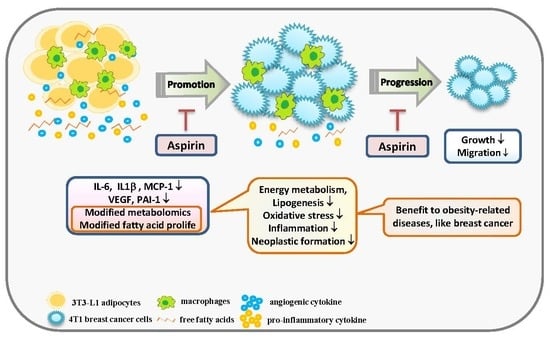
Aspirin Modifies Inflammatory Mediators and Metabolomic Profiles and Contributes to the Suppression of Obesity-Associated Breast Cancer Cell Growth
Abstract
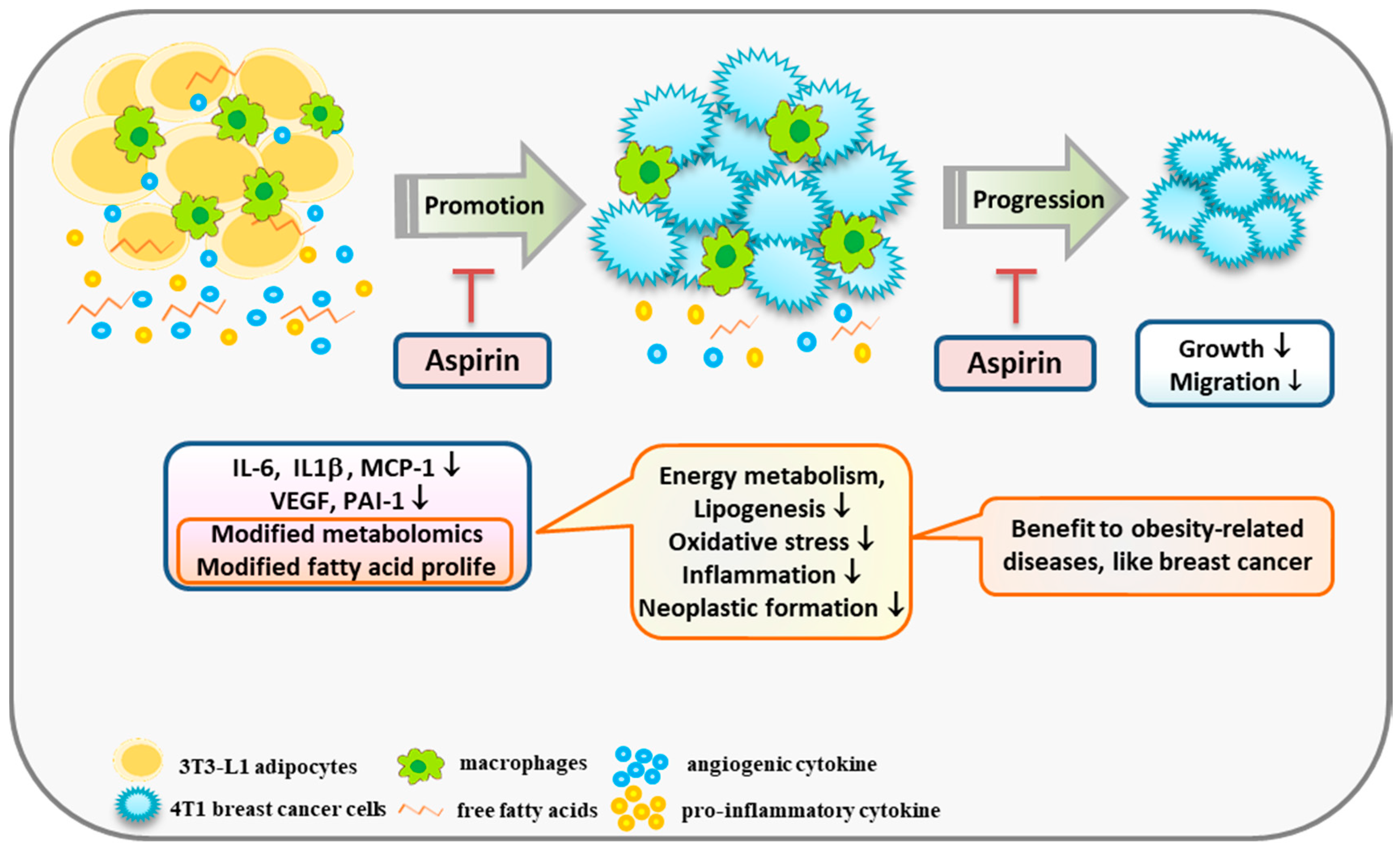
1. Introduction
2. Results
2.1. Aspirin Suppresses Proinflammatory and Angiogenesis-Related Cytokines in 3T3-L1 Adipocytes
2.2. Aspirin Regulates Metabolism in 3T3-L1 Adipocytes
2.3. Aspirin Regulates Fatty Acid Composition in 3T3-L1 Adipocytes
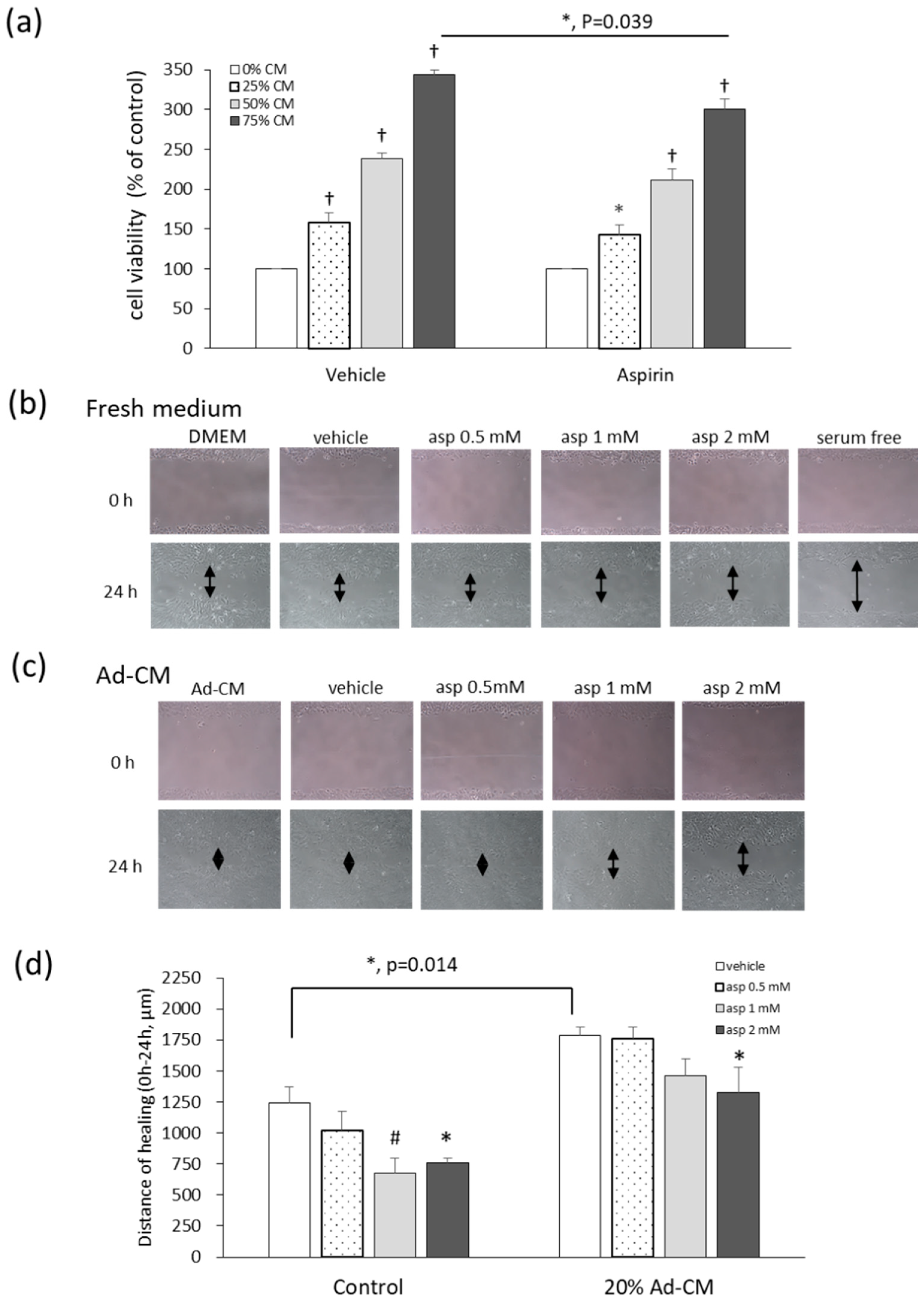
2.4. Aspirin Inhibits Ad-CM-Promoted 4T1 Cell Proliferation and Migration
3. Discussion
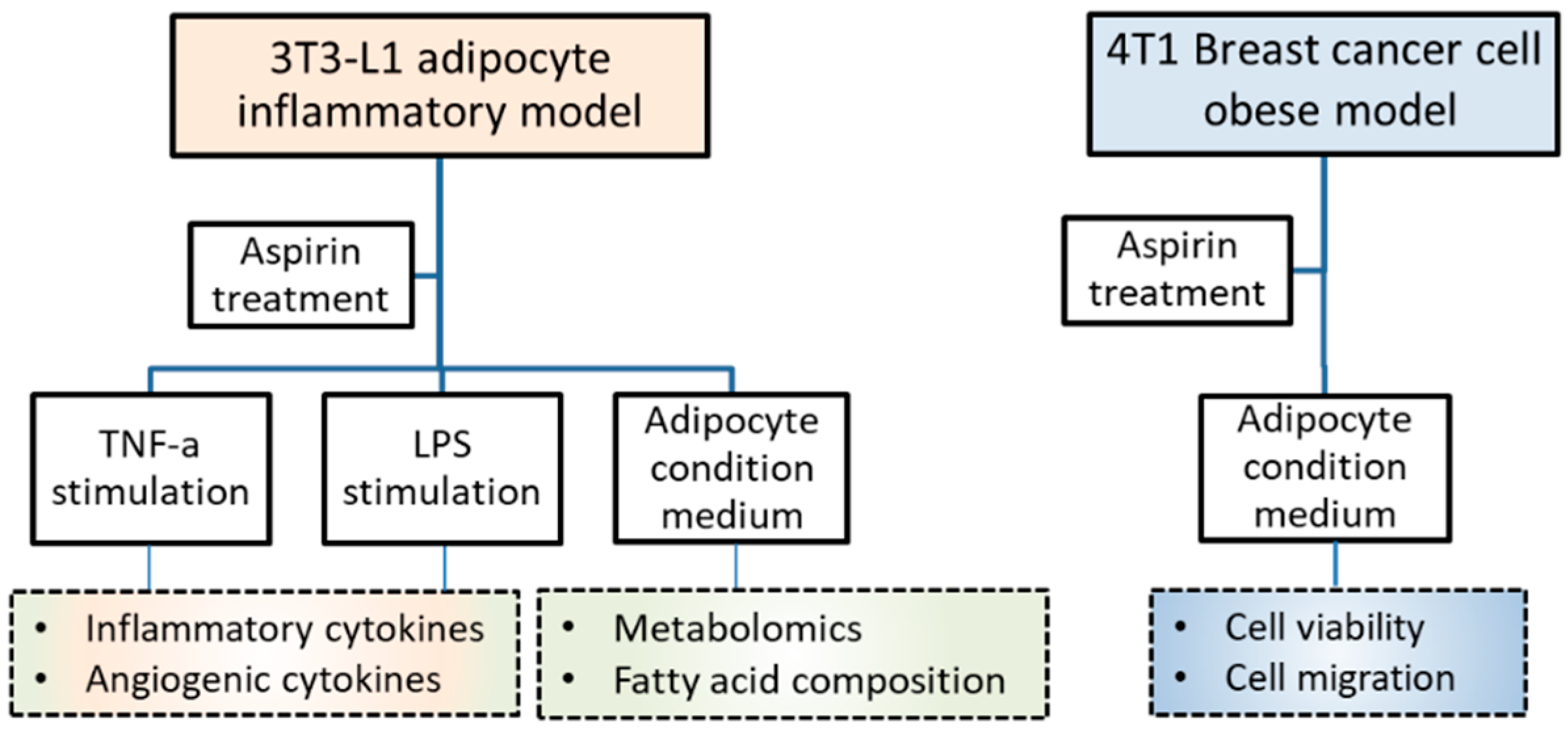
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. T3-L1 Preadipocytes Differentiate into Adipocytes
4.3. Cytokines, Adipokines, and Angiogenic Mediators in 3T3-L1 Adipocytes
4.4. Obesity-Associated Model Set-Up
4.5. Metabolite Analysis of 3T3-L1 Adipocyte-Conditioned Medium
4.6. Metabolite Profiling by Liquid Chromatography–Time of Flight Mass Spectrometry
4.7. Fatty Acid Analysis by GC-MS
4.8. T1 Cell Viability Assay
4.9. T1 Cell Migration Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| Ad | Adipocyte |
| AdA | Adipocyte treated with aspirin |
| Ad-CM | Adipocyte-conditioned medium |
| BCAA | Branched-chain amino acid |
| COX-2 | Cyclooxygenase-2 |
| ELISA | Enzyme-linked immunosorbent assay |
| Fb | Fibroblast |
| GC-MS | Gas chromatography-mass spectrometry |
| IL | Interleukin |
| LC-MS | Liquid chromatography-mass spectrometry |
| LPS | Lipopolysaccharide |
| MCP-1 | Macrophage chemoattractant protein-1 |
| MTT | Methylthiazole tetrazolium |
| PCA | Principal component analysis |
| PAI-1 | Plasminogen activator inhibitor-1 |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-alpha |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Breast cancer. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 7 May 2019).
