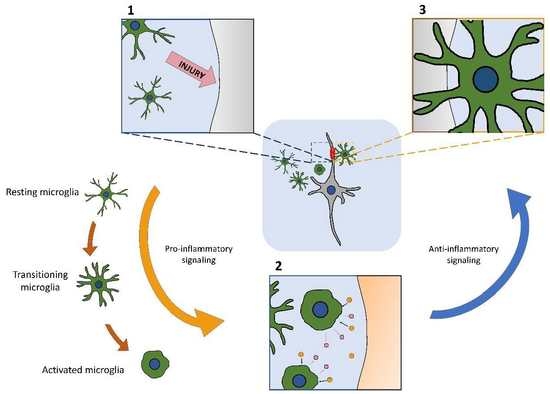
2. Overview of Physiological Functions of Macrophages and Microglia
3. Macrophages and Microglia in Tissue Regeneration in Fish
4. Translational Studies in Fish and Mammalian Models
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayata, P.; Schaefer, A. Innate sensing of mechanical properties of brain tissue by microglia. Innate Immun. 2020, 62, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Tauber, A.I. Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 2003, 4, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Teti, G.; Biondo, C.; Beninati, C. The phagocyte, metchnikoff, and the foundation of immunology. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Sawyer, R.T.; Strausbauch, P.H.; Volkman, A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab. Investig. J. Tech. Methods Pathol. 1982, 46, 165–170. [Google Scholar]
- van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 1972, 46, 845–852. [Google Scholar]
- van Furth, R.; Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968, 128, 415–435. [Google Scholar] [CrossRef]
- Volkman, A.; Chang, N.C.; Strausbauch, P.H.; Morahan, P.S. Differential effects of chronic monocyte depletion on macrophage populations. Lab. Investig. J. Tech. Methods Pathol. 1983, 49, 291–298. [Google Scholar]
- Mantovani, B.; Rabinovitch, M.; Nussenzweig, V. Phagocytosis of immune complexes by macrophages. Different roles of the macrophage receptor sites for complement (C3) and for immunoglobulin (IgG). J. Exp. Med. 1972, 135, 780–792. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Lichanska, A.M.; Hume, D.A. Origins and functions of phagocytes in the embryo. Exp. Hematol. 2000, 28, 601–611. [Google Scholar] [CrossRef]
- Yona, S.; Kim, K.-W.; Wolf, Y.; Milder, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013, 38, 79–91. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: ‘Phagoptosis’. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Elward, K.; Gasque, P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: Emphasis on the critical role of the complement system. Mol. Immunol. 2003, 40, 85–94. [Google Scholar] [CrossRef]
- Janeway, C. Immunogenecity signals 1,2,3... and 0. Immunol. Today 1989, 10, 283–286. [Google Scholar] [CrossRef]
- Parnaik, R.; Raff, M.C.; Scholes, J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 2000, 10, 857–860. [Google Scholar] [CrossRef]
- Fischer, U.; Koppang, E.O.; Nakanishi, T. Teleost T and NK cell immunity. Fish Shellfish Immunol. 2013, 35, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Vera-Jimenez, N.I.; Nielsen, M.E. Carp head kidney leukocytes display different patterns of oxygen radical production after stimulation with PAMPs and DAMPs. Mol. Immunol. 2013, 55, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, M.; Spreafico, R.; Springstead, J.R.; Mendelson, M.M.; Joehanes, R.; Levy, D.; Zanoni, I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 2020, 21, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Serbulea, V.; Upchurch, C.M.; Ahern, K.W.; Bories, G.; Voigt, P.; DeWeese, D.E.; Meher, A.K.; Harris, T.E.; Leitinger, N. Macrophages sensing oxidized DAMPs reprogram their metabolism to support redox homeostasis and inflammation through a TLR2-Syk-ceramide dependent mechanism. Mol. Metab. 2018, 7, 23–34. [Google Scholar] [CrossRef]
- Garapaty, A.; Champion, J.A. Shape of ligand immobilized particles dominates and amplifies the macrophage cytokine response to ligands. PLoS ONE 2019, 14, e0217022. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chun, T. Anti-inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol. Cells 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Arnett, E.; Schlesinger, L.S. Macrophage nuclear receptors: Emerging key players in infectious diseases. PLoS Pathog. 2019, 15, e1007585. [Google Scholar] [CrossRef]
- Gentek, R.; Molawi, K.; Sieweke, M.H. Tissue macrophage identity and self-renewal. Immunol. Rev. 2014, 262, 56–73. [Google Scholar] [CrossRef]
- Lavin, Y.; Winter, D.; Blecher-Gonen, R.; David, E.; Keren-Shaul, H.; Merad, M.; Jung, S.; Amit, I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014, 159, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.W.; Filosa, J.A. A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J. Neuroinflammation 2013, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314. [Google Scholar] [CrossRef]
