Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections
Abstract
1. Introduction
2. General Concepts on Vimentin Structure and Assembly
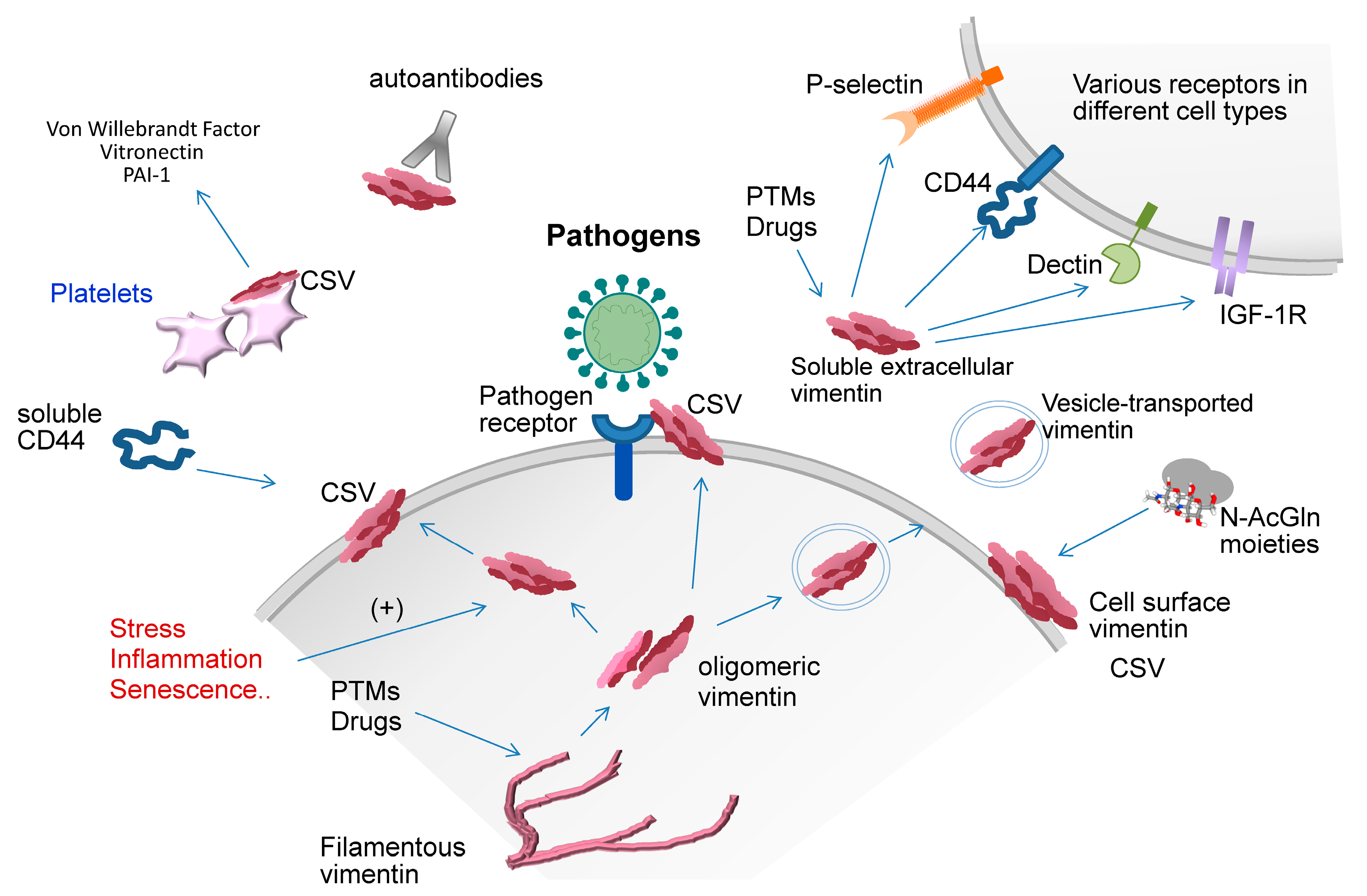
3. Extracellular Vimentin
4. Vimentin in Tissue Damage and Repair
5. Vimentin in Immune Responses
6. Vimentin in Host-Pathogen Interactions
6.1. Bacterial Infections
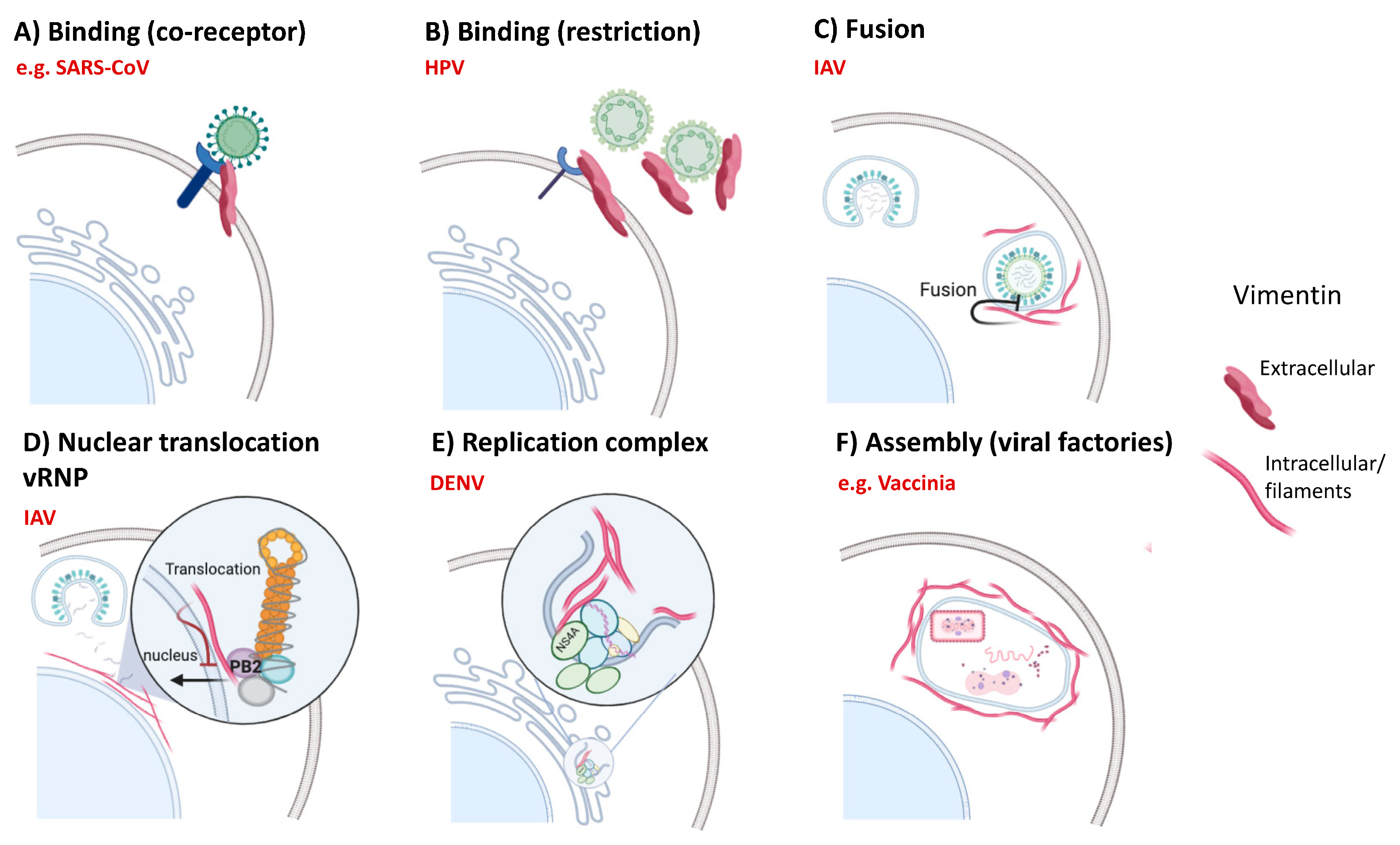
6.2. Viral Infections
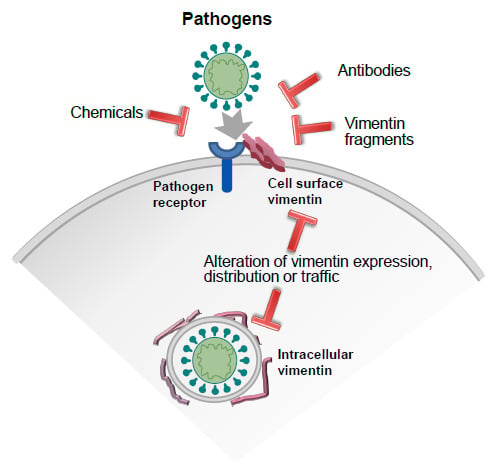
7. Strategies to Modulate Vimentin Function: Focus on Extracellular Vimentin-Pathogen Interactions
7.1. Anti-Vimentin Antibodies
7.2. Chemical Agents
7.3. Other Strategies
8. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin converting enzyme |
| AVA | anti-vimentin autoantibodies |
| BALF | bronchoaveolar lavage fluid |
| CHO | Chinese hamster ovary |
| CSV | cell surface vimentin |
| DENV | dengue virus |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| HMEC | human microvascular endothelial cells |
| HUVEC | human umbilical endothelial cells |
| IAV | influenza A virus |
| ICAM-1 | intercellular adhesion molecule 1 |
| IGF-1R | insulin-like growth factor receptor 1 |
| LAMP1 | lysosomal-associated membrane protein 1 |
| LPS | bacterial lipopolycaccharide |
| mAB | monoclonal antibody |
| N-AcGln | N-acetylglucosamine |
| NRLP3 | nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 |
| NS4A | non-structural protein 4A |
| PAI-1 | plasminogen activator inhibitor 1 |
| PSLG-1 | P-selectin glycoprotein ligand 1 |
| PTM | posttranslational modification |
| PRR | pattern recognition receptor |
| RA | rheumatoid arthritis |
| RIG-I | retinoic acid-inducible gene I |
| ROS | reactive oxygen species |
| SARS-CoV | severe acute respiratory syndrome-related coronavirus |
| TMPRSS2 | transmembrane protease, serine 2 |
| ULF | unit-length filament |
| VCAM-1 | vascular cell adhesion molecule 1 |
| vRNP | viral ribonucleoprotein |
| VWF | Von Willebrandt Factor |
| WFA | withaferin A |
References
- Gan, Z.; Ding, L.; Burckhardt, C.J.; Lowery, J.; Zaritsky, A.; Sitterley, K.; Mota, A.; Costigliola, N.; Starker, C.G.; Voytas, D.F.; et al. Vimentin Intermediate Filaments Template Microtubule Networks to Enhance Persistence in Cell Polarity and Directed Migration. Cell Syst. 2016, 3, 252–263 e258. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Cytoplasmic Intermediate Filaments in Cell Biology. Annu Rev. Cell Dev. Biol 2018, 34, 1–28. [Google Scholar] [CrossRef]
- Duarte, S.; Viedma-Poyatos, A.; Navarro-Carrasco, E.; Martinez, A.E.; Pajares, M.A.; Perez-Sala, D. Vimentin filaments interact with the actin cortex in mitosis allowing normal cell division. Nat. Commun. 2019, 10, 4200. [Google Scholar] [CrossRef] [PubMed]
- Styers, M.L.; Salazar, G.; Love, R.; Peden, A.A.; Kowalczyk, A.P.; Faundez, V. The endo-lysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol. Biol. Cell 2004, 15, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Garzón, B.; Carrasco, M.J.; Cañada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 7287. [Google Scholar] [CrossRef] [PubMed]
- Chernoivanenko, I.S.; Matveeva, E.A.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Mitochondrial membrane potential is regulated by vimentin intermediate filaments. FASEB J. 2015, 29, 820–827. [Google Scholar] [CrossRef]
- Watabe, M.; Nakaki, T. Protein kinase CK2 regulates the formation and clearance of aggresomes in response to stress. J. Cell Sci. 2011, 124, 1519–1532. [Google Scholar] [CrossRef]
- Patteson, A.E.; Vahabikashi, A.; Pogoda, K.; Adam, S.A.; Mandal, K.; Kittisopikul, M.; Sivagurunathan, S.; Goldman, A.; Goldman, R.D.; Janmey, P.A. Vimentin protects cells against nuclear rupture and DNA damage during migration. J. Cell Biol. 2019, 218, 4079–4092. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Caldeira Araujo, H.; Lautenschlager, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef]
- Eckes, B.; Colucci-Guyon, E.; Smola, H.; Nodder, S.; Babinet, C.; Krieg, T.; Martin, P. Impaired wound healing in embryonic and adult mice lacking vimentin. J. Cell Sci. 2000, 113 (Pt. 13), 2455–2462. [Google Scholar]
- Dos Santos, G.; Rogel, M.R.; Baker, M.A.; Troken, J.R.; Urich, D.; Morales-Nebreda, L.; Sennello, J.A.; Kutuzov, M.A.; Sitikov, A.; Davis, J.M.; et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6574. [Google Scholar] [CrossRef] [PubMed]
- Haversen, L.; Sundelin, J.P.; Mardinoglu, A.; Rutberg, M.; Stahlman, M.; Wilhelmsson, U.; Hulten, L.M.; Pekny, M.; Fogelstrand, P.; Bentzon, J.F.; et al. Vimentin deficiency in macrophages induces increased oxidative stress and vascular inflammation but attenuates atherosclerosis in mice. Sci. Rep. 2018, 8, 16973. [Google Scholar] [CrossRef] [PubMed]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin is secreted by activated macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Divanyan, A.; Jourd’heuil, F.L.; Goldman, R.D.; Ridge, K.M.; Jourd’heuil, D.; Lopez-Soler, R.I. Vimentin expression is required for the development of EMT-related renal fibrosis following unilateral ureteral obstruction in mice. Am. J. Physiol. Ren. Physiol. 2018, 315, F769–F780. [Google Scholar] [CrossRef] [PubMed]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Dsouza, K.; Thomas, V.; Mirov, S.; Perez-Sala, D.; Athar, M.; Thannickal, V.J.; et al. Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Lin, H.H.; Tang, M.J.; Wang, Y.K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. [Google Scholar] [CrossRef]
- Richardson, A.M.; Havel, L.S.; Koyen, A.E.; Konen, J.M.; Shupe, J.; Wiles, W.G.T.; Martin, W.D.; Grossniklaus, H.E.; Sica, G.; Gilbert-Ross, M.; et al. Vimentin Is Required for Lung Adenocarcinoma Metastasis via Heterotypic Tumor Cell-Cancer-Associated Fibroblast Interactions during Collective Invasion. Clin. Cancer Res. 2018, 24, 420–432. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Despres, N.; Lapointe, E.; van der Heijden, A.; Lora, M.; Senshu, T.; van Venrooij, W.J.; Menard, H.A. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res. 2004, 6, R142–R150. [Google Scholar] [CrossRef]
- Du, N.; Cong, H.; Tian, H.; Zhang, H.; Zhang, W.; Song, L.; Tien, P. Cell surface vimentin is an attachment receptor for enterovirus 71. J. Virol. 2014, 88, 5816–5833. [Google Scholar] [CrossRef]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.N.; Bruggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef]