- World Health Organization (WHO). Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 May 2019).
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.I.; Hsieh, C.C. Why are women with obesity more likely to develop breast cancer. Future Oncol. 2018, 14, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Frühbeck, G. Adipose tissue immunity and cancer. Front Physiol. 2013, 4, 275. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K. Metabolomics: The greatest omics of them all? Anal. Chem. 2006, 78, 7954–7958. [Google Scholar] [CrossRef]
- Cuperlovic’-Culf, M.; Barnett, D.A.; Culf, A.S.; Chute, I. Cell culture metabolomics applications and future directions. Drug Discov Today 2010, 15, 610–621. [Google Scholar] [CrossRef]
- Cuzick, J.; Otto, F.; Baron, J.A.; Brown, P.H.; Burn, J.; Greenwald, P.; Jankowski, J.; La Vecchia, C.; Meyskens, F.; Senn, H.J.; et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: An international consensus statement. Lancet Oncol. 2009, 10, 501–507. [Google Scholar] [CrossRef]
- Bosetti, C.; Rosato, V.; Gallus, S.; Cuzick, J.; La Vecchia, C. Aspirin and cancer risk: A quantitative review to 2011. Ann. Oncol. 2012, 23, 1403–1415. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Wang, C.H. Aspirin disrupts the crosstalk of angiogenic and inflammatory cytokines between 4T1 breast cancer cells and macrophages. Mediat. Inflamm. 2018, 2018. [Google Scholar] [CrossRef]
- Thorat, M.A.; Cuzick, J. Role of Aspirin in Cancer Prevention. Curr. Oncol. Rep. 2013, 15, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Gao, P.; Sun, J.X.; Song, Y.X.; Tsai, C.; Liu, J.; Chen, X.W.; Chen, P.; Xu, H.M.; Wang, Z.N. Aspirin and nonsteroidal anti-inflammatory drugs after but not before diagnosis are associated with improved breast cancer survival: A meta-analysis. Cancer Cause. Control 2015, 26, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Huang, Y.S. Aspirin breaks the cross-talk between 3T3-L1 adipocytes and 4T1 breast cancer cells by regulating cytokines production. PLoS ONE 2016, 11, e0147161. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Peng, S.H.; Chou, M.J. Obesity enhances carcinogen 7, 12-Dimethylbenz [a] anthracene -induced tumorigenesis in vitro and in vivo. Food Chem. Toxicol. 2017, 110, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Chou, M.J.; Wang, C.H. Lunasin attenuates obesity-related inflammation in RAW264.7 cells and 3T3-L1 adipocytes by inhibiting inflammatory cytokine production. PLoS ONE 2017, 12, e0171969. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Wang, C.H.; Huang, Y.S. Lunasin attenuates obesity-associated metastasis of 4T1 breast cancer cell through anti-inflammatory property. Int. J. Mol. Sci. 2016, 17, E2109. [Google Scholar] [CrossRef] [PubMed]
- Velaei, K.; Samadi, N.; Barazvan, B.; Soleimani Rad, J. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast 2016, 30, 92–100. [Google Scholar] [CrossRef]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The role of the immune system in obesity and insulin resistance. J. Obes. 2013, 2013. [Google Scholar] [CrossRef]
- Ford, N.A.; Lashinger, L.M.; Allott, E.H.; Hursting, S.D. Mechanistic targets and phytochemical strategies for breaking the obesity-cancer link. Front Oncol. 2013, 3, 209. [Google Scholar] [CrossRef]
- Cox, A.R.; Chernis, N.; Masschelin, P.M.; Hartig, S.M. Immune Cells Gate White Adipose Tissue Expansion. Endocrinology 2019, 160, 1645–1658. [Google Scholar] [CrossRef]
