Quantitative Near-Infrared Imaging of Platelets in Platelet-Rich Fibrin (PRF) Matrices: Comparative Analysis of Bio-PRF, Leukocyte-Rich PRF, Advanced-PRF and Concentrated Growth Factors
Abstract
1. Introduction
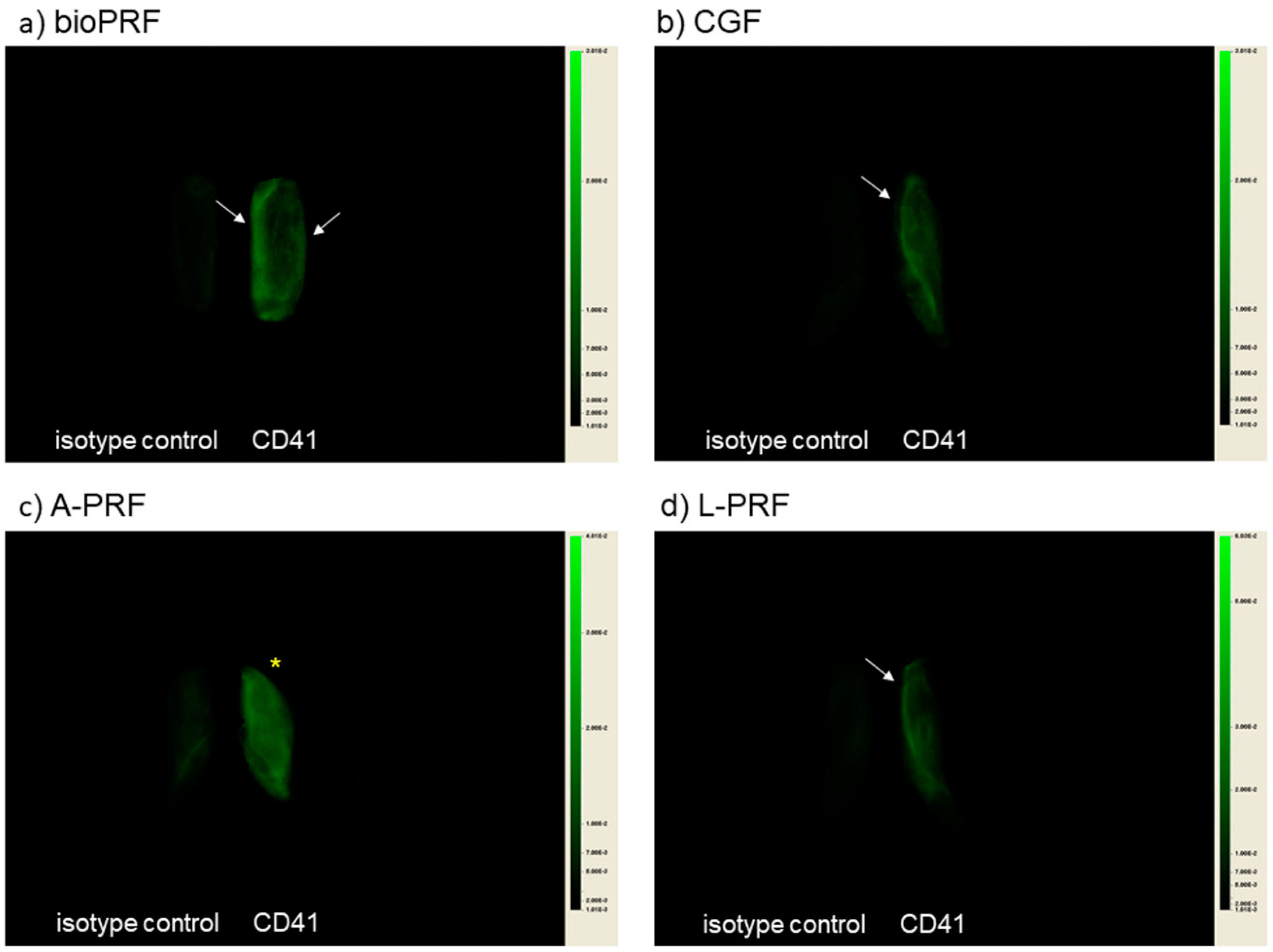
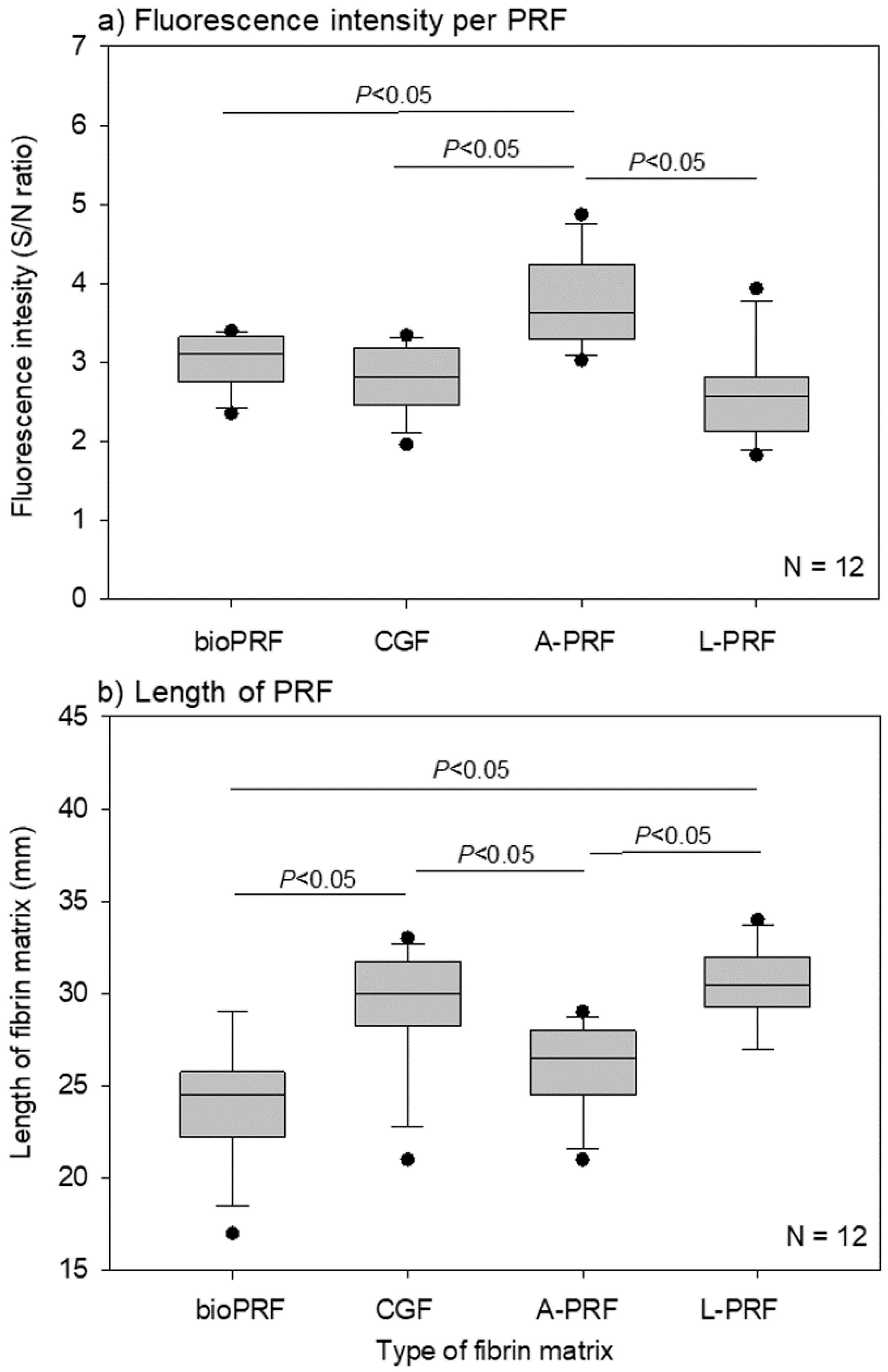