- Strelkov, S.V.; Herrmann, H.; Geisler, N.; Wedig, T.; Zimbelmann, R.; Aebi, U.; Burkhard, P. Conserved segments 1A and 2B of the intermediate filament dimer: Their atomic structures and role in filament assembly. EMBO J. 2002, 21, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Chernyatina, A.A.; Nicolet, S.; Aebi, U.; Herrmann, H.; Strelkov, S.V. Atomic structure of the vimentin central alpha-helical domain and its implications for intermediate filament assembly. Proc. Natl. Acad. Sci. USA 2012, 109, 13620–13625. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.V.; Kreplak, L.; Wedig, T.; Mucke, N.; Svergun, D.I.; Herrmann, H.; Aebi, U.; Strelkov, S.V. Monitoring intermediate filament assembly by small-angle x-ray scattering reveals the molecular architecture of assembly intermediates. Proc. Natl. Acad. Sci. USA 2006, 103, 16206–16211. [Google Scholar] [CrossRef] [PubMed]
- Kirmse, R.; Bouchet-Marquis, C.; Page, C.; Hoenger, A. Three-dimensional cryo-electron microscopy on intermediate filaments. Methods Cell Biol. 2010, 96, 565–589. [Google Scholar] [CrossRef]
- Chernyatina, A.A.; Guzenko, D.; Strelkov, S.V. Intermediate filament structure: The bottom-up approach. Curr. Opin. Cell Biol. 2015, 32, 65–72. [Google Scholar] [CrossRef]
- Kornreich, M.; Avinery, R.; Malka-Gibor, E.; Laser-Azogui, A.; Beck, R. Order and disorder in intermediate filament proteins. FEBS Lett. 2015, 589, 2464–2476. [Google Scholar] [CrossRef]
- Eriksson, J.E.; He, T.; Trejo-Skalli, A.V.; Härmälä-Braskén, A.-S.; Hellman, J.; Chou, Y.-H.; Goldman, R.D. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J. Cell Sci. 2004, 117, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Goldman, R.D. Intermediate filaments mediate cytoskeletal crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Omary, M.B. Post-translational modifications of intermediate filament proteins: Mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Viedma-Poyatos, A.; Pajares, M.A.; Pérez-Sala, D. Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biol. 2020, 101582. [Google Scholar] [CrossRef]
- Chavez, J.; Chung, W.G.; Miranda, C.L.; Singhal, M.; Stevens, J.F.; Maier, C.S. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: Protein carbonylation is diminished by ascorbic acid. Chem. Res. Toxicol. 2010, 23, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mónico, A.; Duarte, S.; Pajares, M.A.; Pérez-Sala, D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019, 23, 101098. [Google Scholar] [CrossRef]
- Tarbet, H.J.; Dolat, L.; Smith, T.J.; Condon, B.M.; O’Brien, E.T., 3rd; Valdivia, R.H.; Boyce, M. Site-specific glycosylation regulates the form and function of the intermediate filament cytoskeleton. eLife 2018, 7, e31807. [Google Scholar] [CrossRef]
- Byun, Y.; Chen, F.; Chang, R.; Trivedi, M.; Green, K.J.; Cryns, V.L. Caspase cleavage of vimentin disrupts intermediate filaments and promotes apoptosis. Cell Death Differ. 2001, 8, 443–450. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, P.; Hu, F.; Yan, R.; He, M.; Li, W.; Xu, J.; Xu, K. Hypotonic Stress Induces Fast, Reversible Degradation of the Vimentin Cytoskeleton via Intracellular Calcium Release. Adv. Sci. (Weinh) 2019, 6, 1900865. [Google Scholar] [CrossRef]
- Singh, B.; Arlinghaus, R.B. Vimentin phosphorylation by p37mos protein kinase in vitro and generation of a 50-kDa cleavage product in v-mos-transformed cells. Virology 1989, 173, 144–156. [Google Scholar] [CrossRef]
- Shoeman, R.L.; Honer, B.; Stoller, T.J.; Kesselmeier, C.; Miedel, M.C.; Traub, P.; Graves, M.C. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc. Natl. Acad. Sci. USA 1990, 87, 6336–6340. [Google Scholar] [CrossRef]
- Frescas, D.; Roux, C.M.; Aygun-Sunar, S.; Gleiberman, A.S.; Krasnov, P.; Kurnasov, O.V.; Strom, E.; Virtuoso, L.P.; Wrobel, M.; Osterman, A.L.; et al. Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proc. Natl. Acad. Sci. USA 2017, 114, E1668–E1677. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.; Tumpara, S.; Wiese, M.; Wrenger, S.; Vijayan, V.; Gueler, F.; Chen, R.; Madyaningrana, K.; Mahadeva, R.; Welte, T.; et al. Alpha1-antitrypsin binds hemin and prevents oxidative activation of human neutrophils: Putative pathophysiological significance. J. Leukoc Biol. 2017, 102, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; He, L.; Huang, S.H. Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem. Biophys. Res. Commun. 2006, 351, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Pall, T.; Pink, A.; Kasak, L.; Turkina, M.; Anderson, W.; Valkna, A.; Kogerman, P. Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS ONE 2011, 6, e29305. [Google Scholar] [CrossRef] [PubMed]
- Da, Q.; Behymer, M.; Correa, J.I.; Vijayan, K.V.; Cruz, M.A. Platelet adhesion involves a novel interaction between vimentin and von Willebrand factor under high shear stress. Blood 2014, 123, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; deWaal, R.M.; Mor-Vaknin, N.; Hibbard, C.; Markovitz, D.M.; Kahn, M.L. The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol. Cell Biol. 2004, 24, 9198–9206. [Google Scholar] [CrossRef]
- Kim, S.; Cho, W.; Kim, I.; Lee, S.H.; Oh, G.T.; Park, Y.M. Oxidized LDL induces vimentin secretion by macrophages and contributes to atherosclerotic inflammation. J. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Avram, D.; Romijn, E.P.; Pap, E.H.; Heck, A.J.; Wirtz, K.W. Identification of proteins in activated human neutrophils susceptible to tyrosyl radical attack. A proteomic study using a tyrosylating fluorophore. Proteomics 2004, 4, 2397–2407. [Google Scholar] [CrossRef]
- Komura, K.; Ise, H.; Akaike, T. Dynamic behaviors of vimentin induced by interaction with GlcNAc molecules. Glycobiology 2012, 22, 1741–1759. [Google Scholar] [CrossRef]
- Laragione, T.; Gianazza, E.; Tonelli, R.; Bigini, P.; Mennini, T.; Casoni, F.; Massignan, T.; Bonetto, V.; Ghezzi, P. Regulation of redox-sensitive exofacial protein thiols in CHO cells. Biol. Chem. 2006, 387, 1371–1376. [Google Scholar] [CrossRef]
- Checconi, P.; Salzano, S.; Bowler, L.; Mullen, L.; Mengozzi, M.; Hanschmann, E.M.; Lillig, C.H.; Sgarbanti, R.; Panella, S.; Nencioni, L.; et al. Redox proteomics of the inflammatory secretome identifies a common set of redoxins and other glutathionylated proteins released in inflammation, influenza virus infection and oxidative stress. PLoS ONE 2015, 10, e0127086. [Google Scholar] [CrossRef]
