Diverse Physiological Functions of Cation Proton Antiporters across Bacteria and Plant Cells
Abstract
1. Introduction
2. Structure of CPAs
3. Physiological Functions of CPAs in E. coli
3.1. CPAs as Na+ Extrusion Systems
3.2. CPAs as pH Regulators at Alkaline pH
3.3. CPAs as Antimicrobial Stress Mechanisms
4. Physiological Function of CPAs in Cyanobacteria
5. Physiological Functions of CPAs in Plants
5.1. CPAs in the Plasma Membrane Are Required for Salt Extrusion and Nutrient Uptake
5.2. Vacuolar CPAs Are Important for Cell Turgor Pressure and Storage of Intracellular K+
5.3. Regulation of Photosynthesis by Multiple CPAs
5.4. Role of Endosome-Localized CPAs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CPA | cation/proton antiporter |
| CpHMD | constant pH molecular dynamics simulations |
| KTN | K+ transport nucleotide binding |
| NHX | Na+/H+ exchanger |
| CHX | cation/H+ exchanger |
| KEA | K+ efflux antiporters |
| NPQ | non-photochemical quenching |
| OEC | oxygen evolving complex |
| TGN | trans-Golgi network |
| PVC | prevacuolar compartment |
| VSR | vacuolar sorting receptor |
| PIN | PIN FORMED |
| ER | endoplasmic reticulum |
References
- Meury, J.; Kepes, A. The regulation of potassium fluxes for the adjustment and maintenance of potassium levels in Escherichia coli. Eur. J. Biochem. 1981, 119, 165–170. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. FEBS J. 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Etherton, B.; Higinbotham, N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science 1960, 131, 409–410. [Google Scholar] [CrossRef]
- Cheeseman, J.M.; Hanson, J.B. Mathematical analysis of the dependence of cell potential on external potassium in corn roots. Plant Physiol. 1979, 63, 1–4. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Sanders, D. Energization of potassium uptake in Arabidopsis thaliana. Planta 1993, 191, 302–307. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Gohara, D.W.; Di Cera, E. Molecular mechanisms of enzyme activation by monovalent cations. J. Biol. Chem. 2016, 291, 20840–20848. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Uozumi, N. Uniquely evolved plant ion channels. FEBS J. 2011, 278, 4261. [Google Scholar] [CrossRef]
- Lo, C.J.; Leake, M.C.; Berry, R.M. Fluorescence measurement of intracellular sodium concentration in single Escherichia coli cells. Biophys. J. 2006, 90, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, N.; Dreyer, I. Structure-function correlates in plant ion channels. In Comprehensive Biophysics; Academic Press: Cambridge, MA, USA, 2012; Volume 6, pp. 234–245. [Google Scholar]
- Brett, C.L.; Donowitz, M.; Rao, R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am. J. Physiol. Cell Physiol. 2005, 288, C223–C229. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Ding, L.; Zhu, J.K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 1996, 8, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, S.; Fishkes, H. Sodium-proton antiport in isolated membrane vesicles of Escherichia coli. Biochemistry 1978, 17, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Postier, B.L.; Burnap, R.L. Polymerase chain reaction-based mutageneses identify key transporters belonging to multigene families involved in Na+ and ph homeostasis of Synechocystis sp. PCC 6803. Mol. Microbiol. 2002, 44, 1493–1506. [Google Scholar] [CrossRef]