- Frost, J.L.; Schafer, D.P. Microglia: Architects of the developing nervous system. Trends Cell Biol. 2016, 26, 587–597. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Lowery, R.L.; Majewska, A.K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernández-Suárez, D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Roediger, B.; Weninger, W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 2014, 291, 22–31. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia biology: One century of evolving concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer ‘if’ but ‘how’. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef] [PubMed]
- van Ham, T.J.; Brady, C.A.; Kalicharan, R.D.; Oosterhof, N.; Kuipers, J.; Veenstra-Algra, A.; Sjollema, K.A.; Peterson, R.T.; Kampinga, H.H.; Giepmans, B.N.G. Intravital correlated microscopy reveals differential macrophage and microglial dynamics during resolution of neuroinflammation. Dis. Models Mech. 2014, 7, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef]
- Shechter, R.; Miller, O.; Yovel, G.; Rosenzweig, N.; London, A.; Ruckh, J.; Kim, K.-W.; Klein, E.; Kalchenko, V.; Bendel, P.; et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 2013, 38, 555–569. [Google Scholar] [CrossRef]
- Herbomel, P.; Thisse, B.; Thisse, C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 2001, 238, 274–288. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Gomez-Perdiguero, E.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Schulz, C.; Gomez-Perdiguero, E.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012, 336, 86–90. [Google Scholar] [CrossRef]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F.; Wittbrodt, J. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nat. Commun. 2014, 5, 4046. [Google Scholar] [CrossRef]
- Peri, F.; Nusslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.; Enikolopov, G.N.; Overstreet-Wadiche, L.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, X.-F.; Liu, C.-S.; Wen, Z.; Du, J.-L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 2012, 23, 1189–1202. [Google Scholar] [CrossRef]
- Sipe, G.O.; Lowery, R.L.; Tremblay, M.-E.; Kelly, E.A.; LaMantia, C.E.; Majewska, A.K. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 2016, 7, 10905. [Google Scholar] [CrossRef] [PubMed]
- Wake, H.; Moorhouse, A.J.; Miyamoto, A.; Nabekura, J. Microglia: Actively surveying and shaping neuronal circuit structure and function. Trends Neurosci. 2013, 36, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hoeffel, G.; Chen, J.; Lavin, Y.; Low, N.; Almeida, F.F.; See, P.; Beaudin, A.E.; Lum, J.; Low, I.; Forsberg, E.C.; et al. C-Myb(+) erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 2015, 42, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Perdiguero, E.; Geissmann, F. The development and maintenance of resident macrophages. Nat. Immunol. 2016, 17, 2–8. [Google Scholar] [CrossRef]
- Greter, M.; Lelios, I.; Pelczar, P.; Hoeffel, G.; Price, J.; Leboeuf, M.; Kündig, T.M.; Frei, K.; Ginhoux, F.; Merad, M.; et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 2012, 37, 1050–1060. [Google Scholar] [CrossRef]
- Wang, Y.; Szretter, K.; Vermi, W.; Gilfillan, S.; Rossini, C.; Cella, M.; Barrow, A.; Diamond, M.S.; Colonna, M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012, 13, 753–760. [Google Scholar] [CrossRef]
- Askew, K.E.; Li, K.; Olmos-Alonso, A.; García-Moreno, F.; Liang, Y.; Richardson, P.; Tipton, T.; Chapman, M.; Riecken, K.; Beccari, S.; et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 2017, 18, 391–405. [Google Scholar] [CrossRef]
- Bruttger, J.; Karram, K.; Wörtge, S.; Regen, T.; Marini, F.; Hoppmann, N.; Klein, M.; Blank, T.; Yona, S.; Wolf, Y.; et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 2015, 43, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; Staszewski, O.; et al. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Braak, H.; Xue, Q.-S.; Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. (Berl.) 2009, 118, 475–485. [Google Scholar] [CrossRef]