- Himbert, C.; Delphan, M.; Scherer, D.; Bowers, L.W.; Hursting, S.; Ulrich, C.M. Signals from the Adipose Microenvironment and the Obesity–Cancer Link—A Systematic Review. Cancer Prev. Res. 2017, 10, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.R.; Watson, C.J. Anti-CCL2: Building a reservoir or opening the floodgates to metastasis? Breast Cancer Res. 2015, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Fortenberry, Y.M.; Brandal, S.M.; Carpentier, G.; Hemani, M.; Pathak, A.P. Intracellular expression of PAI-1 specific aptamers alters breast cancer cell migration, invasion and angiogenesis. PLoS ONE 2016, 11, e0164288. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.H.; Heyn, G.S.; Magalhaes, K.G. The Impact of the Adipose Organ Plasticity on Inflammation and Cancer Progression. Cells 2019, 8, E662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, B.L.; Liu, K.; Tang, N.; Huang, J.; An, Y.; Li, L. Establishment of the insulin resistance induced by inflammatory response in 3T3-L1 preadipocytes cell line. Inflammation 2008, 31, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y. Complex roles of the old drug aspirin in cancer chemoprevention and therapy. Med. Res. Rev. 2019, 39, 114–145. [Google Scholar] [CrossRef]
- Delort, L.; Rossary, A.; Farges, M.C.; Vasson, M.P.; Caldefie-Chezet, F. Leptin, adipocytes and breast cancer: Focus on inflammation and anti-tumor immunity. Life Sci. 2015, 140, 37–48. [Google Scholar] [CrossRef]
- Bellastella, G.; Scappaticcio, L.; Esposito, K.; Giugliano, D.; Ida Maiorino, M. Metabolic Syndrome and Cancer: “The Common Soil Hypothesis”. Diabetes Res. Clin. Pract. 2018, 143, 389–397. [Google Scholar] [CrossRef]
- Lu, J.; Xie, G.; Jia, W.; Jia, W. Metabolomics in human type 2 diabetes research. Front Med. 2013, 7, 4–13. [Google Scholar] [CrossRef]
- Chen, H.H.; Tseng, Y.J.; Wang, S.Y.; Tsai, Y.S.; Chang, C.S.; Kuo, T.C.; Yao, W.J.; Shieh, C.C.; Wu, C.H.; Kuo, P.H. The metabolome profiling and pathway analysis in metabolic healthy and abnormal obesity. Int. J. Obes. (Lond) 2015, 39, 1241–1248. [Google Scholar] [CrossRef]