- Chanroj, S.; Lu, Y.; Padmanaban, S.; Nanatani, K.; Uozumi, N.; Rao, R.; Sze, H. Plant-specific cation/H+ exchanger 17 and its homologs are endomembrane K+ transporters with roles in protein sorting. J. Biol. Chem. 2011, 286, 33931–33941. [Google Scholar] [CrossRef]
- Bassil, E.; Ohto, M.A.; Esumi, T.; Tajima, H.; Zhu, Z.; Cagnac, O.; Belmonte, M.; Peleg, Z.; Yamaguchi, T.; Blumwald, E. The Arabidopsis intracellular Na+ /H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 2011, 23, 224–239. [Google Scholar] [CrossRef]
- Chanroj, S.; Wang, G.; Venema, K.; Zhang, M.W.; Delwiche, C.F.; Sze, H. Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front. Plant Sci. 2012, 3, 25. [Google Scholar] [CrossRef]
- Tsunekawa, K.; Shijuku, T.; Hayashimoto, M.; Kojima, Y.; Onai, K.; Morishita, M.; Ishiura, M.; Kuroda, T.; Nakamura, T.; Kobayashi, H.; et al. Identification and characterization of the Na+/H+ antiporter NhaS3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem. 2009, 284, 16513–16521. [Google Scholar] [CrossRef]
- Tsujii, M.; Kera, K.; Hamamoto, S.; Kuromori, T.; Shikanai, T.; Uozumi, N. Evidence for potassium transport activity of Arabidopsis KEA1-KEA6. Sci. Rep. 2019, 9, 10040. [Google Scholar] [CrossRef]
- Venema, K.; Quintero, F.J.; Pardo, J.M.; Donaire, J.P. The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J. Biol. Chem. 2002, 277, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Sicilia, M.N.; Cagnac, O.; Chanroj, S.; Sze, H.; Rodríguez-Rosales, M.P.; Venema, K. Arabidopsis KEA2, a homolog of bacterial KefC, encodes a K+/H+ antiporter with a chloroplast transit peptide. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Taglicht, D.; Padan, E.; Schuldiner, S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J. Biol. Chem. 1991, 266, 11289–11294. [Google Scholar] [PubMed]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yashiro, S.; Dotson, D.L.; Uzdavinys, P.; Iwata, S.; Sansom, M.S.P.; von Ballmoos, C.; Beckstein, O.; Drew, D.; Cameron, A.D. Crystal structure of the sodium-proton antiporter NhaA dimer and new mechanistic insights. J. Gen. Physiol. 2014, 144, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Michel, H. NhaA: A unique structural fold of secondary active transporters. Isr. J. Chem. 2015, 55, 1233–1239. [Google Scholar] [CrossRef]
- Padan, E.; Kozachkov, L.; Herz, K.; Rimon, A. NhaA crystal structure: Functional-structural insights. J. Exp. Biol. 2009, 212, 1593–1603. [Google Scholar] [CrossRef]
- Maes, M.; Rimon, A.; Kozachkov-Magrisso, L.; Friedler, A.; Padan, E. Revealing the ligand binding site of NhaA Na+/H+ antiporter and its pH dependence. J. Biol. Chem. 2012, 287, 38150–38157. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Dotson, D.L.; Beckstein, O.; Shen, J. Mechanism of pH-dependent activation of the sodium-proton antiporter NhaA. Nat. Commun. 2016, 7, 12940. [Google Scholar] [CrossRef]
- Patiño-Ruiz, M.; Dwivedi, M.; Cǎlinescu, O.; Karabel, M.; Padan, E.; Fendler, K. Replacement of Lys-300 with a glutamine in the NhaA Na+/H+ antiporter of Escherichia coli yields a functional electrogenic transporter. J. Biol. Chem. 2019, 294, 246–256. [Google Scholar] [CrossRef]