- Gronwall, C.; Amara, K.; Hardt, U.; Krishnamurthy, A.; Steen, J.; Engstrom, M.; Sun, M.; Ytterberg, A.J.; Zubarev, R.A.; Scheel-Toellner, D.; et al. Autoreactivity to malondialdehyde-modifications in rheumatoid arthritis is linked to disease activity and synovial pathogenesis. J. Autoimmun. 2017, 84, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Moisan, E.; Girard, D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J. Leukoc Biol. 2006, 79, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Egerer, K.; Gauliard, A.; Luthke, K.; Rudolph, P.E.; Fredenhagen, G.; Berg, W.; Feist, E.; Burmester, G.R. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum 2007, 56, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, E.; Kato, M.; Kudo, Y.; Lee, W.; Hisada, R.; Fujieda, Y.; Oku, K.; Bohgaki, T.; Amengual, O.; Yasuda, S.; et al. Autophagy promotes citrullination of VIM (vimentin) and its interaction with major histocompatibility complex class II in synovial fibroblasts. Autophagy 2020, 16, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Brentville, V.A.; Metheringham, R.L.; Gunn, B.; Symonds, P.; Daniels, I.; Gijon, M.; Cook, K.; Xue, W.; Durrant, L.G. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T cell-mediated antitumor immunity. Cancer Res. 2016, 76, 548–570. [Google Scholar] [CrossRef]
- Deng, L.; Spencer, B.L.; Holmes, J.A.; Mu, R.; Rego, S.; Weston, T.A.; Hu, Y.; Sanches, G.F.; Yoon, S.; Park, N.; et al. The Group B Streptococcal surface antigen I/II protein, BspC, interacts with host vimentin to promote adherence to brain endothelium and inflammation during the pathogenesis of meningitis. PLoS Pathog. 2019, 15, e1007848. [Google Scholar] [CrossRef]
- Hwang, B.; Ise, H. Multimeric conformation of type III intermediate filaments but not the filamentous conformation exhibits high affinity to lipid bilayers. Genes Cells 2020, 25, 413–426. [Google Scholar] [CrossRef]
- Fasipe, T.A.; Hong, S.H.; Da, Q.; Valladolid, C.; Lahey, M.T.; Richards, L.M.; Dunn, A.K.; Cruz, M.A.; Marrelli, S.P. Extracellular Vimentin/VWF (von Willebrand Factor) Interaction Contributes to VWF String Formation and Stroke Pathology. Stroke 2018, 49, 2536–2540. [Google Scholar] [CrossRef]
- Shigyo, M.; Kuboyama, T.; Sawai, Y.; Tada-Umezaki, M.; Tohda, C. Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci. Rep. 2015, 5, 12055. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.S.; Yakubenko, V.P.; Elsori, D.H.; Yadav, S.P.; Willard, B.; Tan, C.D.; Rodriguez, E.R.; Febbraio, M.; Cathcart, M.K. Vimentin is an endogenous ligand for the pattern recognition receptor Dectin-1. Cardiovasc. Res. 2013, 99, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Ise, H.; Kobayashi, S.; Goto, M.; Sato, T.; Kawakubo, M.; Takahashi, M.; Ikeda, U.; Akaike, T. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology 2010, 20, 843–864. [Google Scholar] [CrossRef] [PubMed]
- Ise, H.; Matsunaga, K.; Shinohara, M.; Sakai, Y. Improved Isolation of Mesenchymal Stem Cells Based on Interactions between N-Acetylglucosamine-Bearing Polymers and Cell-Surface Vimentin. Stem Cells Int. 2019, 2019, 4341286. [Google Scholar] [CrossRef]
- Bryant, A.E.; Bayer, C.R.; Huntington, J.D.; Stevens, D.L. Group A streptococcal myonecrosis: Increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J. Infect. Dis. 2006, 193, 1685–1692. [Google Scholar] [CrossRef]
- Yu, Y.T.; Chien, S.C.; Chen, I.Y.; Lai, C.T.; Tsay, Y.G.; Chang, S.C.; Chang, M.F. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 14. [Google Scholar] [CrossRef]
- Cheng, F.; Eriksson, J.E. Intermediate Filaments and the Regulation of Cell Motility during Regeneration and Wound Healing. Cold Spring Harb. Perspect. Biol. 2017, 9, a022046. [Google Scholar] [CrossRef]
- Walker, J.L.; Bleaken, B.M.; Romisher, A.R.; Alnwibit, A.A.; Menko, A.S. In wound repair vimentin mediates the transition of mesenchymal leader cells to a myofibroblast phenotype. Mol. Biol. Cell 2018, 29, 1555–1570. [Google Scholar] [CrossRef]
- Shigyo, M.; Tohda, C. Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci. Rep. 2016, 6, 28293. [Google Scholar] [CrossRef]
- Lam, F.W.; Da, Q.; Guillory, B.; Cruz, M.A. Recombinant Human Vimentin Binds to P-Selectin and Blocks Neutrophil Capture and Rolling on Platelets and Endothelium. J. Immunol. 2018, 200, 1718–1726. [Google Scholar] [CrossRef]
- Gong, D.H.; Dai, Y.; Chen, S.; Wang, X.Q.; Yan, X.X.; Shen, Y.; Liu, J.; Yang, Z.K.; Hu, J.; Yu, L.J.; et al. Secretory vimentin is associated with coronary artery disease in patients and induces atherogenesis in ApoE(-/-) mice. Int. J. Cardiol. 2019, 283, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Barnes, P.F.; Porgador, A.; Roy, S.; Wu, S.; Nanda, J.S.; Griffith, D.E.; Girard, W.M.; Rawal, N.; Shetty, S.; et al. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J. Immunol. 2006, 177, 6192–6198. [Google Scholar] [CrossRef] [PubMed]
- Linder, E.; Helin, H.; Chang, C.M.; Edgington, T.S. Complement-mediated binding of monocytes to intermediate filaments in vitro. Am. J. Pathol. 1983, 112, 267–277. [Google Scholar] [PubMed]
- Linder, E. Binding of C1q and complement activation by vascular endothelium. J. Immunol 1981, 126, 648–658. [Google Scholar]
- Hansson, G.K.; Starkebaum, G.A.; Benditt, E.P.; Schwartz, S.M. Fc-mediated binding of IgG to vimentin-type intermediate filaments in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1984, 81, 3103–3107. [Google Scholar] [CrossRef]
- Ise, H.; Goto, M.; Komura, K.; Akaike, T. Engulfment and clearance of apoptotic cells based on a GlcNAc-binding lectin-like property of surface vimentin. Glycobiology 2012, 22, 788–805. [Google Scholar] [CrossRef] [PubMed]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Pure, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Podor, T.J.; Singh, D.; Chindemi, P.; Foulon, D.M.; McKelvie, R.; Weitz, J.I.; Austin, R.; Boudreau, G.; Davies, R. Vimentin exposed on activated platelets and platelet microparticles localizes vitronectin and plasminogen activator inhibitor complexes on their surface. J. Biol. Chem. 2002, 277, 7529–7539. [Google Scholar] [CrossRef]
- Lazar, M.H.; Christensen, P.J.; Du, M.; Yu, B.; Subbotina, N.M.; Hanson, K.E.; Hansen, J.M.; White, E.S.; Simon, R.H.; Sisson, T.H. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am. J. Respir. Cell Mol. Biol. 2004, 31, 672–678. [Google Scholar] [CrossRef]