- Sabater, D.; Arriarán, S.; del Mar Romero, M.; Agnelli, S.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci. Rep. 2014, 4, 3663. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Rubio, D.; Tovar, A.R.; Noriega, L.G. Emerging perspectives on branched-chain amino acid metabolism during adipocyte differentiation. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 49–57. [Google Scholar] [CrossRef]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA Metabolism and Insulin Sensitivity–Dysregulated by Metabolic Status? Mol. Nutr. Food Res. 2018, 62, e1700756. [Google Scholar] [CrossRef]
- Terakura, D.; Shimizu, M.; Iwasa, J.; Baba, A.; Kochi, T.; Ohno, T.; Kubota, M.; Shirakami, Y.; Shiraki, M.; Takai, K.; et al. Preventive effects of branched-chain amino acid supplementation on the spontaneous development of hepatic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Carcinogenesis 2012, 33, 2499–2506. [Google Scholar] [CrossRef]
- Kim, H.H.; Park, C.S. Methionine cytotoxicity in the human breast cancer cell line MCF-7. In Vitro Cell Dev. Biol. Anim. 2003, 39, 117–119. [Google Scholar] [CrossRef]
- Rauschenbach, M.O.; Zharova, E.I.; Sergeeva, T.I.; Ivanova, V.D.; Probatova, N.A. Blastomogenic activity of p-hydroxyphenyllactic acid in mice. Cancer Res. 1975, 35, 577–585. [Google Scholar]
- Levchuk, A.A.; Faron, R.A.; Khrustalev, S.A.; Raushenbakh, M.O. Effect of the carcinogenic tyrosine metabolite p-hydroxyphenyllactic acid on the ascorbic acid concentration in the organs and blood of mice. Biull. Eksp. Biol. Med. 1986, 102, 462–463. [Google Scholar] [CrossRef]
- The metabolomics innovation centre (TMIC). Showing metabocard for 2-Hydroxycaproic acid (HMDB0001624). Available online: http://www.hmdb.ca/metabolites/HMDB0001624 (accessed on 30 July 2019).
- Khadge, S.; Sharp, J.G.; McGuire, T.R.; Thiele, G.M.; Black, P.; DiRusso, C.; Cook, L.; Klassen, L.W.; Talmadge, J.E. Immune regulation and anti-cancer activity by lipid inflammatory mediators. Int. Immunopharmacol. 2018, 65, 580–592. [Google Scholar] [CrossRef]
