Biosensors for D-Amino Acids: Detection Methods and Applications
Abstract
1. Introduction
2. Biosensors
3. D-Amino Acids
4. Detection of D-AAs in Foods by Electrochemical Biosensors
5. Detection of D-AAs for Biomedical Applications
5.1. Enzymatic Biosensors
5.2. Microbiosensors
5.3. Fluorescence Biosensors
5.4. Alternative Biosensors
6. Summary and Conclusions
7. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAs | α-amino acids |
| Au-NF | Gold nanofilm |
| CNTs | Carbon nanotubes |
| CuNPs | Copper nanoparticles |
| D-AAs | D-enantiomer of α-amino acids |
| DAAO | D-amino acid oxidase |
| DNA/Ag NCs | DNA/silver nanoclusters |
| FAD | Flavin adenine dinucleotide |
| FIA | Flow-injection analysis |
| L-AAs | L-enantiomer of α-amino acids |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MIPs | Molecularly imprinted polymers |
| MWCNTs | Multiwall carbon nanotubes |
| NMDA | N-methyl-D-aspartate |
| PANI | Polyaniline |
| pkDAAO | D-amino acid oxidase from pig kidney |
| PGE | Pencil graphite electrode |
| PO | Pyruvate oxidase |
| PPD | Poly-m-phenylenediamine |
| PTh | Polythiophene |
| RgDAAO | D-amino acid oxidase from Rhodotorula gracilis |
| rGO | Reduced graphene oxide |
| SECM | Scanning electroanalytical technique |
| ZnSNPs | Zinc sulfide nanoparticles |
References
- Pollegioni, P.; Servi, S. Unnatural Amino Acids: Methods and Protocol; Humana Press: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Kalíková, K.; Šlechtová, T.; Tesařová, E. Enantiomeric ratio of amino acids as a tool for determination of aging and disease diagnostics by chromatographic measurement. Separations 2016, 3, 30. [Google Scholar] [CrossRef]
- Friedman, M. Origin, microbiology, nutrition, and pharmacology of D-amino acids. Chem. Biodivers. 2010, 7, 1491–1530. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Brückner, H. Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell. Amino Acids 2006, 31, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Barroso, S.; Santosjj-Delgado, M.J.; Martín-Olivar, C.; Polo-Díez, L.M. Indirect chiral HPLC determination and fluorimetric detection of D-amino acids in milk and oyster samples. J. Dairy Sci. 2006, 89, 82–89. [Google Scholar] [CrossRef]
- Martínez-Girón, A.B.; García-Ruiz, C.; Crego, A.L.; Marina, M.L. Development of an in-capillary derivatization method by CE for the determination of chiral amino acids in dietary supplements and wines. Electrophoresis 2009, 30, 696–704. [Google Scholar] [CrossRef]
- Gogami, Y.; Okada, K.; Oikawa, T. High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 3259–3267. [Google Scholar] [CrossRef]
- Nakano, Y.; Konya, Y.; Taniguchi, M.; Fukusaki, E. Development of a liquid chromatography-tandem mass spectrometry method for quantitative analysis of trace D-amino acids. J. Biosci. Bioeng. 2017, 123, 134–138. [Google Scholar] [CrossRef]
- Marcone, G.L.; Rosini, E.; Crespi, E.; Pollegioni, L. D-amino acids in foods. Appl. Microbiol. Biotechnol. 2020, 104, 555–574. [Google Scholar] [CrossRef]
- Carenzi, G.E.; Sacchi, S.; Abbondi, M.; Pollegioni, L. Direct chromatographic methods for enantioresolution of amino acids: Recent developments. Amino acids 2020. under review. [Google Scholar]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Cammann, K. Bio-sensors based on ion-selective electrodes. Z. Anal. Chem. 1977, 287, 1–9. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. International Union of Pure and Applied Chemistry Compendium of Chemical Terminology; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Patel, S.; Nanda, R.; Sahoo, S.; Mohapatra, E. Biosensors in health care: The milestones achieved in their development towards lab-on-chip-analysis. Biochem. Res. Int. 2016, 2016, 3130469. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors (Basel) 2016, 16, 780. [Google Scholar] [CrossRef]
- Pundir, C.S.; Lata, S.; Narwal, V. Biosensors for determination of D and L- amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef]
- Kacaniklic, V.; Johansson, K.; Marko-Varga, G.; Gorton, L.; Jönsson-Pettersson, G.; Csöregi, E. Amperometric biosensors for detection of L- and D-amino acids based on coimmobilized peroxidase and L- and D-amino acid oxidases in carbon paste electrodes. Electroanalysis 1994, 6, 381–390. [Google Scholar] [CrossRef]
- Sacchi, S.; Pollegioni, L.; Pilone, M.S.; Rossetti, C. Determination of D-amino acids using a D-amino acid oxidase biosensor with spectrophotometric and potentiometric detection. Biotechnol. Tech. 1998, 12, 149–153. [Google Scholar] [CrossRef]
- Rosini, E.; Molla, G.; Rossetti, C.; Pilone, M.S.; Pollegioni, L.; Sacchi, S. A biosensor for all D-amino acids using evolved D-amino acid oxidase. J. Biotechnol. 2008, 135, 377–384. [Google Scholar] [CrossRef]
- Metkar, S.K.; Girigoswami, K. Diagnostic biosensors in medicine—A review. Biocatal. Agric. Biotechnol. 2019, 17, 271–283. [Google Scholar] [CrossRef]
- Thakur, M.S.; Ragavan, K.V. Biosensors in food processing. J. Food Sci. Technol. 2013, 50, 625–641. [Google Scholar] [CrossRef]
- Castillo, J.; Gáspár, S.; Leth, S.; Niculescu, M.; Mortari, A.; Bontidean, I.; Soukharev, V.; Dorneanu, S.A.; Ryabov, A.D.; Csöregi, E. Biosensors for life quality: Design, development and applications. Sens. Actuators B Chem. 2004, 102, 179–194. [Google Scholar] [CrossRef]
- Malima, A.; Siavoshi, S.; Musacchio, T.; Upponi, J.; Yilmaz, C.; Somu, S.; Hartner, W.; Torchilin, V.; Busnaina, A. Highly sensitive microscale in vivo sensor enabled by electrophoretic assembly of nanoparticles for multiple biomarker detection. Lab Chip 2012, 12, 4748–4754. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research, Inc. Available online: https://www.grandviewresearch.com/industry-analysis/biosensors-market (accessed on 8 May 2020).