- Courey, A.J.; Horowitz, J.C.; Kim, K.K.; Koh, T.J.; Novak, M.L.; Subbotina, N.; Warnock, M.; Xue, B.; Cunningham, A.K.; Lin, Y.; et al. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 2011, 118, 2313–2321. [Google Scholar] [CrossRef]
- Schuliga, M.; Grainge, C.; Westall, G.; Knight, D. The fibrogenic actions of the coagulant and plasminogen activation systems in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2018, 97, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Aguilera-Montilla, N.; Corbi, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, F.R.; Craig, J.M.; Singer, B.D.; Files, D.C.; Mock, J.R.; Garibaldi, B.T.; Fallica, J.; Tripathi, A.; Mandke, P.; Gans, J.H.; et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L733–L746. [Google Scholar] [CrossRef] [PubMed]
- Gindele, J.A.; Mang, S.; Pairet, N.; Christ, I.; Gantner, F.; Schymeinsky, J.; Lamb, D.J. Opposing effects of in vitro differentiated macrophages sub-type on epithelial wound healing. PLoS ONE 2017, 12, e0184386. [Google Scholar] [CrossRef]
- Rogel, M.R.; Soni, P.N.; Troken, J.R.; Sitikov, A.; Trejo, H.E.; Ridge, K.M. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 2011, 25, 3873–3883. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Ramos, I.; Fernandez-Sesma, A. Modulating the Innate Immune Response to Influenza A Virus: Potential Therapeutic Use of Anti-Inflammatory Drugs. Front. Immunol. 2015, 6, 361. [Google Scholar] [CrossRef]
- Stevens, C.; Henderson, P.; Nimmo, E.R.; Soares, D.C.; Dogan, B.; Simpson, K.W.; Barrett, J.C.; Wilson, D.C.; Satsangi, J. The intermediate filament protein, vimentin, is a regulator of NOD2 activity. Gut 2013, 62, 695–707. [Google Scholar] [CrossRef]
- De Rivero Vaccari, J.P.; Minkiewicz, J.; Wang, X.; De Rivero Vaccari, J.C.; German, R.; Marcillo, A.E.; Dietrich, W.D.; Keane, R.W. Astrogliosis involves activation of retinoic acid-inducible gene-like signaling in the innate immune response after spinal cord injury. Glia 2012, 60, 414–421. [Google Scholar] [CrossRef]
- Ramos, I.; Bernal-Rubio, D.; Durham, N.; Belicha-Villanueva, A.; Lowen, A.C.; Steel, J.; Fernandez-Sesma, A. Effects of receptor binding specificity of avian influenza virus on the human innate immune response. J. Virol. 2011, 85, 4421–4431. [Google Scholar] [CrossRef]
- Ramos, I.; Fernandez-Sesma, A. Cell receptors for influenza a viruses and the innate immune response. Front. Microbiol. 2012, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Hebrink, D.; Jenson, P.; Kottom, T.; Limper, A.H. Differential Macrophage Polarization from Pneumocystis in Immunocompetent and Immunosuppressed Hosts: Potential Adjunctive Therapy during Pneumonia. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.B.; Guerra, J.; Firek, A.; Langridge, W.H.R. Extracellular vimentin modulates human dendritic cell activation. Mol. Immunol. 2018, 104, 37–46. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Hyman, C.; Muller, J.T.; Loschi, M.; Thangavelu, G.; Saha, A.; Kumari, S.; Reichenbach, D.K.; Smith, M.J.; Zhang, G.; Koehn, B.H.; et al. The vimentin intermediate filament network restrains regulatory T cell suppression of graft-versus-host disease. J. Clin. Investig. 2018, 128, 4604–4621. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.; Henttinen, T.; Merinen, M.; Marttila-Ichihara, F.; Eriksson, J.E.; Jalkanen, S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006, 8, 156–162. [Google Scholar] [CrossRef]
- Tsui, C.; Maldonado, P.; Montaner, B.; Borroto, A.; Alarcon, B.; Bruckbauer, A.; Martinez-Martin, N.; Batista, F.D. Dynamic reorganisation of intermediate filaments coordinates early B-cell activation. Life Sci. Alliance 2018, 1, e201800060. [Google Scholar] [CrossRef]
- Mayet, W.J.; Wandel, E.; Hermann, E.; Dumann, H.; Kohler, H. Antibodies to cytoskeletal components in patients undergoing long-term hemodialysis detected by a sensitive enzyme-linked immunosorbent assay (ELISA). Clin. Nephrol. 1990, 33, 272–278. [Google Scholar]
- Divanyan, T.; Acosta, E.; Patel, D.; Constantino, D.; Lopez-Soler, R.I. Anti-vimentin antibodies in transplant and disease. Hum. Immunol. 2019, 80, 602–607. [Google Scholar] [CrossRef]
- Tilleman, K.; Van Steendam, K.; Cantaert, T.; De Keyser, F.; Elewaut, D.; Deforce, D. Synovial detection and autoantibody reactivity of processed citrullinated isoforms of vimentin in inflammatory arthritides. Rheumatology 2008, 47, 597–604. [Google Scholar] [CrossRef]
- Ytterberg, A.J.; Joshua, V.; Reynisdottir, G.; Tarasova, N.K.; Rutishauser, D.; Ossipova, E.; Haj Hensvold, A.; Eklund, A.; Skold, C.M.; Grunewald, J.; et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: Identification and validation. Ann. Rheum. Dis. 2015, 74, 1772–1777. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Karsdal, M.A.; Vassiliadis, E.; Wichuk, S.; Marcher-Mikkelsen, K.; Lories, R.; Christiansen, C.; Maksymowych, W.P. Circulating citrullinated vimentin fragments reflect disease burden in ankylosing spondylitis and have prognostic capacity for radiographic progression. Arthritis Rheum 2013, 65, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; Godskesen, L.E.; Jensen, M.D.; Van Haaften, W.T.; Klinge, L.G.; Olinga, P.; Dijkstra, G.; Kjeldsen, J.; Karsdal, M.A.; Bay-Jensen, A.C.; et al. Fragments of Citrullinated and MMP-degraded Vimentin and MMP-degraded Type III Collagen Are Novel Serological Biomarkers to Differentiate Crohn’s Disease from Ulcerative Colitis. J. Crohn’s Colitis 2015, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; van Haaften, W.T.; Karsdal, M.A.; Bay-Jensen, A.C.; Olinga, P.; Gronbaek, H.; Hvas, C.L.; Manon-Jensen, T.; Dijkstra, G.; Dige, A. The Citrullinated and MMP-degraded Vimentin Biomarker (VICM) Predicts Early Response to Anti-TNFalpha Treatment in Crohn’s Disease. J. Clin. Gastroenterol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J.; Chang, A.; Ko, K.; Henry Dunand, C.J.; Henderson, S.; Maienschein-Cline, M.; Kaverina, N.; Rovin, B.H.; Salgado Ferrer, M.; Wolfgeher, D.; et al. Vimentin is a dominant target of in situ humoral immunity in human lupus tubulointerstitial nephritis. Arthritis Rheumatol. 2014, 66, 3359–3370. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Cascino, M.D.; Dai, J.; Bermea, R.S.; Ko, K.; Vesselits, M.; Dragone, L.L.; Mor Vaknin, N.; Legendre, M.; Markovitz, D.M.; et al. Anti-vimentin antibodies: A unique antibody class associated with therapy-resistant lupus nephritis. Lupus 2020, 29, 569–577. [Google Scholar] [CrossRef]