- Kanazawa, M.; Ninomiya, I.; Hatakeyama, M.; Takahashi, T.; Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. IJMS 2017, 18, 2135. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Chu, H.X.; Broughton, B.R.S.; Kim, H.A.; Lee, S.; Drummond, G.R.; Sobey, C.G. Evidence that Ly6ChiMonocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke 2015, 46, 1929–1937. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.-Y. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Xiong, X.-Y.; Liu, L.; Yang, Q.-W. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog. Neurobiol. 2016, 142, 23–44. [Google Scholar] [CrossRef]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; E Dailey, M.; Gan, W.-B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef]
- Jonas, R.A.; Yuan, T.-F.; Liang, Y.-X.; Jonas, J.B.; Tay, D.K.C.; Ellis-Behnke, R. The spider effect: Morphological and orienting classification of microglia in response to stimuli in vivo. PLoS ONE 2012, 7, e30763. [Google Scholar] [CrossRef] [PubMed]
- Var, S.R.; Byrd-Jacobs, C.A. Microglial response patterns following damage to the zebrafish olfactory bulb. IBRO Rep. 2019, 7, 70–79. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Harry, G.J.; Kraft, A.D. Microglia in the developing brain: A potential target with lifetime effects. Neurotoxicology 2012, 33, 191–206. [Google Scholar] [CrossRef]
- Wattananit, S.; Tornero, D.; Graubardt, N.; Memanishvili, T.; Monni, E.; Tatarishvili, J.; Miskinyte, G.; Ge, R.; Ahlenius, H.; Lindvall, O.; et al. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J. Neurosci. 2016, 36, 4182–4195. [Google Scholar] [CrossRef]
- Ganzen, L.; Venkatraman, P.; Pang, C.P.; Leung, Y.F.; Zhang, M. Utilizing zebrafish visual behaviors in drug screening for retinal degeneration. Int. J. Mol. Sci. 2017, 18, 1185. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Sakai, C.; Ijaz, S.; Hoffman, E.J. Zebrafish models of neurodevelopmental disorders: Past, present, and future. Front. Mol. Neurosci. 2018, 11, 294. [Google Scholar] [CrossRef]
- Chapouton, P.; Jagasia, R.; Bally-Cuif, L. Adult neurogenesis in non-mammalian vertebrates. BioEssays 2007, 29, 745–757. [Google Scholar] [CrossRef]
- Diotel, N.; Viales, R.R.; Armant, O.; März, M.; Ferg, M.; Rastegar, S.; Strähle, U. Comprehensive expression map of transcription regulators in the adult zebrafish telencephalon reveals distinct neurogenic niches. J. Comp. Neurol. 2015, 523, 1202–1221. [Google Scholar] [CrossRef]
- Kaslin, J.; Ganz, J.; Brand, M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.M.; Belluscio, L. Continuous neural plasticity in the olfactory intrabulbar circuitry. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
- Marks, C.; Cheng, K.; Cummings, D.M.; Belluscio, L. Activity-dependent plasticity in the olfactory intrabulbar map. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 11257–11266. [Google Scholar] [CrossRef]
- Whitlock, K.E. The sense of scents: Olfactory behaviors in the zebrafish. Zebrafish 2006, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Zambusi, A.; Ninkovic, J. Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J. Stem Cells 2020, 12, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Porrello, E.R.; Tan, W.; Mahmoud, A.I.; Hill, J.A.; Bassel-Duby, R.; Sadek, H.A.; Olson, E.N. Macrophages are required for neonatal heart regeneration. J. Clin. Investig. 2014, 124, 1382–13192. [Google Scholar] [CrossRef]
- Petrie, T.A.; Strand, N.S.; Tsung-Yang, C.; Rabinowitz, J.S.; Moon, R. Macrophages modulate adult zebrafish tail fin regeneration. Dev. (Camb. Engl.) 2014, 141, 2581–2591. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhé, B.; Trávníčková, J.; Luz-Crawford, P.; Tejedor, G.; Phan, Q.T.; Duroux-Richard, I.; Levraud, J.-P.; Kissa, K.; Lutfalla, G.; et al. Identification of polarized macrophage subsets in zebrafish. eLife 2015, 4, e07288. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.V.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef]
- Bevan, L.; Lim, Z.W.; Venkatesh, B.; Riley, P.R.; Martin, P.; Richardson, R.J. Specific macrophage populations promote both cardiac scar deposition and subsequent resolution in adult zebrafish. Cardiovasc. Res. 2020, 116, 1357–1371. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.-S.D.P.; Bise, T.; Baier, F.; Marro, J.; Jaźwińska, A. Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart. Open Biol. 2016, 6, 160102. [Google Scholar] [CrossRef]