- De Jonge, B.L.; Gage, D.; Xu, N. The carboxyl terminus of peptidoglycan stem peptides is a determinant for methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 3151–3155. [Google Scholar] [CrossRef]
- Cava, F.; de Pedro, M.A.; Lam, H.; Davis, B.M.; Waldor, M.K. Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 2011, 30, 3442–3453. [Google Scholar] [CrossRef]
- Pidgeon, S.E.; Fura, J.M.; Leon, W.; Birabaharan, M.; Vezenov, D.; Pires, M.M. Metabolic profiling of bacteria by unnatural C-terminated D-amino acids. Angew. Chem. Int. Ed. Engl. 2015, 54, 6158–6162. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, J.J. D-amino acids in animals. Science 1969, 164, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Nishikawa, T.; Oka, T.; Takahashi, K.; Hayashi, T. Determination of free amino acid enantiomers in rat brain and serum by high-performance liquid chromatography after derivatization with N-tert-butyloxycarbonyl-L-cysteine and o-phthaldialdehyde. J. Chromatogr. 1992, 582, 41–48. [Google Scholar] [CrossRef]
- Wolosker, H.; Sheth, K.N.; Takahashi, M.; Mothet, J.P.; Brady, R.O.; Ferris, C.D.; Snyder, S.H. Purification of serine racemase: Biosynthesis of the neuromodulator D-serine. Proc. Natl. Acad. Sci. USA 1999, 96, 721–725. [Google Scholar] [CrossRef]
- Snyder, S.H.; Kim, P.M. D-amino acids as putative neurotransmitters: Focus on D-serine. Neurochem. Res. 2000, 25, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Sacchi, S. Metabolism of the neuromodulator D-serine. Cell. Mol. Life Sci. 2010, 67, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, M.; Martineau, M.; Sacchi, S.; Yatsenko, N.; Radzishevsky, I.; Conrod, S.; Ait Ouares, K.; Wolosker, H.; Pollegioni, L.; Billard, J.M.; et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E204–E213. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Shi, T.; Sweedler, J.V. D-aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 2012, 43, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Errico, F.; Napolitano, F.; Nisticò, R.; Usiello, A. New insights on the role of free D-aspartate in the mammalian brain. Amino Acids 2012, 43, 1861–1871. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Santillo, A.; Chieffi Baccari, G. Current knowledge of D-aspartate in glandular tissues. Amino Acids 2014, 46, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Hamase, K.; Miyoshi, Y.; Yamamoto, R.; Yasuda, K.; Mita, M.; Rakug, H.; Hayashi, T.; Isaka, Y. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci. Rep. 2016, 6, 26137. [Google Scholar] [CrossRef]
- Hesaka, A.; Yasuda, K.; Sakai, S.; Yonishi, H.; Namba-Hamano, T.; Takahashi, A.; Mizui, M.; Hamase, K.; Matsui, R.; Mita, M.; et al. Dynamics of D-serine reflected the recovery course of a patient with rapidly progressive glomerulonephritis. CEN Case Rep. 2019, 8, 297–300. [Google Scholar] [CrossRef]