- Li, Y.; Jia, R.; Liu, Y.; Tang, S.; Ma, X.; Shi, L.; Zhao, J.; Hu, F.; Li, Z. Antibodies against carbamylated vimentin exist in systemic lupus erythematosus and correlate with disease activity. Lupus 2020, 29, 239–247. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Kaiser, Y.; Wolfgeher, D.; Ai, J.; Eklund, A.; Clark, M.R.; Grunewald, J. In Situ Humoral Immunity to Vimentin in HLA-DRB1*03(+) Patients With Pulmonary Sarcoidosis. Front. Immunol. 2018, 9, 1516. [Google Scholar] [CrossRef]
- Li, F.J.; Surolia, R.; Li, H.; Wang, Z.; Kulkarni, T.; Liu, G.; de Andrade, J.A.; Kass, D.J.; Thannickal, V.J.; Duncan, S.R.; et al. Autoimmunity to Vimentin Is Associated with Outcomes of Patients with Idiopathic Pulmonary Fibrosis. J. Immunol. 2017, 199, 1596–1605. [Google Scholar] [CrossRef]
- Ortona, E.; Capozzi, A.; Colasanti, T.; Conti, F.; Alessandri, C.; Longo, A.; Garofalo, T.; Margutti, P.; Misasi, R.; Khamashta, M.A.; et al. Vimentin/cardiolipin complex as a new antigenic target of the antiphospholipid syndrome. Blood 2010, 116, 2960–2967. [Google Scholar] [CrossRef]
- Oldstone, M.B. Molecular mimicry: Its evolution from concept to mechanism as a cause of autoimmune diseases. Monoclon. Antibodies Immunodiagn. Immunother. 2014, 33, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.; Seyer, J.M.; Beachey, E.H. Vimentin-cross-reactive epitope of type 12 streptococcal M protein. Infect. Immun. 1989, 57, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, R.S.; Oldstone, M.B.; Wroblewska, Z.; Frankel, M.E.; Koprowski, H. Molecular mimicry in virus infection: Crossreaction of measles virus phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc. Natl. Acad. Sci. USA 1983, 80, 2346–2350. [Google Scholar] [CrossRef]
- Dales, S.; Fujinami, R.S.; Oldstone, M.B. Infection with vaccinia favors the selection of hybridomas synthesizing autoantibodies against intermediate filaments, one of them cross-reacting with the virus hemagglutinin. J. Immunol. 1983, 131, 1546–1553. [Google Scholar] [PubMed]
- Su, L.; Pan, P.; Yan, P.; Long, Y.; Zhou, X.; Wang, X.; Zhou, R.; Wen, B.; Xie, L.; Liu, D. Role of vimentin in modulating immune cell apoptosis and inflammatory responses in sepsis. Sci. Rep. 2019, 9, 5747. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Valdivia, R.H. Actin and intermediate filaments stabilize the Chlamydia trachomatis vacuole by forming dynamic structural scaffolds. Cell Host Microbe 2008, 4, 159–169. [Google Scholar] [CrossRef]
- He, C.; Kong, L.; Zhou, L.; Xia, J.; Wei, H.; Liu, M.; Peng, H. Host Cell Vimentin Restrains Toxoplasma gondii Invasion and Phosphorylation of Vimentin is Partially Regulated by Interaction with TgROP18. Int. J. Biol. Sci. 2017, 13, 1126–1137. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novoa, R.R.; Calderita, G.; Arranz, R.; Fontana, J.; Granzow, H.; Risco, C. Virus factories: Associations of cell organelles for viral replication and morphogenesis. Biol. Cell 2005, 97, 147–172. [Google Scholar] [CrossRef]
- Babrak, L.; Danelishvili, L.; Rose, S.J.; Kornberg, T.; Bermudez, L.E. The environment of “Mycobacterium avium subsp. hominissuis” microaggregates induces synthesis of small proteins associated with efficient infection of respiratory epithelial cells. Infect. Immun. 2015, 83, 625–636. [Google Scholar] [CrossRef]
- Mak, T.N.; Fischer, N.; Laube, B.; Brinkmann, V.; Metruccio, M.M.; Sfanos, K.S.; Mollenkopf, H.J.; Meyer, T.F.; Bruggemann, H. Propionibacterium acnes host cell tropism contributes to vimentin-mediated invasion and induction of inflammation. Cell Microbiol. 2012, 14, 1720–1733. [Google Scholar] [CrossRef]
- Broers, J.L.; de Leij, L.; Rot, M.K.; ter Haar, A.; Lane, E.B.; Leigh, I.M.; Wagenaar, S.S.; Vooijs, G.P.; Ramaekers, F.C. Expression of intermediate filament proteins in fetal and adult human lung tissues. Differ. Res. Biol. Divers. 1989, 40, 119–128. [Google Scholar] [CrossRef]
- Yang, Y.; Riccio, P.; Schotsaert, M.; Mori, M.; Lu, J.; Lee, D.K.; Garcia-Sastre, A.; Xu, J.; Cardoso, W.V. Spatial-Temporal Lineage Restrictions of Embryonic p63(+) Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Dev. Cell 2018, 44, 752–761 e754. [Google Scholar] [CrossRef] [PubMed]
- Bastounis, E.E.; Yeh, Y.T.; Theriot, J.A. Matrix stiffness modulates infection of endothelial cells by Listeria monocytogenes via expression of cell surface vimentin. Mol. Biol. Cell 2018, 29, 1571–1589. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Halvorsen, E.M.; Ammendolia, D.A.; Mor-Vaknin, N.; O’Riordan, M.X.D.; Brumell, J.H.; Markovitz, D.M.; Higgins, D.E. Invasion of the Brain by Listeria monocytogenes Is Mediated by InlF and Host Cell Vimentin. mBio 2018, 9, e00160-18. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Chi, F.; Peng, L.; Bo, T.; Zhang, B.; Liu, L.Q.; Wu, X.; Mor-Vaknin, N.; Markovitz, D.M.; Cao, H.; et al. Vimentin, a Novel NF-kappaB Regulator, Is Required for Meningitic Escherichia coli K1-Induced Pathogen Invasion and PMN Transmigration across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0162641. [Google Scholar] [CrossRef]
- Guignot, J.; Servin, A.L. Maintenance of the Salmonella-containing vacuole in the juxtanuclear area: A role for intermediate filaments. Microb. Pathog. 2008, 45, 415–422. [Google Scholar] [CrossRef]
- Icenogle, L.M.; Hengel, S.M.; Coye, L.H.; Streifel, A.; Collins, C.M.; Goodlett, D.R.; Moseley, S.L. Molecular and biological characterization of Streptococcal SpyA-mediated ADP-ribosylation of intermediate filament protein vimentin. J. Biol. Chem. 2012, 287, 21481–21491. [Google Scholar] [CrossRef]
- Rohrbeck, A.; Schroder, A.; Hagemann, S.; Pich, A.; Holtje, M.; Ahnert-Hilger, G.; Just, I. Vimentin mediates uptake of C3 exoenzyme. PLoS ONE 2014, 9, e101071. [Google Scholar] [CrossRef]
- Rohrbeck, A.; Holtje, M.; Adolf, A.; Oms, E.; Hagemann, S.; Ahnert-Hilger, G.; Just, I. The Rho ADP-ribosylating C3 exoenzyme binds cells via an Arg-Gly-Asp motif. J. Biol. Chem. 2017, 292, 17668–17680. [Google Scholar] [CrossRef]
- Schafer, G.; Graham, L.M.; Lang, D.M.; Blumenthal, M.J.; Bergant Marusic, M.; Katz, A.A. Vimentin Modulates Infectious Internalization of Human Papillomavirus 16 Pseudovirions. J. Virol. 2017, 91, e00307–e00317. [Google Scholar] [CrossRef]