- Huang, W.-C.; Yang, C.-C.; Chen, I.-H.; Liu, Y.-M.L.; Chang, S.-J.; Chuang, Y.-J. Treatment of glucocorticoids inhibited early immune responses and impaired cardiac repair in adult zebrafish. PLoS ONE 2013, 8, e66613. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, H.-Y.; Kim, A.R.; Kim, W.-H.; Cho, H.; Um, J.; Seo, Y.; Kang, W.S.; Jin, S.-W.; Kim, M.C.; et al. Natural product derivative BIO promotes recovery after myocardial infarction via unique modulation of the cardiac microenvironment. Sci. Rep. 2016, 6, 30726. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-L.; Marín-Juez, R.; Moura, P.L.; Kuenne, C.; Lai, J.K.H.; Tsedeke, A.T.; Guenther, S.; Looso, M.; Stainier, D.Y. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.; Rao, K.S.; Opie, G.W.; Kühn, B.; Tsang, M. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 2018, 145, dev157206. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Morejón, A.; García-Redondo, A.B.; Reuter, H.; Marques, I.J.; Bates, T.; Galardi-Castilla, M.; Große, A.; Manig, S.; Langa, X.; Ernst, A.; et al. Wilms tumor 1b expression defines a pro-regenerative macrophage subtype and is required for organ regeneration in the zebrafish. Cell Rep. 2019, 28, 1296–1306.e6. [Google Scholar] [CrossRef]
- Simões, F.C.; Cahill, T.J.; Kenyon, A.; Gavriouchkina, D.; Vieira, J.M.; Sun, X.; Pezzolla, D.; Ravaud, C.; Masmanian, E.; Weinberger, M.; et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 2020, 11, 600. [Google Scholar] [CrossRef]
- Tahara, N.; Brush, M.; Kawakami, Y. Cell migration during heart regeneration in zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2016, 245, 774–787. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Shi, Y.; Zhang, W.-Q.; Wen, Z. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360. [Google Scholar] [CrossRef]
- Loynes, C.A.; Martin, J.S.; Robertson, A.; Trushell, D.M.I.; Ingham, P.W.; Whyte, M.K.B.; Renshaw, S.A. Pivotal advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J. Leukoc. Biol. 2010, 87, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Geurtzen, K.; Vernet, A.; Freidin, A.; Knopf, F.; Hofbauer, L.C.; Rauner, M.; E Schneider, J.; Brand, M. Immune suppressive and bone inhibitory effects of prednisolone in growing and regenerating zebrafish tissues. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Geurtzen, K.; Knopf, F. Adult zebrafish injury models to study the effects of prednisolone in regenerating bone tissue. J. Vis. Exp. 2018, 58429. [Google Scholar] [CrossRef] [PubMed]
- A Carrillo, S.; Anguita-Salinas, C.; A Peña, O.; Morales, R.A.; Muñoz-Sánchez, S.; Muñoz-Montecinos, C.; Paredes-Zúñiga, S.; Tapia, K.; Allende, M.L. Macrophage recruitment contributes to regeneration of mechanosensory hair cells in the zebrafish lateral line. J. Cell. Biochem. 2016, 117, 1880–1889. [Google Scholar] [CrossRef]
- Hirose, K.; Rutherford, M.A.; Warchol, M.E. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear. Res. 2017, 352, 70–81. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Thompson, D.L.; Kuah, M.-K.; Wong, K.-L.; Wu, K.L.; Linn, S.A.; Jewett, E.M.; Shu-Chien, A.C.; Barald, K.F. The cytokine macrophage migration inhibitory factor (MIF) acts as a neurotrophin in the developing inner ear of the zebrafish, Danio rerio. Dev. Biol. 2012, 363, 84–94. [Google Scholar] [CrossRef]
- Weber, L.J.; Marcy, H.K.; Shen, Y.-C.; Tomkovich, S.; Brooks, K.M.; Hilk, K.E.; Barald, K.F. The role of jab1, a putative downstream effector of the neurotrophic cytokine macrophage migration inhibitory factor (MIF) in zebrafish inner ear hair cell development. Exp. Neurol. 2018, 301, 100–109. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.-P.; Li, Y.-J.; Wang, M.; Chen, L.; Hu, B. Suppression of inflammation delays hair cell regeneration and functional recovery following lateral line damage in zebrafish larvae. bioRxiv 2020, 962753. [Google Scholar] [CrossRef]