- Masrati, G.; Dwivedi, M.; Rimon, A.; Gluck-Margolin, Y.; Kessel, A.; Ashkenazy, H.; Mayrose, I.; Padan, E.; Ben-Tal, N. Broad phylogenetic analysis of cation/proton antiporters reveals transport determinants. Nat. Commun. 2018, 9, 4205. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Maisler, N.; Taglicht, D.; Karpel, R.; Schuldiner, S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J. Biol. Chem. 1989, 264, 20297–20302. [Google Scholar] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Ivey, D.M.; Guffanti, A.A.; Zemsky, J.; Pinner, E.; Karpel, R.; Padan, E.; Schuldiner, S.; Krulwich, T.A. Cloning and characterization of a putative Ca2+/H+ antiporter gene from Escherichia coli upon functional complementation of Na+/H+ antiporter- deficient strains by the overexpressed gene. J. Biol. Chem. 1993, 268, 11296–11303. [Google Scholar] [PubMed]
- Sakuma, T.; Yamada, N.; Saito, H.; Kakegawa, T.; Kobayashi, H. pH dependence of the function of sodium ion extrusion systems in Escherichia coli. Biochim. Biophys. Acta Bioenerg. 1998, 1363, 231–237. [Google Scholar] [CrossRef]
- Inaba, K.; Kuroda, T.; Shimamoto, T.; Kayahara, T.; Tsuda, M.; Tsuchiya, T. Lithium toxicity and Na+(Li+)/H+ antiporter in Escherichia coli. Biol. Pharm. Bull. 1994, 17, 395–398. [Google Scholar] [CrossRef]
- Naseem, R.; Holland, I.B.; Jacq, A.; Wann, K.T.; Campbell, A.K. pH and monovalent cations regulate cytosolic free Ca2+ in E. coli. Biochim. Biophys. Acta Biomembr. 2008, 1778, 1415–1422. [Google Scholar] [CrossRef]
- Radchenko, M.V.; Tanaka, K.; Waditee, R.; Oshimi, S.; Matsuzaki, Y.; Fukuhara, M.; Kobayashi, H.; Takabe, T.; Nakamura, T. Potassium/proton antiport system of Escherichia coli. J. Biol. Chem. 2006, 281, 19822–19829. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–79. [Google Scholar]
- Taglicht, D.; Padan, E.; Schuldiner, S. Proton-sodium stoichiometry of NhaA, an electrogenic antiporter from Escherichia coli. J. Biol. Chem. 1993, 268, 5382–5387. [Google Scholar]
- Pinner, E.; Padan, E.; Schuldiner, S. Kinetic properties of NhaB, a Na+/H+ antiporter from Escherichia coli. J. Biol. Chem. 1994, 269, 26274–26279. [Google Scholar]
- Lewinson, O.; Padan, E.; Bibi, E. Alkalitolerance: A biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 14073–14078. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.R.; Law, C.J. Multidrug resistance protein MdtM adds to the repertoire of antiporters involved in alkaline pH homeostasis in Escherichia coli. BMC Microbiol. 2013, 13, 1987. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.R.; Law, C.J. Functional and biochemical characterisation of the Escherichia coli major facilitator superfamily multidrug transporter MdtM. Biochimie 2012, 94, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.R.; Law, C.J. The major facilitator superfamily transporter MdtM contributes to the intrinsic resistance of Escherichia coli to quaternary ammonium compounds. J. Antimicrob. Chemother. 2013, 68, 831–839. [Google Scholar] [CrossRef]
- Paul, S.; Alegre, K.O.; Holdsworth, S.R.; Rice, M.; Brown, J.A.; Mcveigh, P.; Kelly, S.M.; Law, C.J. A single-component multidrug transporter of the major facilitator superfamily is part of a network that protects Escherichia coli from bile salt stress. Mol. Microbiol. 2014, 92, 872–884. [Google Scholar] [CrossRef]
- Edgar, R.; Bibi, E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J. Bacteriol. 1997, 179, 2274–2280. [Google Scholar] [CrossRef]