- Vinknes, K.J.; Elshorbagy, A.K.; Drevon, C.A.; Nurk, E.; Tell, G.S.; Nygård, O.; Vollset, S.E.; Refsum, H. Associations between plasma polyunsaturated fatty acids, plasma stearoyl-CoA desaturase indices and body fat. Obesity 2013, 21, E512–E519. [Google Scholar] [CrossRef]
- Ilag, L.L. Are long-chain polyunsaturated fatty acids the link between the immune system and the microbiome towards modulating cancer? Medicines 2018, 5, E102. [Google Scholar] [CrossRef]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current evidence linking polyunsaturated Fatty acids with cancer risk and progression. Front Oncol. 2013, 3, 224. [Google Scholar] [CrossRef]
- Amaral, M.E.A.; Nery, L.R.; Leite, C.E.; de Azevedo Junior, W.F.; Campos, M.M. Pre-clinical effects of metformin and aspirin on the cell lines of different breast cancer subtypes. Investig. New Drugs 2018, 36, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef]
- Mazid, M.A.; Chowdhury, A.A.; Nagao, K.; Nishimura, K.; Jisaka, M.; Nagaya, T.; Yokota, K. Endogenous 15-deoxy-D12,14-prostaglandin J2 synthesized by adipocytes during maturation phase contributes to upregulation of fat storage. FEBS Lett. 2006, 580, 6885–6890. [Google Scholar] [CrossRef]
- Su, Y.F.; Yang, S.H.; Lee, Y.H.; Wu, B.C.; Huang, S.C.; Liu, C.M.; Chen, S.L.; Pan, Y.F.; Chou, S.S.; Chou, M.Y.; et al. Aspirin-induced inhibition of adipogenesis was p53-dependentand associated with inactivation of pentose phosphate pathway. Eur. J. Pharmacol. 2014, 738, 101–110. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). National Institutes of Health, Aspirin to reduce cancer risk. Available online: https://www.cancer.gov/about-cancer/causes-prevention/research/aspirin-cancer-risk (accessed on 5 May 2017).