- Bollaerts, I.; Van Houcke, J.; Beckers, A.; Lemmens, K.; Vanhunsel, S.; De Groef, L.; Moons, L. Prior exposure to immunosuppressors sensitizes retinal microglia and accelerates optic nerve regeneration in zebrafish. Mediat. Inflamm. 2019, 2019, 6135795. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Sun, C.; Hunter, S.S.; New, D.D.; Stenkamp, D.L. Regeneration associated transcriptional signature of retinal microglia and macrophages. Sci. Rep. 2019, 9, 4768. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ueda, Y.; Ohshima, T. Wnt signaling regulates proliferation and differentiation of radial glia in regenerative processes after stab injury in the optic tectum of adult zebrafish. Glia 2018, 66, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.J.; Nagashima, M.; Li, J.; Kakuk-Atkins, L.; Ashrafzadeh, M.; Hyde, D.R.; Hitchcock, P.F. Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish. Glia 2020, 68, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Green, L.A.; Nebiolo, J.C.; Smith, C.J. Microglia exit the CNS in spinal root avulsion. PLoS Biol. 2019, 17, e3000159. [Google Scholar] [CrossRef]
- Morsch, M.; Radford, R.; Lee, A.; Don, E.K.; Badrock, A.P.; Hall, T.; Cole, N.; Chung, R. In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front. Cell. Neurosci. 2015, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, J.; Yang, Y.-J.; Maurer, G.W.; Barreiro-Iglesias, A.; Tsarouchas, T.M.; Wehner, D.; Sieger, D.; Becker, T.; Becker, T. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Development 2016, 143, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Becker, T. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J. Comp. Neurol. 2001, 433, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hui, S.P. Axonal regeneration in zebrafish spinal cord. Regen. Oxf. Engl. 2018, 5, 43–60. [Google Scholar] [CrossRef]
- Peng, S.-X.; Yao, L.; Cui, C.; Zhao, H.-D.; Liu, C.-J.; Li, Y.-H.; Wang, L.-F.; Huang, S.-B.; Shen, Y.-Q. Semaphorin4D promotes axon regrowth and swimming ability during recovery following zebrafish spinal cord injury. Neuroscience 2017, 351, 36–46. [Google Scholar] [CrossRef]
- Strand, N.S.; Hoi, K.K.; Phan, T.M.; Ray, C.A.; Berndt, J.D.; Moon, R. Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem. Biophys. Res. Commun. 2016, 477, 952–956. [Google Scholar] [CrossRef]
- Baumgart, E.V.; Barbosa, J.S.; Bally-Cuif, L.; Götz, M.; Ninkovic, J. Stab wound injury of the zebrafish telencephalon: A model for comparative analysis of reactive gliosis. Glia 2011, 60, 343–357. [Google Scholar] [CrossRef]
- Caldwell, L.J.; Davies, N.O.; Cavone, L.; Mysiak, K.S.; Semenova, S.A.; Panula, P.; Armstrong, D.; Becker, T.; Becker, T. Regeneration of dopaminergic neurons in adult zebrafish depends on immune system activation and differs for distinct populations. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 4694–4713. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- März, M.; Schmidt, R.; Rastegar, S.; Strahle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 2011, 240, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Oosterhof, N.; Holtman, I.R.; Kuil, L.E.; Van Der Linde, H.C.; Boddeke, E.W.; Eggen, B.J.; Van Ham, T.J. Identification of a conserved and acute neurodegeneration-specific microglial transcriptome in the zebrafish. Glia 2017, 65, 138–149. [Google Scholar] [CrossRef]
- Yin, G.; Du, M.; Li, R.; Li, K.; Huang, X.; Duan, D.; Ai, X.; Yao, F.; Zhang, L.; Hu, Z.; et al. Glia maturation factor beta is required for reactive gliosis after traumatic brain injury in zebrafish. Exp. Neurol. 2018, 305, 129–138. [Google Scholar] [CrossRef]
- Briona, L.K.; Poulain, F.E.; Mosimann, C.; Dorsky, R.I. Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev. Biol. 2015, 403, 15–21. [Google Scholar] [CrossRef]
- Hackam, A.S.; Garcia, A.L.; Udeh, A.; Kalahasty, K. A growing field: The regulation of axonal regeneration by Wnt signaling. Neural Regen. Res. 2018, 13, 43–52. [Google Scholar] [CrossRef]
- McPherson, A.D.; Barrios, J.P.; Luks-Morgan, S.J.; Manfredi, J.P.; Bonkowsky, J.L.; Douglass, A.D.; Dorsky, R.I. Motor behavior mediated by continuously generated dopaminergic neurons in the zebrafish hypothalamus recovers after cell ablation. Curr. Biol. 2016, 26, 263–269. [Google Scholar] [CrossRef]
- Bhattarai, P.; Thomas, A.K.; Cosacak, M.I.; Papadimitriou, C.; Mashkaryan, V.; Froc, C.; Reinhardt, S.; Kurth, T.; Dahl, A.; Zhang, Y.; et al. IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon amyloid-β42 aggregation in adult zebrafish brain. Cell Rep. 2016, 17, 941–948. [Google Scholar] [CrossRef]