- Tanudjaja, E.; Hoshi, N.; Su, Y.-H.; Hamamoto, S.; Uozumi, N. Kup-mediated Cs+ uptake and Kdp-driven K+ uptake coordinate to promote cell growth during excess Cs+ conditions in Escherichia coli. Sci. Rep. 2017, 7, 2122. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Nikolaev, Y.; McLaggan, D.; Maclean, M.; Booth, I.R. Survival during exposure to the electrophilic reagent N-ethylmaleimide in Escherichia coli: Role of KefB and KefC potassium channels. J. Bacteriol. 1997, 179, 1007–1012. [Google Scholar] [CrossRef]
- Ferguson, G.P.; McLaggan, D.; Booth, I.R. Potassium channel activation by glutathione-S-conjugates in Escherichia coli: Protection against methylglyoxal is mediated by cytoplasmic acidification. Mol. Microbiol. 1995, 17, 1025–1033. [Google Scholar] [CrossRef]
- Miller, S.; Ness, L.S.; Wood, C.M.; Fox, B.C.; Booth, I.R. Identification of an ancillary protein, YabF, required for activity of the KefC glutathione-gated potassium efflux system in Escherichia coli. J. Bacteriol. 2000, 182, 6536–6540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roosild, T.P.; Castronovo, S.; Healy, J.; Miller, S.; Pliotas, C.; Rasmussen, T.; Bartlett, W.; Conway, S.J.; Booth, I.R. Mechanism of ligand-gated potassium efflux in bacterial pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 19784–19789. [Google Scholar] [CrossRef] [PubMed]
- Roosild, T.P.; Castronovo, S.; Miller, S.; Li, C.; Rasmussen, T.; Bartlett, W.; Gunasekera, B.; Choe, S.; Booth, I.R. KTN (RCK) domains regulate K+ channels and transporters by controlling the dimer-hinge conformation. Structure 2009, 17, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Verkhovskaya, M.L.L.; Barquera, B.; Wikström, M. Deletion of one of two Escherichia coli genes encoding putative Na+/H+ exchangers (ycgO) perturbs cytoplasmic alkali cation balance at low osmolarity. Microbiology 2001, 147, 3005–3013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, R.; Shimada, T.; Mishra, V.K.; Upreti, S.; Sardesai, A.A. Growth inhibition by external potassium of Escherichia coli lacking PtsN (EIIANtr) is caused by potassium limitation mediated by YcgO. J. Bacteriol. 2016, 198, 1868–1882. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystems II and I in cyanobacteria. Photosynth. Res. 2008, 98, 529–539. [Google Scholar] [CrossRef]
- Inaba, M.; Sakamoto, A.; Murata, N. Functional expression in Escherichia coli of low-affinity and high-affinity Na+(Li+)/H+ antiporters of Synechocystis. J. Bacteriol. 2001, 183, 1376–1384. [Google Scholar] [CrossRef]
- Elanskaya, I.V.; Karandashova, I.V.; Bogachev, A.V.; Hagemann, M. Functional analysis of the Na+/H+ antiporter encoding genes of the cyanobacterium Synechocystis PCC 6803. Biochemistry (Mosc.) 2002, 67, 432–440. [Google Scholar] [CrossRef]
- Mikkat, S.; Milkowski, C.; Hagemann, M. The gene sll0273 of the cyanobacterium Synechocystis sp. strain PCC6803 encodes a protein essential for growth at low Na+/K.+ ratios. Plant Cell Environ. 2000, 23, 549–559. [Google Scholar] [CrossRef]
- Hamada, A.; Hibino, T.; Nakamura, T.; Takabe, T. Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long c-terminal tail important for the carrier activity. Plant Physiol. 2001, 125, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Billini, M.; Stamatakis, K.; Sophianopoulou, V. Two members of a network of putative Na+/H+ antiporters are involved in salt and pH tolerance of the freshwater cyanobacterium Synechococcus elongatus. J. Bacteriol. 2008, 190, 6318–6329. [Google Scholar] [CrossRef] [PubMed]