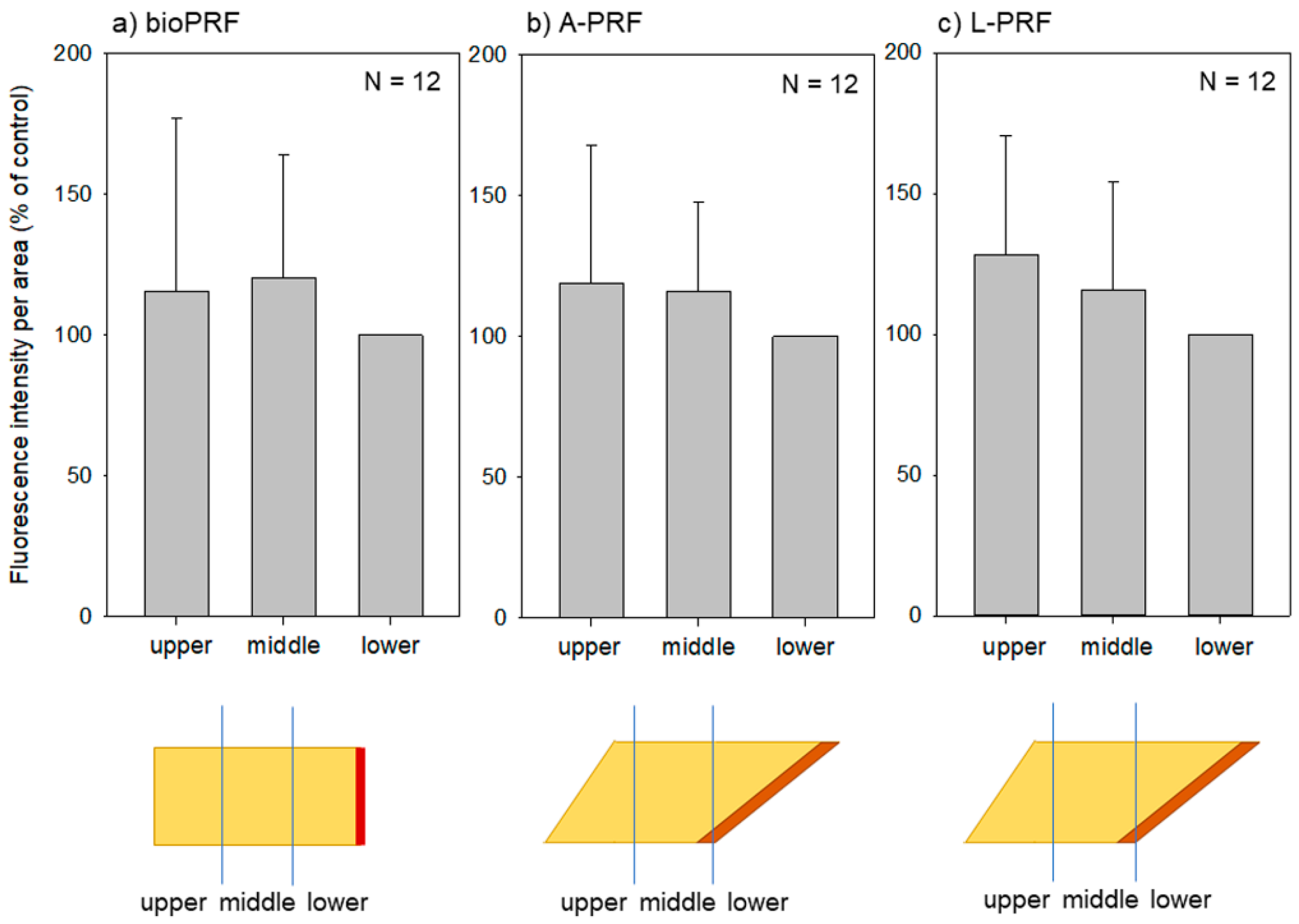
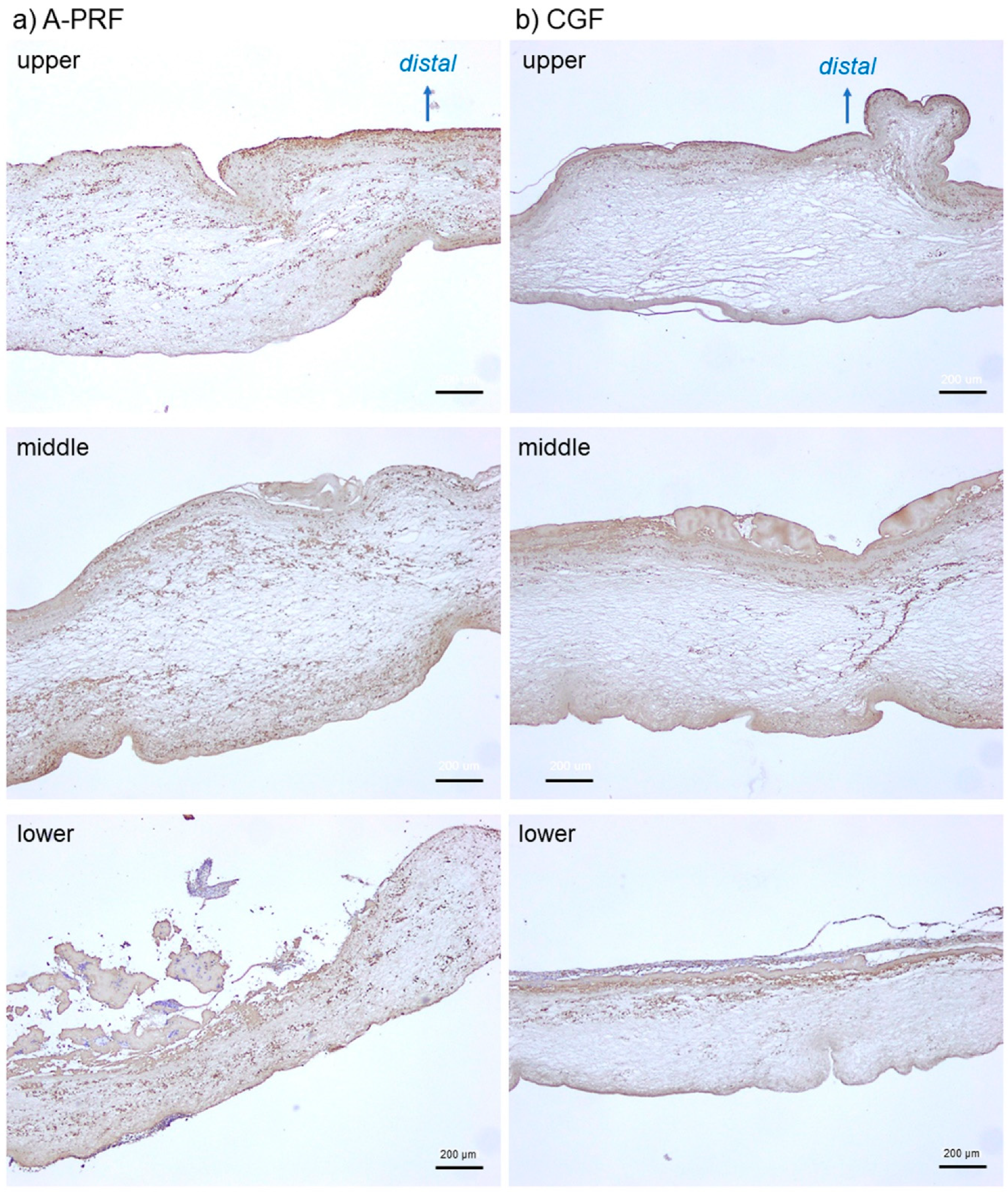
2. Results
3. Discussion