- Bhattarai, P.; Thomas, A.K.; Cosacak, M.I.; Papadimitriou, C.; Mashkaryan, V.; Zhang, Y.; Kizil, C. Modeling amyloid-β42 toxicity and neurodegeneration in adult zebrafish brain. J. Vis. Exp. 2017, 56014. [Google Scholar] [CrossRef]
- Bhattarai, P.; Cosacak, M.I.; Mashkaryan, V.; Demir, S.; Popova, S.D.; Govindarajan, N.; Brandt, K.; Zhang, Y.; Chang, W.; Ampatzis, K.; et al. Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer’s model of adult zebrafish brain. PLoS Biol. 2020, 18, e3000585. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, L.; Kordes, S.; Reinhardt, P.; Glatza, M.; Baumann, M.; Drexler, H.C.; Menninger, S.; Zischinsky, G.; Eickhoff, J.; Fröb, C.; et al. Dual inhibition of gsk3β and cdk5 protects the cytoskeleton of neurons from neuroinflammatory-mediated degeneration in vitro and in vivo. Stem Cell Rep. 2019, 12, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Cosacak, M.I.; Papadimitriou, C.; Kizil, C. Regeneration, plasticity, and induced molecular programs in adult zebrafish brain. BioMed Res. Int. 2015, 2015, 769763. [Google Scholar] [CrossRef] [PubMed]
- Kizil, C.; Dudczig, S.; Kyritsis, N.; Machate, A.; Blaesche, J.; Kroehne, V.; Brand, M. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 2012, 7, 27. [Google Scholar] [CrossRef]
- Kizil, C.; Kyritsis, N.; Dudczig, S.; Kroehne, V.; Freudenreich, D.; Kaslin, J.; Brand, M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of gata3. Dev. Cell 2012, 23, 1230–1237. [Google Scholar] [CrossRef]
- Urbã¡n, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef]
- Blume, Z.I.; Lambert, J.M.; Lovel, A.G.; Mitchell, D.M. Microglia in the developing retina couple phagocytosis with the progression of apoptosis via P2RY12 signaling. Dev. Dyn. 2020, 249, 723–740. [Google Scholar] [CrossRef]
- Hamilton, N.; Rutherford, H.A.; Petts, J.J.; Isles, H.M.; Weber, T.; Henneke, M.; Gärtner, J.; Dunning, M.J.; Renshaw, S.A. The failure of microglia to digest developmental apoptotic cells contributes to the pathology of RNASET2-deficient leukoencephalopathy. Glia 2020, 68, 1531–1545. [Google Scholar] [CrossRef]
- Sharma, C.; Kang, S.C. Garcinol pacifies acrylamide induced cognitive impairments, neuroinflammation and neuronal apoptosis by modulating GSK signaling and activation of pCREB by regulating cathepsin B in the brain of zebrafish larvae. Food Chem. Toxicol. 2020, 138, 111246. [Google Scholar] [CrossRef]
- Eyo, U.B.; Dailey, M.E. Microglia: Key elements in neural development, plasticity, and pathology. J. Neuroimmune Pharmacol. 2013, 8, 494–509. [Google Scholar] [CrossRef]
- Fang, L.; Miller, Y.I. Regulation of lipid rafts, angiogenesis and inflammation by AIBP. Curr. Opin. Lipidol. 2019, 30, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Svahn, A.; Graeber, M.B.; Ellett, F.E.; Lieschke, G.J.; Rinkwitz, S.; Bennett, M.R.; Becker, T.S. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev. Neurobiol. 2013, 73, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Babin, P.; Goizet, C.; Raldúa, D. Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog. Neurobiol. 2014, 118, 36–58. [Google Scholar] [CrossRef]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C. Modeling environmentally-induced motor neuron degeneration in zebrafish. Sci. Rep. 2018, 8, 4890. [Google Scholar] [CrossRef] [PubMed]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C.A. Investigating microglia during motor neuron degeneration using a zebrafish model. Micron 2020, 133, 102852. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.N.; Franklin, R. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain J. Neurol. 2009, 132 Pt 2, 288–295. [Google Scholar] [CrossRef]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831. [Google Scholar] [CrossRef]
- Barbosa, J.S.; Sanchez-Gonzalez, R.; Di Giaimo, R.; Baumgart, E.V.; Theis, F.J.; Götz, M.; Ninkovic, J. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 2015, 348, 789. [Google Scholar] [CrossRef]
- Moritz, C.; Berardi, F.; Abate, C.; Peri, F. Live imaging reveals a new role for the sigma-1 (σ1) receptor in allowing microglia to leave brain injuries. Neurosci. Lett. 2015, 591, 13–18. [Google Scholar] [CrossRef]