- Hashimoto, K.; Engberg, G.; Shimizu, E.; Nordin, C.; Lindström, L.H.; Iyo, M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 767–769. [Google Scholar] [CrossRef]
- Ohnuma, T.; Sakai, Y.; Maeshima, H.; Hatano, T.; Hanzawa, R.; Abe, S.; Kida, S.; Shibata, N.; Suzuki, T.; Arai, H. Changes in plasma glycine, L-serine, and D-serine levels in patients with schizophrenia as their clinical symptoms improve: Results from the Juntendo University Schizophrenia Projects (JUSP). Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008, 32, 1905–1912. [Google Scholar] [CrossRef]
- Sacchi, S.; Rosini, E.; Pollegioni, L.; Molla, G. D-amino acid oxidase inhibitors as a novel class of drugs for schizophrenia therapy. Curr. Pharm. Des. 2013, 19, 2499–2511. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A.; et al. D-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef] [PubMed]
- Guercio, G.D.; Panizzutti, R. Potential and challenges for the clinical use of D-serine as a cognitive enhancer. Front. Psychiatry 2018, 5, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Pantazopoulos, H.; Huang, C.C.Y.; Muszynski, K.; Harvey, T.L.; Uno, Y.; Rorabaugh, J.M.; Galloway, C.R.; Botz-Zapp, C.; Berretta, S.; et al. Neurotoxic astrocytes express the D-serine synthesizing enzyme, serine racemase, in Alzheimer’s disease. Neurobiol. Dis. 2019, 130, 104511. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and microbiology of D-amino acids. Agric. Food Chem. 1999, 47, 3457–3479. [Google Scholar] [CrossRef]
- Pollegioni, L.; Rosini, E.; Molla, G. Advances in enzymatic synthesis of D-amino acids. Int. J. Mol. Sci. 2020, 21, 3206. [Google Scholar] [CrossRef]
- Szökő, É.; Vincze, I.; Tábi, T. Chiral separations for D-amino acid analysis in biological samples. J. Pharm. Biomed. Anal. 2016, 130, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Piubelli, L.; Sacchi, S.; Pilone, M.S.; Molla, G. Physiological functions of D-amino acid oxidases: From yeast to humans. Cell. Mol. Life Sci. 2007, 64, 1373–1394. [Google Scholar] [CrossRef]
- Molla, G.; Piubelli, L.; Volontè, F.; Pilone, M.S. Enzymatic detection of D-amino acids. Methods Mol. Biol. 2012, 794, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Molla, G.; Porrini, D.; Job, V.; Motteran, L.; Vegezzi, C.; Campaner, S.; Pilone, M.S.; Pollegioni, L. Role of arginine 285 in the active site of Rhodotorula gracilis D-amino acid oxidase. A site-directed mutagenesis study. J. Biol. Chem. 2000, 275, 24715–24721. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, S.; Lorenzi, S.; Molla, G.; Pilone, M.S.; Rossetti, C.; Pollegioni, L. Engineering the substrate specificity of D-amino-acid oxidase. J. Biol. Chem. 2002, 277, 27510–27516. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Molla, G. New biotech applications from evolved D-amino acid oxidases. Trends Biotechnol. 2011, 29, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Frattini, L.; Rosini, E.; Pollegioni, L.; Pilone, M.S. Analyzing the D-amino acid content in biological samples by engineered enzymes. J. Chrom. B 2011, 879, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Saam, J.; Rosini, E.; Molla, G.; Schulten, K.; Pollegioni, L.; Ghisla, S. O2 reactivity of flavoproteins: Dynamic access of dioxygen to the active site and role of a H+ relay system in D-amino acid oxidase. J. Biol. Chem. 2010, 285, 24439–24446. [Google Scholar] [CrossRef] [PubMed]
- Rosini, E.; Molla, G.; Ghisla, S.; Pollegioni, L. On the reaction of D-amino acid oxidase with dioxygen: O2 diffusion pathways and enhancement of reactivity. FEBS J. 2011, 278, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Váradi, M.; Adányi, N.; Szabó, E.E.; Trummer, N. Determination of the ratio of D- and L-amino acids in brewing by an immobilised amino acid oxidase enzyme reactor coupled to amperometric detection. Biosens. Bioelectron. 1999, 14, 335–340. [Google Scholar] [CrossRef]
- Inaba, Y.; Mizukami, K.; Hamada-Sato, N.; Kobayashi, T.; Imada, C.; Watanabe, E. Development of a D-alanine sensor for the monitoring of a fermentation using the improved selectivity by the combination of D-amino acid oxidase and pyruvate oxidase. Biosens. Bioelectron. 2003, 19, 423–431. [Google Scholar] [CrossRef]
- Sarkar, P.; Tothill, I.E.; Setford, S.J.; Turner, A.P. Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst 1999, 124, 865–870. [Google Scholar] [CrossRef]
- Wcisło, M.; Compagnone, D.; Trojanowicz, M. Enantioselective screen-printed amperometric biosensor for the determination of D-amino acids. Bioelectrochemistry 2007, 71, 91–98. [Google Scholar] [CrossRef]
- Lata, S.; Batra, B.; Kumar, P.; Pundir, C.S. Construction of an amperometric D-amino acid biosensor based on D-amino acid oxidase/carboxylated mutliwalled carbon nanotube/copper nanoparticles/polyalinine modified gold electrode. Anal. Biochem. 2013, 437, 1–9. [Google Scholar] [CrossRef]
- Lata, S.; Batra, B.; Pundir, C.S. Construction of D-amino acid biosensor based on D-amino acid oxidase immobilized onto poly (indole-5-carboxylic acid)/zinc sulfide nanoparticles hybrid film. Process Biochem. 2012, 47, 2131–2138. [Google Scholar] [CrossRef]