- Wu, W.; Pante, N. Vimentin plays a role in the release of the influenza A viral genome from endosomes. Virology 2016, 497, 41–52. [Google Scholar] [CrossRef]
- Huang, S.Y.; Huang, C.H.; Chen, C.J.; Chen, T.W.; Lin, C.Y.; Lin, Y.T.; Kuo, S.M.; Huang, C.G.; Lee, L.A.; Chen, Y.H.; et al. Novel Role for miR-1290 in Host Species Specificity of Influenza A Virus. Mol. Ther. Nucleic Acids 2019, 17, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.S.; Chu, J.J. Cellular vimentin regulates construction of dengue virus replication complexes through interaction with NS4A protein. J. Virol. 2014, 88, 1897–1913. [Google Scholar] [CrossRef] [PubMed]
- Risco, C.; Rodriguez, J.R.; Lopez-Iglesias, C.; Carrascosa, J.L.; Esteban, M.; Rodriguez, D. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 2002, 76, 1839–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, B.; Xu, J.; Chang, Q.; McNutt, M.A.; Korteweg, C.; Gong, E.; Gu, J. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am. J. Pathol. 2007, 170, 538–545. [Google Scholar] [CrossRef]
- Mossel, E.C.; Wang, J.; Jeffers, S.; Edeen, K.E.; Wang, S.; Cosgrove, G.P.; Funk, C.J.; Manzer, R.; Miura, T.A.; Pearson, L.D.; et al. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology 2008, 372, 127–135. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; O’Meara, M.J.; Guo, J.Z.; Swaney, D.L.; Tummino, T.A.; Hüttenhain, R.; et al. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug-Repurposing. BioRxiv 2020, 10.1101. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Shalek, A.K.; Ordovas-Montanes, J.; Network, H.L.B. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181, 1016–1035. [Google Scholar] [CrossRef] [PubMed]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.; Stosiek, P. The expression of vimentin in epithelial cells from human nasal mucosa. Eur. Arch. Otorhinolaryngol. 1990, 248, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, C.B.; Jhanji, V.; Xu, C.; Yuan, X.L.; Liang, J.J.; Huang, Y.; Cen, L.P.; Ng, T.K. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye 2020, 34, 1212–1219. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904. [Google Scholar] [CrossRef]
- Yang, J.; Zou, L.; Yang, Y.; Yuan, J.; Hu, Z.; Liu, H.; Peng, H.; Shang, W.; Zhang, X.; Zhu, J.; et al. Superficial vimentin mediates DENV-2 infection of vascular endothelial cells. Sci. Rep. 2016, 6, 38372. [Google Scholar] [CrossRef]
- Liang, J.J.; Yu, C.Y.; Liao, C.L.; Lin, Y.L. Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol. 2011, 13, 1358–1370. [Google Scholar] [CrossRef]
- Das, S.K.; Gupta, I.; Cho, Y.K.; Zhang, X.; Uehara, H.; Muddana, S.K.; Bernhisel, A.A.; Archer, B.; Ambati, B.K. Vimentin knockdown decreases corneal opacity. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4030–4040. [Google Scholar] [CrossRef]
- Kim, J.K.; Fahad, A.M.; Shanmukhappa, K.; Kapil, S. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J. Virol. 2006, 80, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, K.J.; Destito, G.; Plummer, E.M.; Trauger, S.A.; Siuzdak, G.; Manchester, M. Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog. 2009, 5, e1000417. [Google Scholar] [CrossRef] [PubMed]
- Kavathekar, V.K.; Dhanavade, M.J.; Sonawane, K.D.; Balakrishnan, A. Role of cell surface vimentin in Chandipura virus replication in Neuro-2a cells. Virus Res. 2020, 285, 198014. [Google Scholar] [CrossRef]
- Turkki, P.; Laajala, M.; Flodstrom-Tullberg, M.; Marjomaki, V. Human Enterovirus Group B Viruses Rely on Vimentin Dynamics for Efficient Processing of Viral Nonstructural Proteins. J. Virol. 2020, 94, e01393-01319. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ling, Y.; Li, P.; Sun, P.; Cao, Y.; Bai, X.; Li, K.; Fu, Y.; Zhang, J.; Li, D.; et al. Cellular Vimentin Interacts with Foot-and-Mouth Disease Virus Nonstructural Protein 3A and Negatively Modulates Viral Replication. J. Virol. 2020, JVI.00273-20. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, S.; Windsor, M.; Nagata, K.I.; Inagaki, M.; Wileman, T. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 2005, 79, 11766–11775. [Google Scholar] [CrossRef] [PubMed]
- Nitahara-Kasahara, Y.; Fukasawa, M.; Shinkai-Ouchi, F.; Sato, S.; Suzuki, T.; Murakami, K.; Wakita, T.; Hanada, K.; Miyamura, T.; Nishijima, M. Cellular vimentin content regulates the protein level of hepatitis C virus core protein and the hepatitis C virus production in cultured cells. Virology 2009, 383, 319–327. [Google Scholar] [CrossRef]
- DeBoer, J.; Wojtkiewicz, M.S.; Haverland, N.; Li, Y.; Harwood, E.; Leshen, E.; George, J.W.; Ciborowski, P.; Belshan, M. Proteomic profiling of HIV-infected T-cells by SWATH mass spectrometry. Virology 2018, 516, 246–257. [Google Scholar] [CrossRef]
- Fernandez-Ortega, C.; Ramirez, A.; Casillas, D.; Paneque, T.; Ubieta, R.; Dubed, M.; Navea, L.; Castellanos-Serra, L.; Duarte, C.; Falcon, V.; et al. Identification of Vimentin as a Potential Therapeutic Target against HIV Infection. Viruses 2016, 8, 98. [Google Scholar] [CrossRef]
- Snasel, J.; Shoeman, R.; Horejsi, M.; Hruskova-Heidingsfeldova, O.; Sedlacek, J.; Ruml, T.; Pichova, I. Cleavage of vimentin by different retroviral proteases. Arch. Biochem. Biophys. 2000, 377, 241–245. [Google Scholar] [CrossRef]
- Alldridge, L.C.; O’Farrell, M.K.; Dealtry, G.B. Interferon beta increases expression of vimentin at the messenger RNA and protein levels in differentiated embryonal carcinoma (PSMB) cells. Exp. Cell Res. 1989, 185, 387–393. [Google Scholar] [CrossRef]
- Lv, N.; Gao, Y.; Guan, H.; Wu, D.; Ding, S.; Teng, W.; Shan, Z. Inflammatory mediators, tumor necrosis factor-alpha and interferon-gamma, induce EMT in human PTC cell lines. Oncol. Lett. 2015, 10, 2591–2597. [Google Scholar] [CrossRef] [PubMed]
- Geisler, F.; Leube, R.E. Epithelial Intermediate Filaments: Guardians against Microbial Infection? Cells 2016, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Denes, C.E.; Miranda-Saksena, M.; Cunningham, A.L.; Diefenbach, R.J. Cytoskeletons in the Closet-Subversion in Alphaherpesvirus Infections. Viruses 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Ridge, K.M.; Shumaker, D.; Robert, A.; Hookway, C.; Gelfand, V.I.; Janmey, P.A.; Lowery, J.; Guo, M.; Weitz, D.A.; Kuczmarski, E.; et al. Methods for Determining the Cellular Functions of Vimentin Intermediate Filaments. Methods Enzym. 2016, 568, 389–426. [Google Scholar] [CrossRef]