- Waditee, R.; Hossain, G.S.; Tanaka, Y.; Nakamura, T.; Shikata, M.; Takano, J.; Takabe, T.; Takabe, T. Isolation and functional characterization of Ca2+/H+ antiporters from cyanobacteria. J. Biol. Chem. 2004, 279, 4330–4338. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Spalding, E.P. Protection of plasma membrane K+ transport by the salt overly sensitive1 Na+/H+ antiporter during salinity stress. Plant Physiol. 2004, 136, 2548–2555. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- An, R.; Chen, Q.J.; Chai, M.F.; Lu, P.L.; Su, Z.; Qin, Z.X.; Chen, J.; Wang, X.C. AtNHX8, a member of the monovalent cation:proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J. 2007, 49, 718–728. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, N.H.; Motes, C.M.; Blancaflor, E.B.; Moore, M.; Gonzales, N.; Padmanaban, S.; Sze, H.; Ward, J.M.; Hirschi, K.D. AtCHX13 is a plasma membrane K+ transporter. Plant Physiol. 2008, 148, 796–807. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Motes, C.M.; Park, S.; Hirschi, K.D. CHX14 is a plasma membrane K-efflux transporter that regulates K+ redistribution in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 2223–2238. [Google Scholar] [CrossRef]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 2011, 23, 3482–3497. [Google Scholar] [CrossRef]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; de Luca, A.; Fernández, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Aharon, G.S.; Sottosanto, J.B.; Blumwald, E. Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc. Natl. Acad. Sci. USA. 2005, 102, 16107–16112. [Google Scholar] [CrossRef]
- Gruwel, M.L.H.; Rauw, V.L.; Loewen, M.; Abrams, S.R. Effects of sodium chloride on plant cells; A 31P and 23Na NMR system to study salt tolerance. Plant Sci. 2001, 160, 785–794. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Yu, M.; Zhang, Y.; Wu, Y.; Zhang, H. Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ. 2008, 31, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tang, R.; Zhang, Y.; Wang, C.; Lv, Q.; Gao, X.; Li, W.B.; Zhang, H. AtNHX3 is a vacuolar K+/H+ antiporter required for low-potassium tolerance in Arabidopsis thaliana. Plant Cell Environ. 2010, 33, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Liu, H.; Gao, X.S.; Zhang, H. Knock-out of Arabidopsis AtNHX4 gene enhances tolerance to salt stress. Biochem. Biophys. Res. Commun. 2009, 382, 637–641. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Kunz, H.-H.; Gierth, M.; Herdean, A.; Satoh-Cruz, M.; Kramer, D.M.; Spetea, C.; Schroeder, J.I. Plastidial transporters KEA1, -2, and -3 are essential for chloroplast osmoregulation, integrity, and pH regulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 7480–7845. [Google Scholar] [CrossRef]
- Armbruster, U.; Carrillo, L.R.; Venema, K.; Pavlovic, L.; Schmidtmann, E.; Kornfeld, A.; Jahns, P.; Berry, J.A.; Kramer, D.M.; Jonikas, M.C. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. 2014, 5, 5439. [Google Scholar] [CrossRef]
- Armbruster, U.; Leonelli, L.; Galvis, V.C.; Strand, D.; Quinn, E.H.; Jonikas, M.C.; Niyogi, K.K. Regulation and levels of the thylakoid K+/H+ antiporter KEA3 shape the dynamic response of photosynthesis in fluctuating light. Plant Cell Physiol. 2016, 57, 1557–1567. [Google Scholar] [CrossRef]