- Lata, S.; Pundir, C.S. Fabrication of an amperometric D-amino acid biosensor based on nickel hexacyanoferrate polypyrrole hybrid film deposited on glassy carbon electrode. Bioprocess Biosyst. Eng. 2013, 36, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Batalla, P.; Hernández-Ferrer, J.; Martínez, M.T.; Escarpa, A. Graphene oxide nanoribbon-based sensors for the simultaneous bio-electrochemical enantiomeric resolution and analysis of amino acid biomarkers. Biosens. Bioelectron. 2015, 68, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Batalla, P.; Martín, A.; López, M.Á.; González, M.C.; Escarpa, A. Enzyme-based microfluidic chip coupled to graphene electrodes for the detection of D-amino acid enantiomer-biomarkers. Anal. Chem. 2015, 87, 5074–5078. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Enzymatic biosensor based on entrapment of D-amino acid oxidase on gold nanofilm/MWCNTs nanocomposite modified glassy carbon electrode by sol-gel network: Analytical applications for D-alanine in human serum. Enzyme Microb. Technol. 2017, 100, 20–27. [Google Scholar] [CrossRef]
- García-Carmona, L.; Moreno-Guzmán, M.; González, M.C.; Escarpa, A. Class enzyme-based motors for “on the fly” enantiomer analysis of amino acids. Biosens. Bioelectron. 2017, 96, 275–280. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of D-amino acid oxidase and hemoglobin as efficient bio-nanocomposite in fabrication of dopamine bi-enzyme biosensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 637–645. [Google Scholar] [CrossRef]
- Nieh, C.H.; Kitazumi, Y.; Shirai, O.; Kano, K. Sensitive D-amino acid biosensor based on oxidase/peroxidase system mediated by pentacyanoferrate-bound polymer. Biosens. Bioelectron. 2013, 47, 350–355. [Google Scholar] [CrossRef][Green Version]
- Pernot, P.; Maucler, C.; Tholance, Y.; Vasylieva, N.; Debilly, G.; Pollegioni, L.; Cespuglio, R.; Marinesco, S. D-serine diffusion through the blood-brain barrier: Effect on D-serine compartmentalization and storage. Neurochem. Int. 2012, 60, 837–845. [Google Scholar] [CrossRef]
- Vasylieva, N.; Barnych, B.; Meiller, A.; Maucler, C.; Pollegioni, L.; Lin, J.S.; Barbier, D.; Marinesco, S. Covalent enzyme immobilization by poly(ethylene glycol) diglycidyl ether (PEGDE) for microelectrode biosensor preparation. Biosens. Bioelectron. 2011, 26, 3993–4000. [Google Scholar] [CrossRef]
- Polcari, D.; Kwan, A.; Van Horn, M.R.; Danis, L.; Pollegioni, L.; Ruthazer, E.S.; Mauzeroll, J. Disk-shaped amperometric enzymatic biosensor for in vivo detection of D-serine. Anal. Chem. 2014, 86, 3501–3507. [Google Scholar] [CrossRef] [PubMed]
- Campos-Beltrán, D.; Konradsson-Geuken, Å.; Quintero, J.E.; Marshall, L. Amperometric self-referencing ceramic based microelectrode arrays for D-serine detection. Biosensors (Basel) 2018, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Liu, P.; Yang, L.; Jiang, X.; Luo, D.; Yang, D. Non-invasive detection of gastric cancer relevant D-amino acids with luminescent DNA/silver nanoclusters. Nanoscale 2017, 9, 19367–19373. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, Y.; Yang, J.; Guo, Y.; Zeng, X.; Zhou, S.; Cheng, J.; Zhang, Y. An aptamer-based fluorescence bio-sensor for chiral recognition of arginine enantiomers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 200, 330–338. [Google Scholar] [CrossRef] [PubMed]