- Strouhalova, K.; Prechova, M.; Gandalovicova, A.; Brabek, J.; Gregor, M.; Rosel, D. Vimentin Intermediate Filaments as Potential Target for Cancer Treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef]
- Weidle, U.H.; Maisel, D.; Klostermann, S.; Schiller, C.; Weiss, E.H. Intracellular Proteins Displayed on the Surface of Tumor Cells as Targets for Therapeutic Intervention with Antibody-related Agents. J. Cell Sci. 2011, 8, 49–64. [Google Scholar]
- Noh, H.; Yan, J.; Hong, S.; Kong, L.Y.; Gabrusiewicz, K.; Xia, X.; Heimberger, A.B.; Li, S. Discovery of cell surface vimentin targeting mAb for direct disruption of GBM tumor initiating cells. Oncotarget 2016, 7, 72021–72032. [Google Scholar] [CrossRef]
- Babic, I.; Nurmemmedov, E.; Yenugonda, V.M.; Juarez, T.; Nomura, N.; Pingle, S.C.; Glassy, M.C.; Kesari, S. Pritumumab, the first therapeutic antibody for glioma patients. Hum. Antibodies 2018, 26, 95–101. [Google Scholar] [CrossRef]
- Hugwil, A.V. The meaning of the anti-cancer antibody CLN-IgG (Pritumumab) generated by human x human hybridoma technology against the cyto-skeletal protein, vimentin, in the course of the treatment of malignancy. Med. Hypotheses 2013, 81, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Huet, D.; Bagot, M.; Loyaux, D.; Capdevielle, J.; Conraux, L.; Ferrara, P.; Bensussan, A.; Marie-Cardine, A. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J. Immunol. 2006, 176, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib Study of PEGylated Recombinant Human Hyaluronidase and Gemcitabine in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Ha, Y.; Ha, S.K.; Han, D.U.; Kim, D.W.; Moon, W.K.; Chae, C. Antiviral effect of Saccharomyces cerevisiae beta-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. J. Vet. Med. BInfect. Dis. Vet. Public Health 2004, 51, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Higashino-Kameda, M.; Yabe-Wada, T.; Matsuba, S.; Takeda, K.; Anzawa, K.; Mochizuki, T.; Makimura, K.; Saijo, S.; Iwakura, Y.; Toga, H.; et al. A critical role of Dectin-1 in hypersensitivity pneumonitis. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016, 65, 235–244. [Google Scholar] [CrossRef]
- Mohan, R.; Bargagna-Mohan, P. The Use of Withaferin A to Study Intermediate Filaments. Methods Enzym. 2016, 568, 187–218. [Google Scholar] [CrossRef]
- Kaschula, C.H.; Tuveri, R.; Ngarande, E.; Dzobo, K.; Barnett, C.; Kusza, D.A.; Graham, L.M.; Katz, A.A.; Rafudeen, M.S.; Parker, M.I.; et al. The garlic compound ajoene covalently binds vimentin, disrupts the vimentin network and exerts anti-metastatic activity in cancer cells. BMC Cancer 2019, 19, 248. [Google Scholar] [CrossRef]
- Ermakova, S.; Choi, B.Y.; Choi, H.S.; Kang, B.S.; Bode, A.M.; Dong, Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005, 280, 16882–16890. [Google Scholar] [CrossRef]
- Miyai, S.; Yamaguchi, A.; Iwasaki, T.; Shamsa, F.; Ohtsuki, K. Biochemical characterization of epigallocatechin-3-gallate as an effective stimulator for the phosphorylation of its binding proteins by glycogen synthase kinase-3beta in vitro. Biol. Pharm. Bull. 2010, 33, 1932–1937. [Google Scholar] [CrossRef]
- Hsu, S. Compounds Derived from Epigallocatechin-3-Gallate (EGCG) as a Novel Approach to the Prevention of Viral Infections. Inflamm. Allergy Drug Targets 2015, 14, 13–18. [Google Scholar] [CrossRef]
- Yue, Q.; Feng, L.; Cao, B.; Liu, M.; Zhang, D.; Wu, W.; Jiang, B.; Yang, M.; Liu, X.; Guo, D. Proteomic Analysis Revealed the Important Role of Vimentin in Human Cervical Carcinoma HeLa Cells Treated With Gambogic Acid. Mol. Cell Proteom. 2016, 15, 26–44. [Google Scholar] [CrossRef]
- Li, D.; Song, X.Y.; Yue, Q.X.; Cui, Y.J.; Liu, M.; Feng, L.X.; Wu, W.Y.; Jiang, B.H.; Yang, M.; Qu, X.B.; et al. Proteomic and bioinformatic analyses of possible target-related proteins of gambogic acid in human breast carcinoma MDA-MB-231 cells. Chin. J. Nat. Med. 2015, 13, 41–51. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, G.; Ji, Y.; Zhua, H.; Lv, C.; Jiang, W. Protective role of gambogic acid in experimental pulmonary fibrosis in vitro and in vivo. Phytomed.: Int. J. Phytother. Phytopharm. 2016, 23, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Trogden, K.P.; Battaglia, R.A.; Kabiraj, P.; Madden, V.J.; Herrmann, H.; Snider, N.T. An image-based small-molecule screen identifies vimentin as a pharmacologically relevant target of simvastatin in cancer cells. FASEB J. 2018, 32, 2841–2854. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.; Tawab, A.; Marie-Cardine, A.; Bagot, M.; Boumsell, L.; Bensussan, A. Increased expression of a novel early activation surface membrane receptor in cutaneous T cell lymphoma cells. J. Investig. Derm. 2001, 116, 731–738. [Google Scholar] [CrossRef]
- Glassy, M.C.; Hagiwara, H. Summary analysis of the pre-clinical and clinical results of brain tumor patients treated with pritumumab. Hum. Antibodies 2009, 18, 127–137. [Google Scholar] [CrossRef]
- Sager, P.R.; Matheson, D.W. Mechanisms of neurotoxicity related to selective disruption of microtubules and intermediate filaments. Toxicology 1988, 49, 479–492. [Google Scholar] [CrossRef]
- Arocena, M. Effect of acrylamide on the cytoskeleton and apoptosis of bovine lens epithelial cells. Cell Biol. Int. 2006, 30, 1007–1012. [Google Scholar] [CrossRef]
- Galigniana, M.D.; Scruggs, J.L.; Herrington, J.; Welsh, M.J.; Carter-Su, C.; Housley, P.R.; Pratt, W.B. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol. Endocrinol. 1998, 12, 1903–1913. [Google Scholar] [CrossRef]
- Stamatakis, K.; Sánchez-Gómez, F.J.; Pérez-Sala, D. Identification of novel protein targets for modification by 15-deoxy-Δ12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J. Am. Soc. Nephrol. 2006, 17, 89–98. [Google Scholar] [CrossRef]