- Chen, W.Y.; Holmes, M.D. Role of Aspirin in Breast Cancer Survival. Curr. Oncol. Rep. 2017, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef]
- Lu, L.; Shi, L.; Zeng, J.; Wen, Z. Aspirin as a potential modality for the chemoprevention of breast cancer: A dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget 2017, 8, 40389–40401. [Google Scholar] [CrossRef]

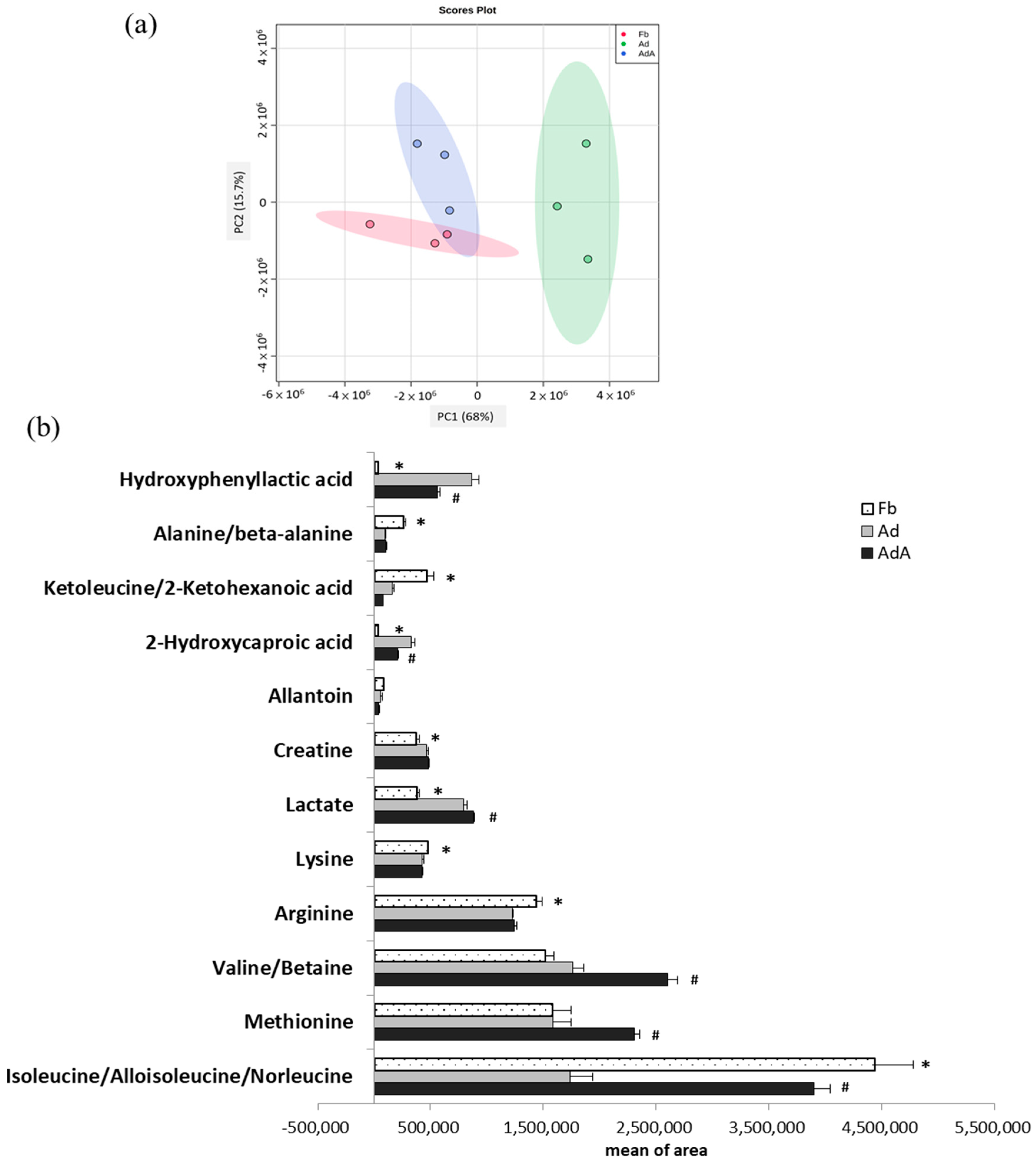

| Metabolites | Ad/Fb 1 | AdA/Ad 1 | Major Metabolic Pathway 3 | |||
|---|---|---|---|---|---|---|
| ANOVA 2 | Fold 2 | LSD 2 | Fold | LSD | ||
| Hydroxyphenyllactic acid | <0.001 | 26.8 | <0.001 | 0.7 | 0.002 | phenylalanine metabolism |
| 2-Hydroxycaproic acid | <0.001 | 9.01 | <0.001 | 0.63 | 0.003 | fatty acid metabolism |
| Lactate | <0.001 | 2.09 | <0.001 | 1.11 | 0.030 | energy metabolism metabolite |
| Creatine | 0.012 | 1.25 | 0.010 | 1.02 | 0.664 | participates in enzymatic reactions |
| Isoleucine/Alloisoleucine /Norleucine | <0.001 | 0.39 | 0.002 | 2.25 | 0.001 | valine, leucine, and isoleucine metabolism |
| Ketoleucine/2-Ketohexanoic acid | 0.001 | 0.35 | 0.001 | 0.48 | 0.169 | leucine biosynthesis |
| Valine/Betaine | <0.001 | 1.16 | 0.091 | 1.48 | <0.001 | valine, leucine, and isoleucine metabolism |
| Arginine | 0.007 | 0.85 | 0.004 | 1.01 | 0.755 | urea cycle/arginine biosynthesis |
| Methionine | 0.013 | 1.00 | 1.000 | 1.46 | 0.008 | cysteine and methionine metabolism |
| Alanine/beta-alanine | <0.001 | 0.37 | <0.001 | 1.09 | 0.586 | beta-alanine metabolism |