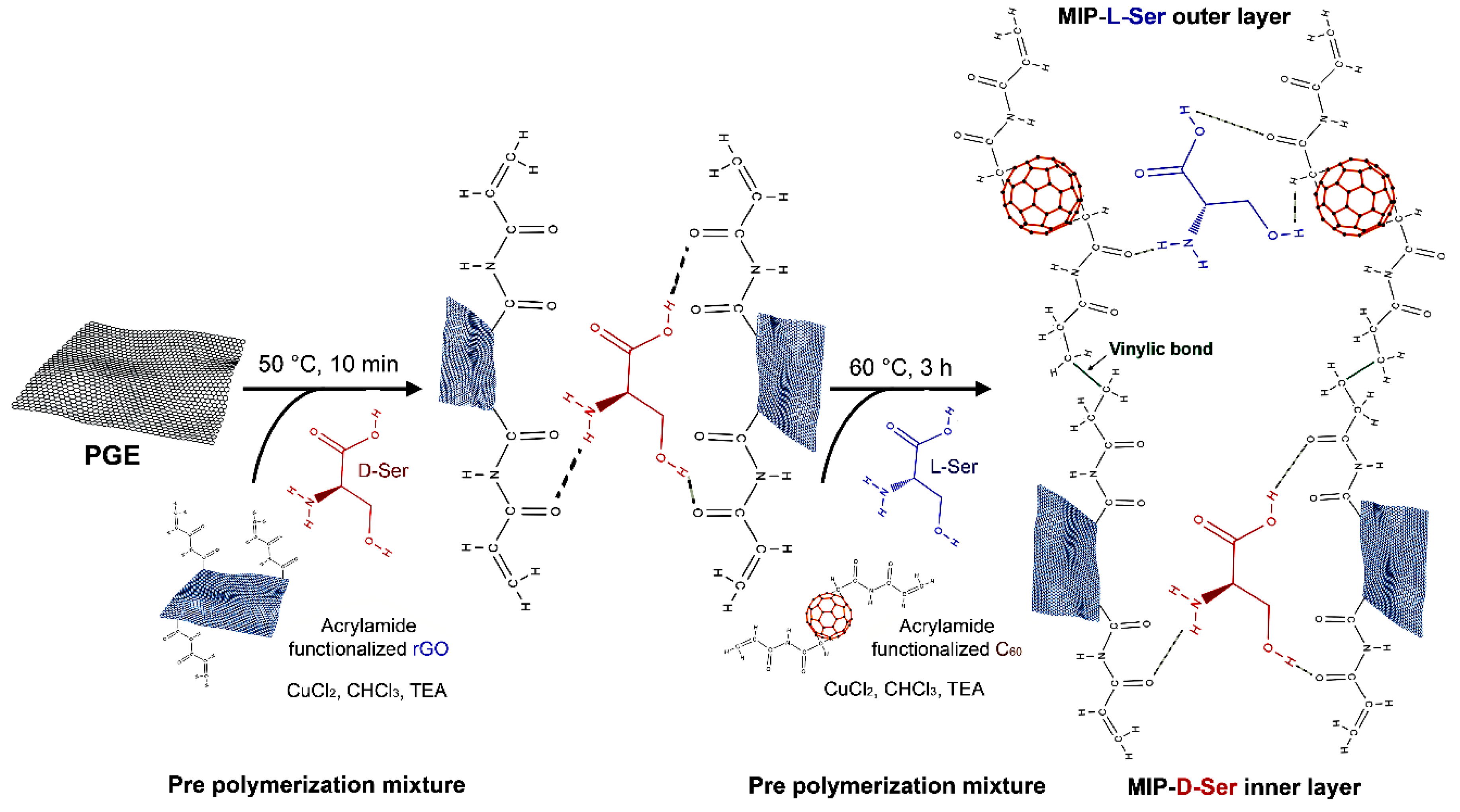
- Jaiswal, S.; Singh, R.; Singh, K.; Fatma, S.; Prasad, B.B. Enantioselective analysis of D- and L- serine on a layer-by-layer imprinted electrochemical sensor. Biosens. Bioelectron. 2019, 124-125, 176–183. [Google Scholar] [CrossRef]
- Pernot, P.; Mothet, J.P.; Schuvailo, O.; Soldatkin, A.; Pollegioni, L.; Pilone, M.; Adeline, M.T.; Cespuglio, R.; Marinesco, S. Characterization of a yeast D-amino acid oxidase microbiosensor for D-serine detection in the central nervous system. Anal. Chem. 2008, 80, 1589–1597. [Google Scholar] [CrossRef]
- Maucler, C.; Pernot, P.; Vasylieva, N.; Pollegioni, L.; Marinesco, S. In vivo D-serine hetero-exchange through alanine-serine-cysteine (ASC) transporters detected by microelectrode biosensors. ACS Chem. Neurosci. 2013, 4, 772–781. [Google Scholar] [CrossRef]
- Zou, C.; Crux, S.; Marinesco, S.; Montagna, E.; Sgobio, C.; Shi, Y.; Shi, S.; Zhu, K.; Dorostkar, M.M.; Müller, U.C.; et al. Amyloid precursor protein maintains constitutive and adaptive plasticity of dendritic spines in adult brain by regulating D-serine homeostasis. EMBO J. 2016, 35, 2213–2222. [Google Scholar] [CrossRef]
- Papouin, T.; Haydon, P.G. D-serine measurements in brain slices or other tissue explants. Bio Protoc. 2018, 8, e2698. [Google Scholar] [CrossRef] [PubMed]
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jégo, P.; Vigneron, P.A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y.; et al. Impairment of glycolysis-derived L-serine production in astrocytes contributes to cognitive deficits in Alzheimer’s disease. Cell Metab. 2020, 31, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Polcari, D.; Perry, S.C.; Pollegioni, L.; Geissler, M.; Mauzeroll, J. Localized detection of D-serine using an enzymatic amperometric biosensor and scanning electrochemical microscopy. ChemElectroChem 2017, 4, 920–926. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z. Chemiluminescence flow biosensor for determination of total D-amino acid in serum with immobilized reagents. Sensor Actuat. B-Chem. 2000, 69, 70–74. [Google Scholar] [CrossRef]
- Staiano, M.; Strianese, M.; Varriale, A.; Di Giovanni, S.; di Mase, D.S.; Dell’Angelo, V.; Ruggiero, G.; Labella, T.; Pellecchia, C.; D’Auria, S. D-serine-dehydratase from Saccaromyces cerevisiae: A pyridoxal 5′- phosphate-dependent enzyme for advanced biotech applications. Protein Pept. Lett. 2012, 19, 592–595. [Google Scholar] [CrossRef]