- Santoro, M.G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997, 5, 276–281. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Khuan Abby Ho, Y.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Grin, B.; Mahammad, S.; Wedig, T.; Cleland, M.M.; Tsai, L.; Herrmann, H.; Goldman, R.D. Withaferin a alters intermediate filament organization, cell shape and behavior. PLoS ONE 2012, 7, e39065. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, W.; Li, Y.; Niu, L.; Ye, H.; Chen, L. The Natural Compound Withaferin A Covalently Binds to Cys239 of beta-Tubulin to Promote Tubulin Degradation. Mol. Pharm. 2019, 96, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bollong, M.J.; Pietila, M.; Pearson, A.D.; Sarkar, T.R.; Ahmad, I.; Soundararajan, R.; Lyssiotis, C.A.; Mani, S.A.; Schultz, P.G.; Lairson, L.L. A vimentin binding small molecule leads to mitotic disruption in mesenchymal cancers. Proc. Natl. Acad. Sci. USA 2017, 114, E9903–E9912. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Mollinedo, F. Inhibition of isoprenoid biosynthesis induces apoptosis in human promyelocytic HL-60 cells. Biochem. Biophys. Res. Commun. 1994, 199, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Collado-Escobar, D.; Mollinedo, F. Intracellular Alkalinization Suppresses Lovastatin-induced Apoptosis in HL-60 Cells through the Inactivation of a pH-dependent Endonuclease. J. Biol. Chem. 1995, 270, 6235–6242. [Google Scholar] [CrossRef]
- Kanugula, A.K.; Dhople, V.M.; Volker, U.; Ummanni, R.; Kotamraju, S. Fluvastatin mediated breast cancer cell death: A proteomic approach to identify differentially regulated proteins in MDA-MB-231 cells. PLoS ONE 2014, 9, e108890. [Google Scholar] [CrossRef]
- Fedson, D.S.; Opal, S.M.; Rordam, O.M. Hiding in Plain Sight: An Approach to Treating Patients with Severe COVID-19 Infection. mBio 2020, 11, e00398-00320. [Google Scholar] [CrossRef]
- Esposito, A.M.; Cheung, P.; Swartz, T.H.; Li, H.; Tsibane, T.; Durham, N.D.; Basler, C.F.; Felsenfeld, D.P.; Chen, B.K. A high throughput Cre-lox activated viral membrane fusion assay identifies pharmacological inhibitors of HIV entry. Virology 2016, 490, 6–16. [Google Scholar] [CrossRef]
- Españo, E.; Nam, J.H.; Song, E.J.; Song, D.; Lee, C.K.; Kim, J.K. Lipophilic statins inhibit Zika virus production in Vero cells. Sci. Rep. 2019, 9, 11461. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Broedersz, C.P.; Rowat, A.C.; Wedig, T.; Herrmann, H.; Mackintosh, F.C.; Weitz, D.A. Divalent cations crosslink vimentin intermediate filament tail domains to regulate network mechanics. J. Mol. Biol. 2010, 399, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Monico, A.; Zorrilla, S.; Rivas, G.; Perez-Sala, D. Zinc Differentially Modulates the Assembly of Soluble and Polymerized Vimentin. Int. J. Mol. Sci. 2020, 21, 2426. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, Y.; Su, W.; Huang, S.; Lu, W.; Zhou, Y.; Gao, J.; Zhao, W.; Zhang, B.; Wu, X. Enterovirus A71 VP1 Variation A289T Decreases the Central Nervous System Infectivity via Attenuation of Interactions between VP1 and Vimentin In Vitro and In Vivo. Viruses 2019, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, S.; Waheed, A.A.; Dabbagh, D.; Zhou, Z.; Trinite, B.; Wang, Z.; Yu, J.; Wang, D.; Li, F.; et al. PSGL-1 restricts HIV-1 infectivity by blocking virus particle attachment to target cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9537–9545. [Google Scholar] [CrossRef] [PubMed]
- Zamay, T.N.; Kolovskaya, O.S.; Glazyrin, Y.E.; Zamay, G.S.; Kuznetsova, S.A.; Spivak, E.A.; Wehbe, M.; Savitskaya, A.G.; Zubkova, O.A.; Kadkina, A.; et al. DNA-aptamer targeting vimentin for tumor therapy in vivo. Nucleic Acid Ther. 2014, 24, 160–170. [Google Scholar] [CrossRef]
- Jalalian, S.H.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Targeted co-delivery of epirubicin and NAS-24 aptamer to cancer cells using selenium nanoparticles for enhancing tumor response in vitro and in vivo. Cancer Lett. 2018, 416, 87–93. [Google Scholar] [CrossRef]
- Yoon, S.; Armstrong, B.; Habib, N.; Rossi, J.J. Blind SELEX Approach Identifies RNA Aptamers That Regulate EMT and Inhibit Metastasis. Mol. Cancer Res. MCR 2017, 15, 811–820. [Google Scholar] [CrossRef]
| Macromolecules | Clinical Use | Putative Effect | Specificity for Vimentin | References |
|---|---|---|---|---|
| Expression vectors, wt and mutants/fragments | Mimic/inhibit | Very high | [6] | |
| siRNAs | Inhibit expression | High | [20,129] | |
| Recombinant vimentin and fragments | Mimic/compete vimentin release or exposure | High | [44,66] | |
| Soluble CD44 | Compete for vimentin binding | High | [44] | |
| Pritumumab (anti-vimentin mAb) | Clinic, Phase II | Membrane vimentin binding | Very high | [169,170] |
| SC5 anti-vimentin mAb | Membrane vimentin binding | Very high | [171] | |
| Anti-Cell surface vimentin (CSV) 86C mAb | Membrane vimentin binding and internalization | Very high | [168] | |
| Anti-citrullinated Vimentin antibodies | Diagnostic | Biomarker | Very high | [19] |
| Hyaluronic acid (CTX-100) | Phase II | Compete with vimentin for CD44 | Moderate- Low | NCT00993707 * |
| PEGPH20 (Pegylated Hyaluronidase) | Phase I | Reduce hyaluronan levels | Moderate- Low | [172] |
| ß-glucans (Proglucamune) | Dietary supplement | Dectin-1 agonist | Low | [173,174] |
| Dectin-1 blocking antibodies | Block Dectin-1 signals | High | [62] | |
| Small Molecules | Clinical Use | Putative Effect | Specificity for Vimentin | References |
| Withaferin A | Withania Somnifera extract (WSE; Sensoril®) | Reduce vimentin levels, binds region of C328, phosphorylation | Moderate | [16,175] |
| Ajoene | Garlic oil & pure studies | Disrupt vimentin network and functions, bind C328 | Low | [176] |
| Epigallocathechin gallate | Dietary supplement trials | Inhibit vimentin phosphorylation | Low | [177,178,179] |
| Gambogic acid | Traditional Asian medicine | Vimentin cleavage | Low | [180,181,182] |
| Simvastatin | Clinic | Vimentin distribution; viral entry inhibition; anti-inflammatory | Low | [183] NCT04348695 * |
| Cantharidin | Phase I-IV | Vimentin distribution; antiviral | Low | [183] |
| Carvedilol | Phase I-IV | Vimentin distribution | Low | [183] |
| Ivermectin | Phase I-IV | Vimentin distribution; COVID-19 and Dengue treatment | Low | [183] NCT04343092 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. https://doi.org/10.3390/ijms21134675
Ramos I, Stamatakis K, Oeste CL, Pérez-Sala D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. International Journal of Molecular Sciences. 2020; 21(13):4675. https://doi.org/10.3390/ijms21134675
Chicago/Turabian StyleRamos, Irene, Konstantinos Stamatakis, Clara L. Oeste, and Dolores Pérez-Sala. 2020. "Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections" International Journal of Molecular Sciences 21, no. 13: 4675. https://doi.org/10.3390/ijms21134675
APA StyleRamos, I., Stamatakis, K., Oeste, C. L., & Pérez-Sala, D. (2020). Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. International Journal of Molecular Sciences, 21(13), 4675. https://doi.org/10.3390/ijms21134675