| Lysine | 0.033 | 0.90 | 0.021 | 1.00 | 0.993 | biotin metabolism, carnitine synthesis |
| Allantoin | 0.043 | 0.71 | 0.103 | 0.70 | 0.213 | product of the oxidation of uric acid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-C.; Chiu, H.-H.; Wang, C.-H.; Kuo, C.-H. Aspirin Modifies Inflammatory Mediators and Metabolomic Profiles and Contributes to the Suppression of Obesity-Associated Breast Cancer Cell Growth. Int. J. Mol. Sci. 2020, 21, 4652. https://doi.org/10.3390/ijms21134652
Hsieh C-C, Chiu H-H, Wang C-H, Kuo C-H. Aspirin Modifies Inflammatory Mediators and Metabolomic Profiles and Contributes to the Suppression of Obesity-Associated Breast Cancer Cell Growth. International Journal of Molecular Sciences. 2020; 21(13):4652. https://doi.org/10.3390/ijms21134652
Chicago/Turabian StyleHsieh, Chia-Chien, Huai-Hsuan Chiu, Chih-Hsuan Wang, and Ching-Hua Kuo. 2020. "Aspirin Modifies Inflammatory Mediators and Metabolomic Profiles and Contributes to the Suppression of Obesity-Associated Breast Cancer Cell Growth" International Journal of Molecular Sciences 21, no. 13: 4652. https://doi.org/10.3390/ijms21134652
APA StyleHsieh, C.-C., Chiu, H.-H., Wang, C.-H., & Kuo, C.-H. (2020). Aspirin Modifies Inflammatory Mediators and Metabolomic Profiles and Contributes to the Suppression of Obesity-Associated Breast Cancer Cell Growth. International Journal of Molecular Sciences, 21(13), 4652. https://doi.org/10.3390/ijms21134652