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K.; et al. Possible role of D-serine in the pathophysiology of Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 385–388. [Google Scholar] [CrossRef]
- Nuzzo, T.; Feligioni, M.; Cristino, L.; Pagano, I.; Marcelli, S.; Iannuzzi, F.; Imperatore, R.; D’Angelo, L.; Petrella, C.; Carella, M.; et al. Free D-aspartate triggers NMDA receptor-dependent cell death in primary cortical neurons and perturbs JNK activation, Tau phosphorylation, and protein SUMOylation in the cerebral cortex of mice lacking D-aspartate oxidase activity. Exp. Neurol. 2019, 317, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Piubelli, L.; Pollegioni, L.; Rabattoni, V.; Mauri, M.; Princiotta-Cariddi, L.; Versino, M.; Sacchi, S. Serum D-serine levels are altered in early phases of Alzheimer’s disease: Towards a precocious biomarker. Transl. Psychiatry 2020, in press. [Google Scholar]
- Wang, Y.C.; Stevens, A.L.; Han, J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef]
- Belluzo, M.S.; Ribone, M.E.; Lagier, C.M. Assembling amperometric biosensors for clinical diagnostics. Sensors 2008, 8, 1366–1399. [Google Scholar] [CrossRef] [PubMed]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef] [PubMed]
- Medvedovici, A.; Bacalum, E.; David, V. Sample preparation for large-scale bioanalytical studies based on liquid chromatographic techniques. Biomed. Chromatogr. 2018, 32, e4137. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, K.H.; Vellingiri, K.; Samaddar, P.; Kumar, P.; Deep, A.; Kumar, N. Hybrid porous thin films: Opportunities and challenges for sensing applications. Biosens. Bioelectron. 2018, 104, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.F.; Giacomelli, C.E.; Garcia, C.D. Interaction of D-amino acid oxidase with carbon nanotubes: Implications in the design of biosensors. Anal. Chem. 2009, 81, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, S.; Cappelletti, P.; Giovannardi, S.; Pollegioni, L. Evidence for the interaction of D-amino acid oxidase with pLG72 in a glial cell line. Mol. Cell. Neurosci. 2011, 48, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, P.; Campomenosi, P.; Pollegioni, L.; Sacchi, S. The degradation (by distinct pathways) of human D-amino acid oxidase and its interacting partner pLG72-two key proteins in D-serine catabolism in the brain. FEBS J. 2014, 281, 708–723. [Google Scholar] [CrossRef]
- Nuzzo, T.; Sacchi, S.; Errico, F.; Keller, S.; Palumbo, O.; Florio, E.; Punzo, D.; Napolitano, F.; Copetti, M.; Carella, M.; et al. Decreased free D-aspartate levels are linked to enhanced D-aspartate oxidase activity in the dorsolateral prefrontal cortex of schizophrenia patients. NPJ Schizophr. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Mothet, J.P.; Billard, J.M.; Pollegioni, L.; Coyle, J.T.; Sweedler, J.V. Investigating brain D-serine: Advocacy for good practices. Acta Physiol. 2019, 226, e13257. [Google Scholar] [CrossRef] [PubMed]