- Oosterhof, N.; Boddeke, E.; Van Ham, T.J. Immune cell dynamics in the CNS: Learning from the zebrafish. Glia 2015, 63, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.M.; Voigt, M.M. Peripheral glia have a pivotal role in the initial response to axon degeneration of peripheral sensory neurons in zebrafish. PLoS ONE 2014, 9, e103283. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Gokhan, S.; Dai, X.; Wei, S.; Enikolopov, G.N.; Lin, H.; Mehler, M.F.; Stanley, R. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 2012, 367, 100–113. [Google Scholar] [CrossRef]
- Pons, V.; Laflamme, N.; Préfontaine, P.; Rivest, S. Role of macrophage colony-stimulating factor receptor on the proliferation and survival of microglia following systemic nerve and cuprizone-induced injuries. Front. Immunol. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Stanley, E.R.; Chitu, V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 2014, 6, a021857. [Google Scholar] [CrossRef]
- Pons, V.; Rivest, S. New therapeutic avenues of mCSF for brain diseases and injuries. Front. Cell. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef]
- Najafi, A.R.; Crapser, J.; Jiang, S.; Ng, W.; Mortazavi, A.; West, B.L.; Green, K. A limited capacity for microglial repopulation in the adult brain. Glia 2018, 66, 2385–2396. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Xiong, S.; Sun, F.; Qin, G.; Hu, G.; Wang, J.; Zhao, L.; Liang, Y.-X.; Wu, T.; et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 2018, 21, 530–540. [Google Scholar] [CrossRef]
- Li, Q.; Lan, X.; Han, X.; Wang, J. Expression of Tmem119/Sall1 and Ccr2/CD69 in FACS-sorted microglia- and monocyte/macrophage-enriched cell populations after intracerebral hemorrhage. Front. Cell. Neurosci. 2019, 12, 520. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Lee, R.J.; West, B.; Green, K. Characterizing newly repopulated microglia in the adult mouse: Impacts on animal behavior, cell morphology, and neuroinflammation. PLoS ONE 2015, 10, e0122912. [Google Scholar] [CrossRef]
- Cherry, J.D.; Tripodis, Y.; Alvarez, V.E.; Huber, B.; Kiernan, P.T.; Daneshvar, D.; Mez, J.; Montenigro, P.; Solomon, T.M.; Alosco, M.L.; et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Nagamoto-Combs, K.; McNeal, D.W.; Morecraft, R.J.; Combs, C.K. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J. Neurotrauma 2007, 24, 1719–1742. [Google Scholar] [CrossRef] [PubMed]
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383. [Google Scholar] [CrossRef]
- Smith, D.H.; Chen, X.-H.; Pierce, J.E.S.; Wolf, J.A.; Trojanowski, J.Q.; Graham, D.I.; McIntosh, T.K. Progressive atrophy and neuron death for one year following brain trauma in the rat. J. Neurotrauma 1997, 14, 715–727. [Google Scholar] [CrossRef]
- Rice, R.A.; Pham, J.; Lee, R.J.; Najafi, A.R.; West, B.; Green, K. Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia 2017, 65, 931–944. [Google Scholar] [CrossRef]
- Caggiano, A.; Brunjes, P. Microglia and the developing olfactory bulb. Neuroscience 1993, 52, 717–724. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chien, H.F.; Jiangshieh, Y.F.; Wu, C.H. Microglia in the olfactory bulb of rats during postnatal development and olfactory nerve injury with zinc sulfate: A lectin labeling and ultrastrucutural study. Neurosci. Res. 2003, 45, 325–333. [Google Scholar] [CrossRef]
- Kulbe, J.R.; Hall, E.D. Chronic traumatic encephalopathy-integration of canonical traumatic brain injury secondary injury mechanisms with tau pathology. Prog. Neurobiol. 2017, 158, 15–44. [Google Scholar] [CrossRef]
- Scheib, J.; Byrd-Jacobs, C. Zebrafish astroglial morphology in the olfactory bulb is altered with repetitive peripheral damage. Front. Neuroanat. 2020, 14, 4. [Google Scholar] [CrossRef]
- Kotter, M.R.N.; Zhao, C.; Van Rooijen, N.; Franklin, R. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 2005, 18, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.; Mehalow, A.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates cns synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.E.L.; Calinescu, A.-A.; Hitchcock, P.F. Identification of the molecular signatures integral to regenerating photoreceptors in the retina of the zebra fish. J. Ocul. Biol. Dis. Inform. 2008, 1, 73–84. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, J.F.; Poché, R.A. Awakening the regenerative potential of the mammalian retina. Development 2019, 146, dev182642. [Google Scholar] [CrossRef]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T.A. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13717–13722. [Google Scholar] [CrossRef]