- Wang, C.; Yamamoto, H.; Narumiya, F.; Munekage, Y.N.; Finazzi, G.; Szabo, I.; Shikanai, T. Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J. 2017, 89, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shikanai, T. Modification of Activity of the Thylakoid H+/K+ antiporter KEA3 disturbs ∆pH-dependent regulation of photosynthesis. Plant Physiol. 2019, 181, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Stephan, A.B.; Kunz, H.-H.; Yang, E.; Schroeder, J.I. Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters. Proc. Natl. Acad. Sci. USA 2016, 113, E5242–E5249. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Sicilia, M.N.; Aboukila, A.; Armbruster, U.; Cagnac, O.; Schumann, T.; Kunz, H.-H.; Jahns, P.; Rodríguez-Rosales, M.P.; Sze, H.; Venema, K. Envelope K+/H+ antiporters AtKEA1 and AtKEA2 function in plastid development. Plant Physiol. 2016, 172, 441–449. [Google Scholar] [CrossRef]
- Müller, M.; Kunz, H.H.; Schroeder, J.I.; Kemp, G.; Young, H.S.; Ekkehard Neuhaus, H. Decreased capacity for sodium export out of Arabidopsis chloroplasts impairs salt tolerance, photosynthesis and plant performance. Plant J. 2014, 78, 646–658. [Google Scholar] [CrossRef]
- Furumoto, T.; Yamaguchi, T.; Ohshima-Ichie, Y.; Nakamura, M.; Tsuchida-Iwata, Y.; Shimamura, M.; Ohnishi, J.; Hata, S.; Gowik, U.; Westhoff, P.; et al. A plastidial sodium-dependent pyruvate transporter. Nature 2011, 476, 472–475. [Google Scholar] [CrossRef]
- Nomura, H.; Shiina, T. Calcium signaling in plant endosymbiotic organelles: Mechanism and role in physiology. Mol. Plant 2014, 7, 1094–1104. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; Jin, H.; Zhang, T.; Lai, J.; Zhou, X.; Zhang, S.; Liu, S.; Duan, X.; Wang, H.; et al. A Putative Chloroplast-Localized Ca2+/H+ Antiporter CCHA1 Is involved in Calcium and pH homeostasis and required for PSII function in arabidopsis. Mol. Plant 2016, 9, 1183–1196. [Google Scholar] [CrossRef]
- Schneider, A.; Steinberger, I.; Herdean, A.; Gandini, C.; Eisenhut, M.; Kurz, S.; Morper, A.; Hoecker, N.; Rühle, T.; Labs, M.; et al. The evolutionarily conserved protein PHOTOSYNTHESIS AFFECTED MUTANT71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis. Plant Cell 2016, 28, 892–910. [Google Scholar] [CrossRef]
- Reguera, M.; Bassil, E.; Tajima, H.; Wimmer, M.; Chanoca, A.; Otegui, M.S.; Paris, N.; Blumwald, E. pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell 2015, 27, 1200–1217. [Google Scholar] [CrossRef]
- Dragwidge, J.M.; Ford, B.A.; Ashnest, J.R.; Das, P.; Gendall, A.R. Two endosomal NHX-Type Na+/H+ antiporters are involved in auxin-mediated development in Arabidopsis Thaliana. Plant Cell Physiol. 2018, 59, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Cellier, F.; Conéjéro, G.; Ricaud, L.; Doan, T.L.; Lepetit, M.; Gosti, F.; Casse, F. Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J. 2004, 39, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Chanroj, S.; Padmanaban, S.; Czerny, D.D.; Jauh, G.Y.; Sze, H. K+ transporter AtCHX17 with its hydrophilic C tail localizes to membranes of the secretory/endocytic system: Role in reproduction and seed set. Mol. Plant 2013, 6, 1226–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chanroj, S.; Zulkifli, L.; Johnson, M.A.; Uozumi, N.; Cheung, A.; Sze, H. Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 2011, 23, 81–93. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, T.; Zhang, X.; Fan, L.; Quintero, F.J.; Zhao, H.; Su, X.; Li, X.; Villalta, I.; Mendoza, I.; et al. K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol. 2018, 178, 1657–1678. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, R.J.; Yang, X.; Zheng, X.; Shao, Q.; Tang, Q.L.; Fu, A.; Luan, S. Golgi-localized cation/proton exchangers regulate ionic homeostasis and skotomorphogenesis in Arabidopsis. Plant Cell Environ. 2019, 42, 673–687. [Google Scholar] [CrossRef]