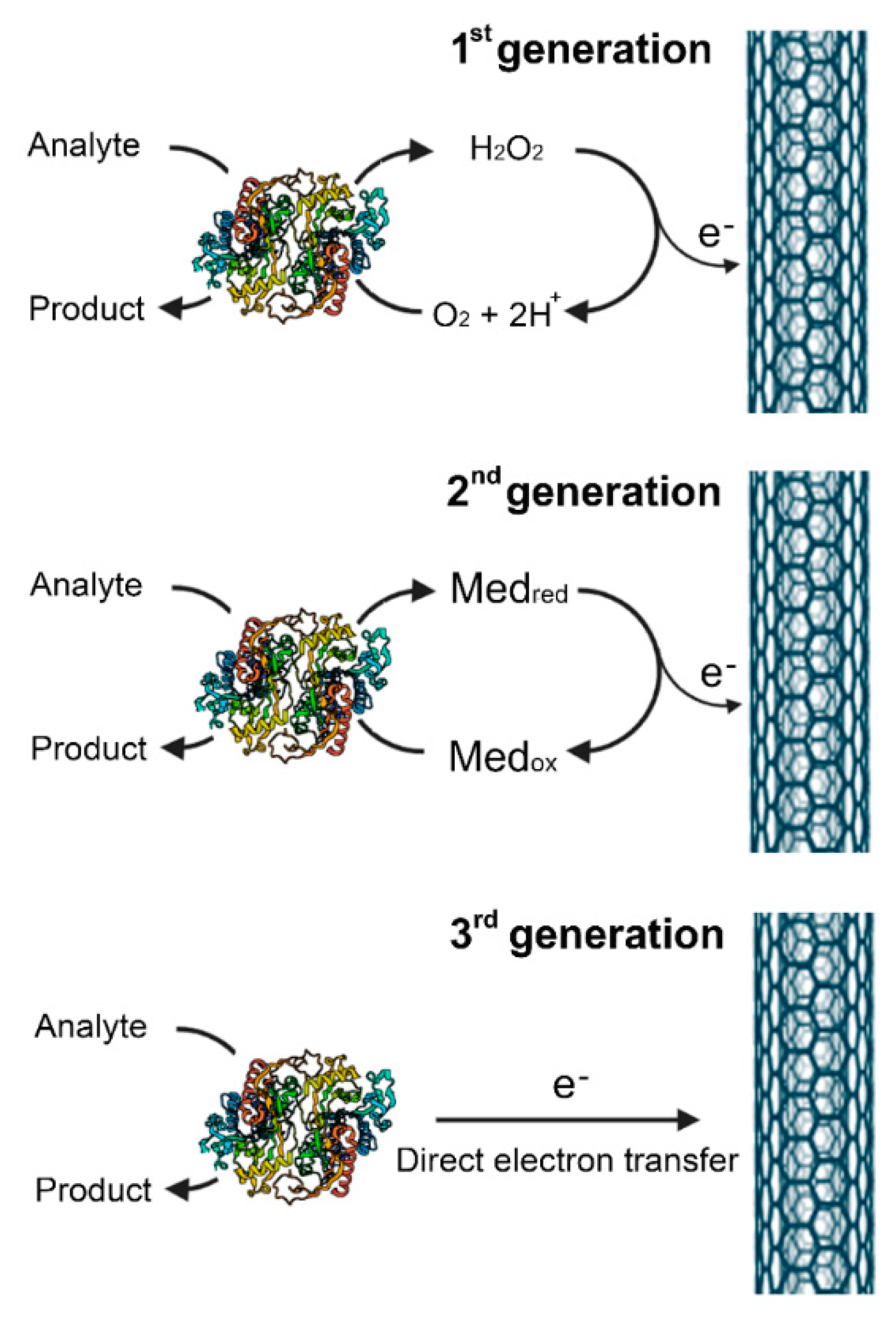
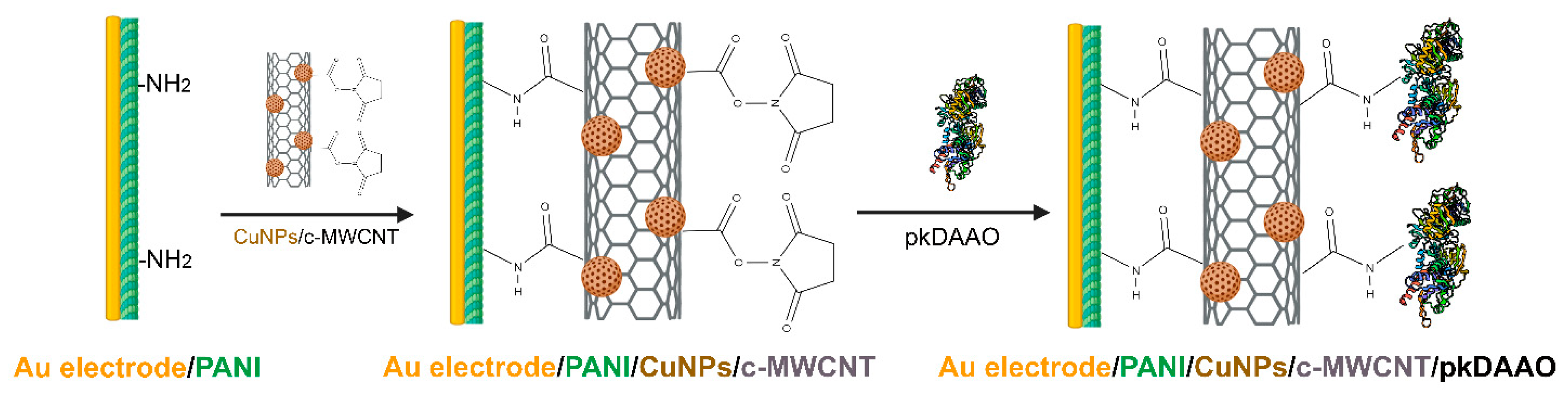
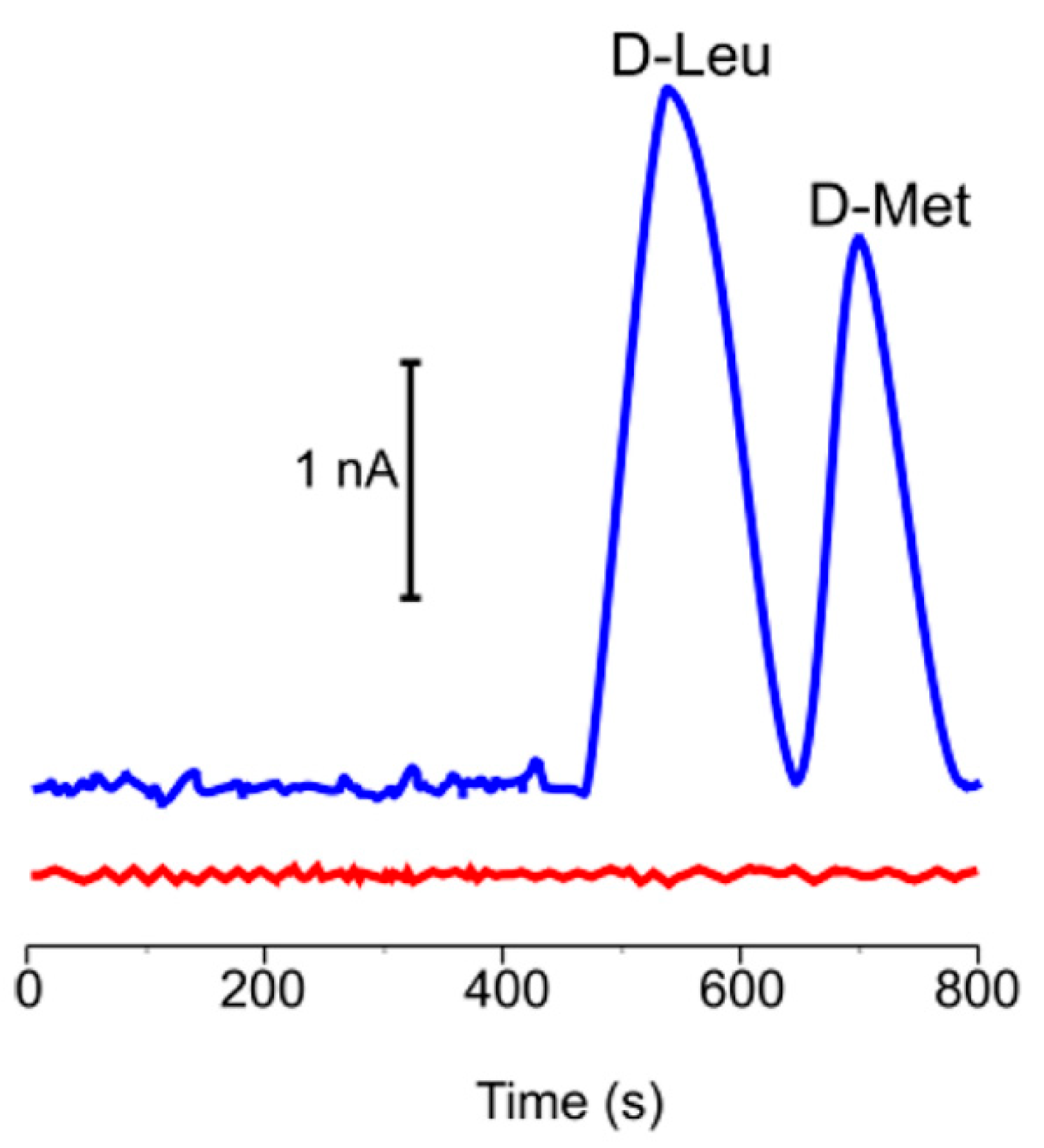
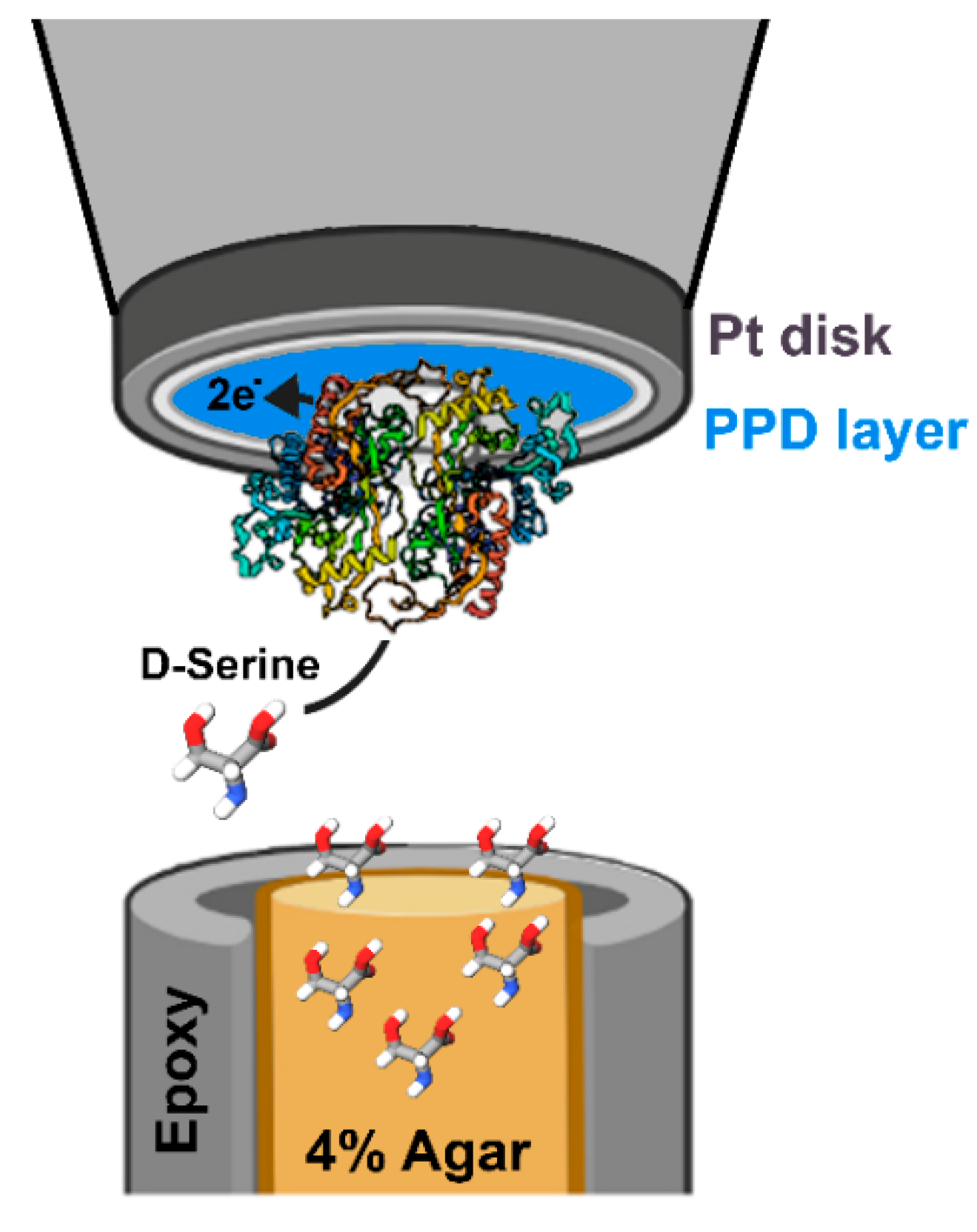
| DAAO Source | Optimal Temperature (°C) | Response Time | LOD (mM) | Detected D-AAs | Application | Ref. |
|---|---|---|---|---|---|---|
| R. gracilis | 25 | 5 min | 0.15 | Ala | Milk | [21] |
| 25 | 15 min | 0.25 | Ala, Gln, Glu, Lys, Met | Grana Padano cheese | [22] | |
| Pig kidney | 40 | 1 min | 0.2 | Ala, His, Ile, Leu, Met, Phe, Pro, Trp, Val | Beer fermentation | [59] |
| 25 | 12 min | 0.05 | Ala | Fish sauce | [60] | |
| 25 | 4 min | 0.47 | Ala, Arg, Met, Phe, Pro, Val | Milk | [61] | |
| 25 | 3 min | 0.001–0.03 | Ala | Milk, fruit juice | [62] | |
| 30 | 2 s | 0.0002 | Ala | Fruit juice | [63] | |
| Goat kidney | 35 | 3 s | 0.001 | Ala | Fruit juice | [64] |
| Bioreceptor | Assay Technique | Response Time | LOD (μM) | Biological Sample | Ref. |
|---|---|---|---|---|---|
| pkDAAO | Amperometric | 6 min | 60 | Urine | [66] |
| Amperometric | 6 min | 1000 | [67] | ||
| Amperometric | 10 s | 0.02 | Serum | [68] | |
| Amperometric | 2 min | 6 | V. cholerae cultures | [69] | |
| Amperometric | 5 s | 0.04 | Serum | [70] | |
| Amperometric | 50 s | 2 | Urine | [71] | |
| DAAO from goat kidney | Amperometric | 1 s | 1.5 | Serum, urine | [65] |
| RgDAAO | Amperometric | 2 s | 0.016 | Rat frontal cortex | [72] |
| Amperometric | 4 s | 0.008 | Rat frontal cortex | [73] | |
| Amperometric | 2 min | 0.6 | Xenopus laevis brain | [74] | |
| Amperometric | 10 s | 0.17 | Rat brain | [75] | |
| DNA | Fluorimetric | 60 min | 0.1-1 | Saliva | [76] |
| Aptamer | Fluorimetric | 45 min | 0.002 | Urine | [77] |
| Dual imprinted polymer | Amperometric | 3 min | 2.3 | Serum, brain, drugs | [78] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosini, E.; D’Antona, P.; Pollegioni, L. Biosensors for D-Amino Acids: Detection Methods and Applications. Int. J. Mol. Sci. 2020, 21, 4574. https://doi.org/10.3390/ijms21134574
Rosini E, D’Antona P, Pollegioni L. Biosensors for D-Amino Acids: Detection Methods and Applications. International Journal of Molecular Sciences. 2020; 21(13):4574. https://doi.org/10.3390/ijms21134574
Chicago/Turabian StyleRosini, Elena, Paola D’Antona, and Loredano Pollegioni. 2020. "Biosensors for D-Amino Acids: Detection Methods and Applications" International Journal of Molecular Sciences 21, no. 13: 4574. https://doi.org/10.3390/ijms21134574
APA StyleRosini, E., D’Antona, P., & Pollegioni, L. (2020). Biosensors for D-Amino Acids: Detection Methods and Applications. International Journal of Molecular Sciences, 21(13), 4574. https://doi.org/10.3390/ijms21134574