- White, D.T.; Sengupta, S.; Saxena, M.T.; Xu, Q.; Hanes, J.; Ding, D.; Ji, H.; Mumm, J.S. Immunomodulation-accelerated neuronal regeneration following selective rod photoreceptor cell ablation in the zebrafish retina. Proc. Natl. Acad. Sci. USA 2017, 114, E3719–E3728. [Google Scholar] [CrossRef]
- Rappert, A.; Bechmann, I.; Pivneva, T.; Mahlo, J.; Biber, K.; Nolte, C.; Kovac, A.D.; Gerard, C.; Boddeke, H.W.G.M.; Nitsch, R.; et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J. Neurosci. 2004, 24, 8500–8509. [Google Scholar] [CrossRef]
- Cullheim, S.; Thams, S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res. Rev. 2007, 55, 89–96. [Google Scholar] [CrossRef]
- Streit, W.J. Microglia and neuroprotection: Implications for Alzheimer’s disease. Brain Res. Rev. 2005, 48, 234–239. [Google Scholar] [CrossRef]
- Pisanu, A.; Lecca, D.; Mulas, G.; Wardas, J.; Simbula, G.; Spiga, S.; Carta, A. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γ agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson’s disease. Neurobiol. Dis. 2014, 71, 280–291. [Google Scholar] [CrossRef]
- Qin, M.; Wong, A.; Séguin, D.; Gerlai, R. Induction of social behavior in zebrafish: Live versus computer animated fish as stimuli. Zebrafish 2014, 11, 185–197. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, P.; Thomas, A.K.; Zhang, Y.; Kizil, C. The effects of aging on Amyloid-β42-induced neurodegeneration and regeneration in adult zebrafish brain. Neurogenesis 2017, 4, e1322666. [Google Scholar] [CrossRef] [PubMed]
- Ellett, F.E.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef]
- Mazzolini, J.; Le Clerc, S.; Morisse, G.; Coulonges, C.; Kuil, L.E.; Van Ham, T.J.; Zagury, J.; Sieger, D. Gene expression profiling reveals a conserved microglia signature in larval zebrafish. Glia 2020, 68, 298–315. [Google Scholar] [CrossRef]
- Kim, M.J.; Kang, K.H.; Kim, C.-H.; Choi, S.-Y. Real-time imaging of mitochondria in transgenic zebrafish expressing mitochondrially targeted GFP. Biotechniques 2008, 45, 331–334. [Google Scholar] [CrossRef]
- O’Donnell, K.C.; Vargas, M.E.; Sagasti, A. WldS and PGC-1α regulate mitochondrial transport and oxidation state after axonal injury. J. Neurosci. 2013, 33, 14778–14790. [Google Scholar] [CrossRef]
- Villegas, R.; Martin, S.M.; O’Donnell, K.C.; A Carrillo, S.; Sagasti, A.; Allende, M.L. Dynamics of degeneration and regeneration in developing zebrafish peripheral axons reveals a requirement for extrinsic cell types. Neural Dev. 2012, 7, 19. [Google Scholar] [CrossRef]
| Tissue | Life Stage | Cell Type | References |
|---|---|---|---|
| Heart | Adult | Macrophage | [91,92,93,94,95,96,97,98,99] |
| Fin | Larvae | Macrophage | [89,97,100,101], |
| Fin | Adult | Macrophage | [87,97,102,103] |
| Hair Cell | Larvae | Macrophage | [104,105,106,107,108] |
| Retina | Adult | Microglia | [109,110,111,112] |
| Spinal Cord | Larvae | Microglia | [90,113,114,115] |
| Spinal Cord | Adult | Microglia | [116,117,118,119] |
| Brain (telencephalon, olfactory bulb) | Adult | Microglia | [72,85,120,121,122,123,124,125] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Var, S.R.; Byrd-Jacobs, C.A. Role of Macrophages and Microglia in Zebrafish Regeneration. Int. J. Mol. Sci. 2020, 21, 4768. https://doi.org/10.3390/ijms21134768
Var SR, Byrd-Jacobs CA. Role of Macrophages and Microglia in Zebrafish Regeneration. International Journal of Molecular Sciences. 2020; 21(13):4768. https://doi.org/10.3390/ijms21134768
Chicago/Turabian StyleVar, Susanna R., and Christine A. Byrd-Jacobs. 2020. "Role of Macrophages and Microglia in Zebrafish Regeneration" International Journal of Molecular Sciences 21, no. 13: 4768. https://doi.org/10.3390/ijms21134768
APA StyleVar, S. R., & Byrd-Jacobs, C. A. (2020). Role of Macrophages and Microglia in Zebrafish Regeneration. International Journal of Molecular Sciences, 21(13), 4768. https://doi.org/10.3390/ijms21134768