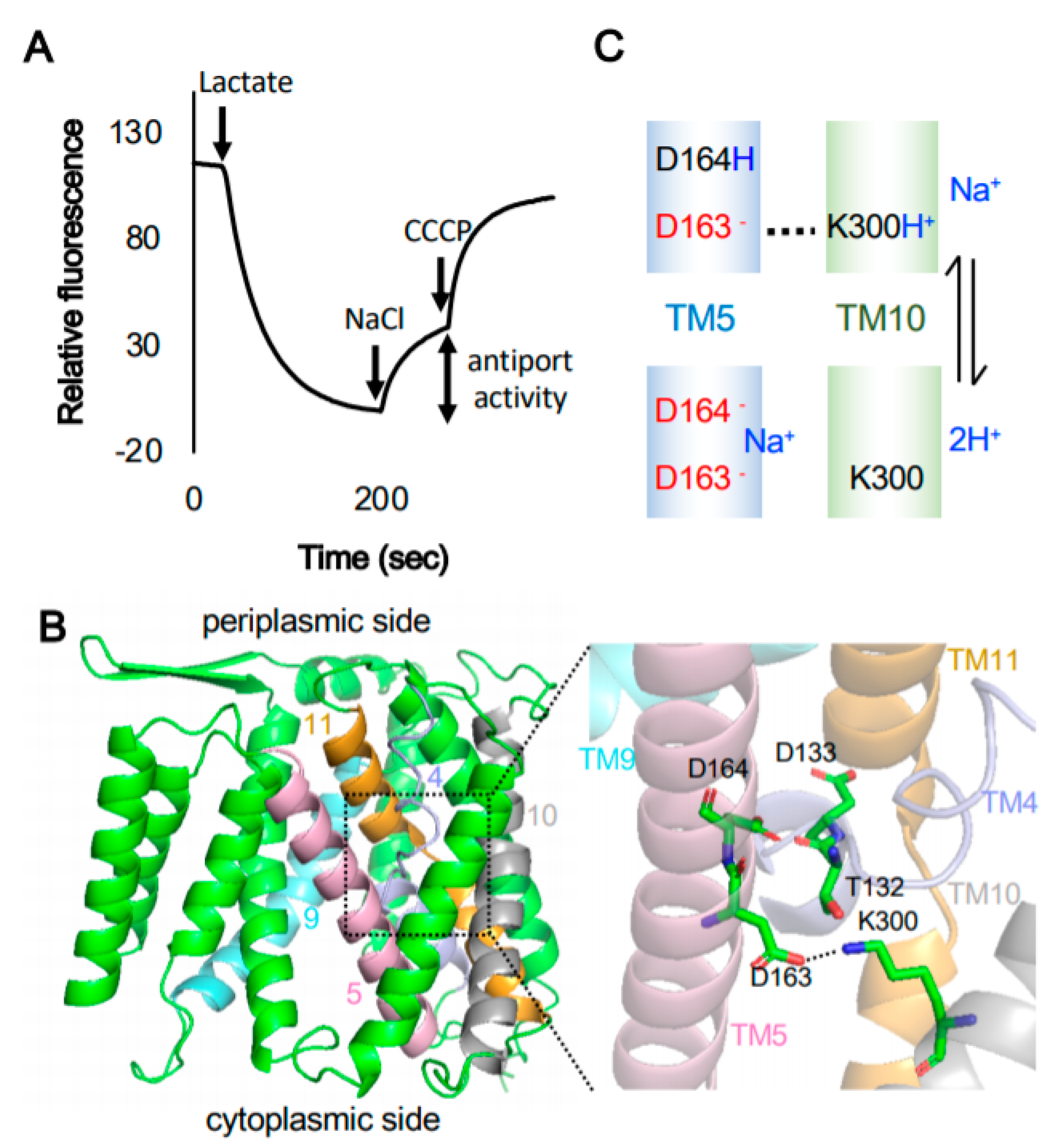
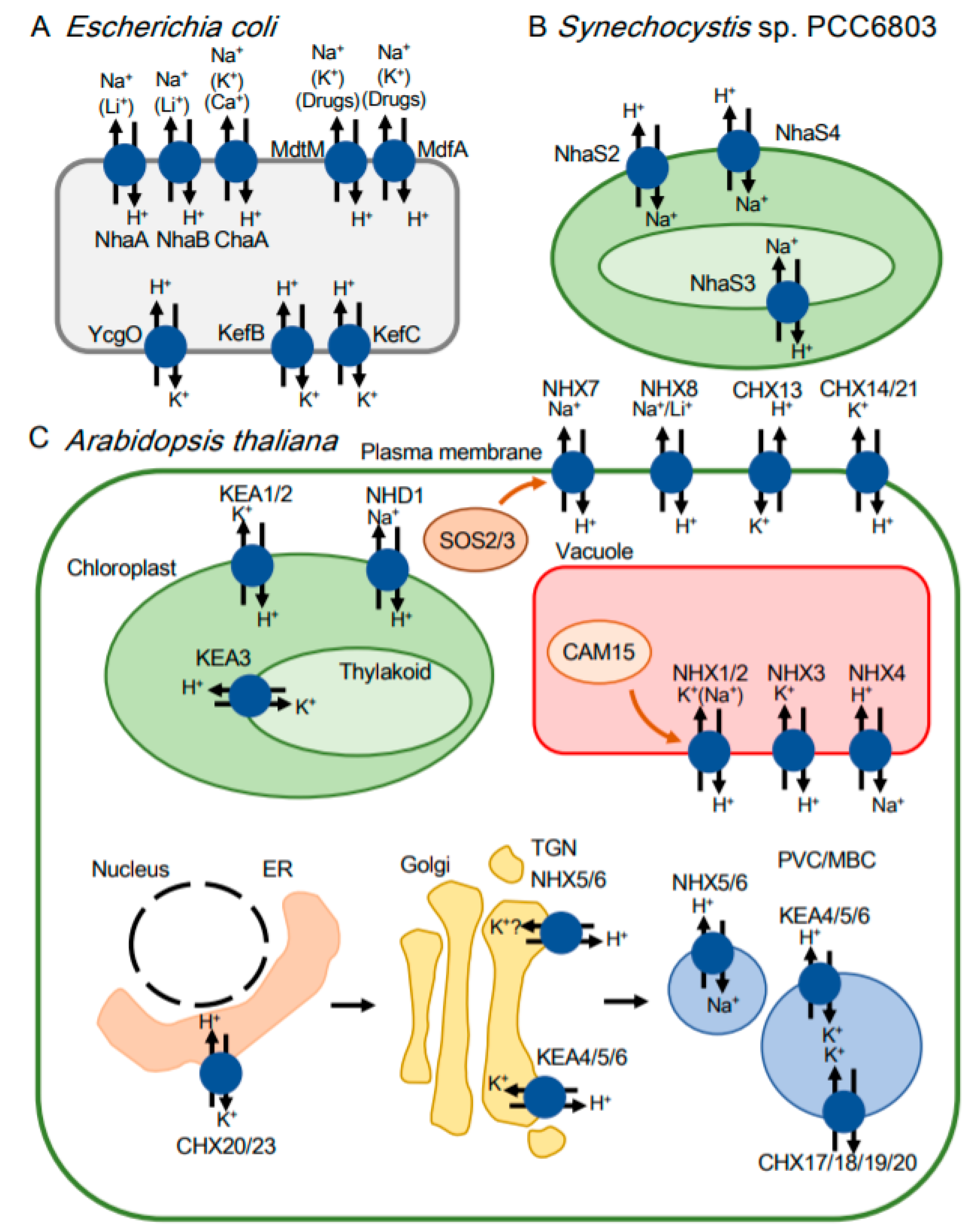
| Transporter | Activity | Physiological Function | Family | Reference |
|---|---|---|---|---|
| NhaA | Na+(Li+)/H+ antiporter | Na+ extrusion, alkaline pH homeostasis | NhaA | (Taglicht et al., 1991) |
| NhaB | Na+(Li+)/H+ antiporter | Na+ extrusion, alkaline pH homeostasis | NhaB | (Pinner et al., 1993, Shimamoto et al., 1994) |
| ChaA | Na+(Ca+, K+)/H+ antiporter | Na+ extrusion, alkaline pH homeostasis, KCl salinity tolerance | CaCA | (Ivey et al., 1993, Radchenko et al., 2006) |
| KefB | glutathione-regulated K+ efflux (K+/H+ antiporter) | electrophilic stress tolerance | CPA2 | (Ferguson et al., 1997, Elmore et al., 1997) |
| KefC | glutathione-regulated K+ efflux (K+/H+ antiporter) | electrophilic stress tolerance | CPA2 | (Ferguson et al., 1997, Elmore et al., 1997) |
| MdfA | drugs (Na+,K+)/H+ antiporter | antimicrobial resistance, alkaline pH homeostasis | DHA1 | (Lewinson et al., 2004, Lewinson et al., 2003) |
| MdtM | drugs (Na+,K+, Rb+, Li+)/H+ antiporter | antimicrobial resistance, alkaline pH homeostasis | DHA1 | (Holdsworth et al., 2013) |
| YcgO | K+/H+ antiporter | growth at low osmolarity | CPA1 | (Sharma et al., 2016, Verkhovskaya et al., 2001) |
| Prokaryote Isoform | Membrane Location | Activity | Physiological Function | Family | Reference |
|---|---|---|---|---|---|
| NhaS1 (Syncechocystis sp. pcc6803) | - | Na+ (Li+)/H+ antiporter | - | CPA1 | (Inaba et al., 2001, Hamada et al., 2001, Waditee et al., 2006) |
| NhaS2 (Syncehocystis sp. pcc6803) | - | - | Na+ influx | CPA1 | (Mikkat et al., 2000, Wang et al., 2002) |
| NhaS3 (Syncehocystis sp. pcc6803) | Thylakoid membrane | Na+/H+ antiporter | Na+ homeostasis, adaptation to osmotic stress | CPA2 | (Wang et al., 2002, Tsunekawa et al., 2009) |
| NhaS4 (Syncehocystis sp. pcc6803) | - | - | Na+ influx | CPA2 | (Wang et al., 2002) |
| SynCAX (Syncehocystis sp. pcc6803) | - | Ca2+/H+ antiporter | alkaline homeostasis, adaptation to salt stress | - | (Waditee et al., 2004) |
| NhaS2 (Syncechococcus sp. strain PCC 7942) | - | - | Na+ influx | CPA1 | (Billini et al., 2008) |
| NhaS3 (Syncechococcus sp. strain PCC 7942) | - | Na+/H+ antiporter | Na+ homeostasis | CPA2 | (Billini et al., 2008) |
| Plant Isoform | Membrane Location | Activity | Physiological Function | Family | Reference |
|---|---|---|---|---|---|
| NHX1, NHX2 | Vacuole | K+(Na+)/H+ antiporter | maintaining cell turgor pressure, adaptation to excess K+ conditions, flowering | CPA1 | (Shi and Zhu, 2002, Bassil et al., 2011, Barragan et al., 2012) |
| NHX3 | Vacuole | K+(Na+)/H+ antiporter | adaptation to low K+ conditions | CPA1 | (Liu et al., 2008, Liu et al., 2010) |
| NHX4 | Vacuole | Na+/H+ antiporter | Na+ homeostasis | CPA1 | (Li et al., 2009) |
| NHX5, NHX6 | Golgi, Trans-golgi network, prevacuolar compartment | - | protein sorting to vacuole, adaptation to salt stress, root extention, redistribution of auxin | CPA1 | (Bassil et al., 2011, Reguela et al., 2015, Dragwidge et al., 2018) |
| NHX7 (SOS1) | Plasma membrane | Na+/H+ antiporter | Na+ extrusion, maintaining intracellular K+ content | CPA1 | (Wu et al., 1996, Shi et al., 2002, Qiu et al., 2002, Qi and Spalding, 2004, Ariga et al., 2013) |
| NHX8 | Plasma membrane | (Li+)/H+ antiporter | Li+ extrusion | CPA1 | (An et al., 2007) |
| CHX13 | Plasma membrane | K+/H+ antiporter | K+ uptake | CPA2 | (Zhao et al., 2008) |
| CHX14 | Plasma membrane | K+/H+ antiporter | K+ extrusion | CPA2 | (Zhao et al., 2015) |
| CHX17 | Prevacuolar compartment | K+/H+ antiporter | protein sorting to vacuole, K+ homeostasis, seed development | CPA2 | (Cellier et al., 2004, Chanroj et al., 2011, Chanroj et al., 2013) |
| CHX18, CHX19 | Prevacuolar compartment | K+/H+ antiporter | - | CPA2 | (Chanroj et al., 2011) |
| CHX20 | Prevacuolar compartment, ER | K+/H+ antiporter | protein sorting to vacuole | CPA2 | (Chanroj et al., 2011) |
| CHX21 | Plasma membrane | - | adaptation to high Na+ (K+) conditions, pollen tube growth | CPA2 | (Hall et al., 2006, Evans et al., 2011) |
| CHX23 | ER | K+/H+ antiporter | pollen tube growth | CPA2 | (Lu et al., 2011, Evans et al., 2011) |
| KEA1, KEA2 | Plastid envelope | K+/H+ antiporter | chloroplast development, adaptation to hyper-osmotic stress | CPA2 | (Kunz et al., 2014, Aranda Sicilia et al., 2016, Stephan et al., 2016, Tsujii et al., 2019) |
| KEA3 | Plastid thylakoid membrane | K+/H+ antiporter | fine-tuning of photosynthesis, chloroplast development, adaptation to hyper-osmotic stress | CPA2 | (Kunz et al., 2014, Armbruster et al., 2014, 2016, Stephan et al., 2016, Wang et al., 2017, 2019, Tsujii et al., 2019) |
| KEA4, KEA5, KEA6 | Golgi, Trans-golgi network, prevacuolar compartment | K+/H+ antiporter | maintaining ion homeostasis in low K+ conditions, high K+(Na+) stress, pH regulation in vacuole | CPA2 | (Zhu et al., 2018, Wang et al., 2018, Tsujii et al., 2019) |
| NHD1 | Plastid envelope | Na+/H+ antiporter | Na+ extrusion from chloroplast | NhaD | (Maria et al., 2014) |
| CCHA1 (PAM71) | Plastid thylakoid membrane | - | Photosynthetic regulation, regulation of cytosolic pH in guard cells | - | (Wang et al., 2016, Schneider et al., 2016) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujii, M.; Tanudjaja, E.; Uozumi, N. Diverse Physiological Functions of Cation Proton Antiporters across Bacteria and Plant Cells. Int. J. Mol. Sci. 2020, 21, 4566. https://doi.org/10.3390/ijms21124566
Tsujii M, Tanudjaja E, Uozumi N. Diverse Physiological Functions of Cation Proton Antiporters across Bacteria and Plant Cells. International Journal of Molecular Sciences. 2020; 21(12):4566. https://doi.org/10.3390/ijms21124566
Chicago/Turabian StyleTsujii, Masaru, Ellen Tanudjaja, and Nobuyuki Uozumi. 2020. "Diverse Physiological Functions of Cation Proton Antiporters across Bacteria and Plant Cells" International Journal of Molecular Sciences 21, no. 12: 4566. https://doi.org/10.3390/ijms21124566
APA StyleTsujii, M., Tanudjaja, E., & Uozumi, N. (2020). Diverse Physiological Functions of Cation Proton Antiporters across Bacteria and Plant Cells. International Journal of Molecular Sciences, 21(12), 4566. https://doi.org/10.3390/ijms21124566