3.1. Advantages and Limitations of Our Imaging Method
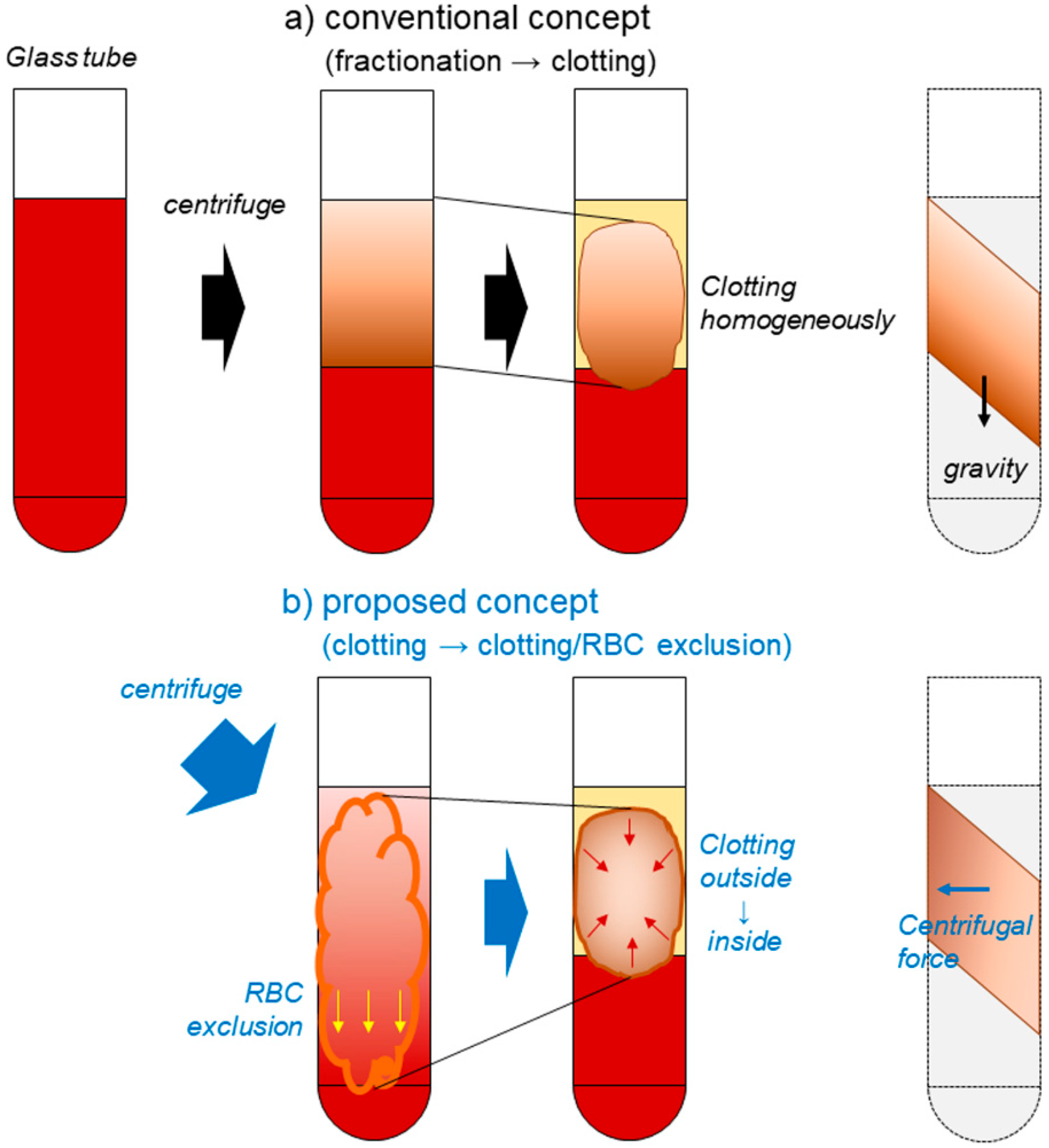
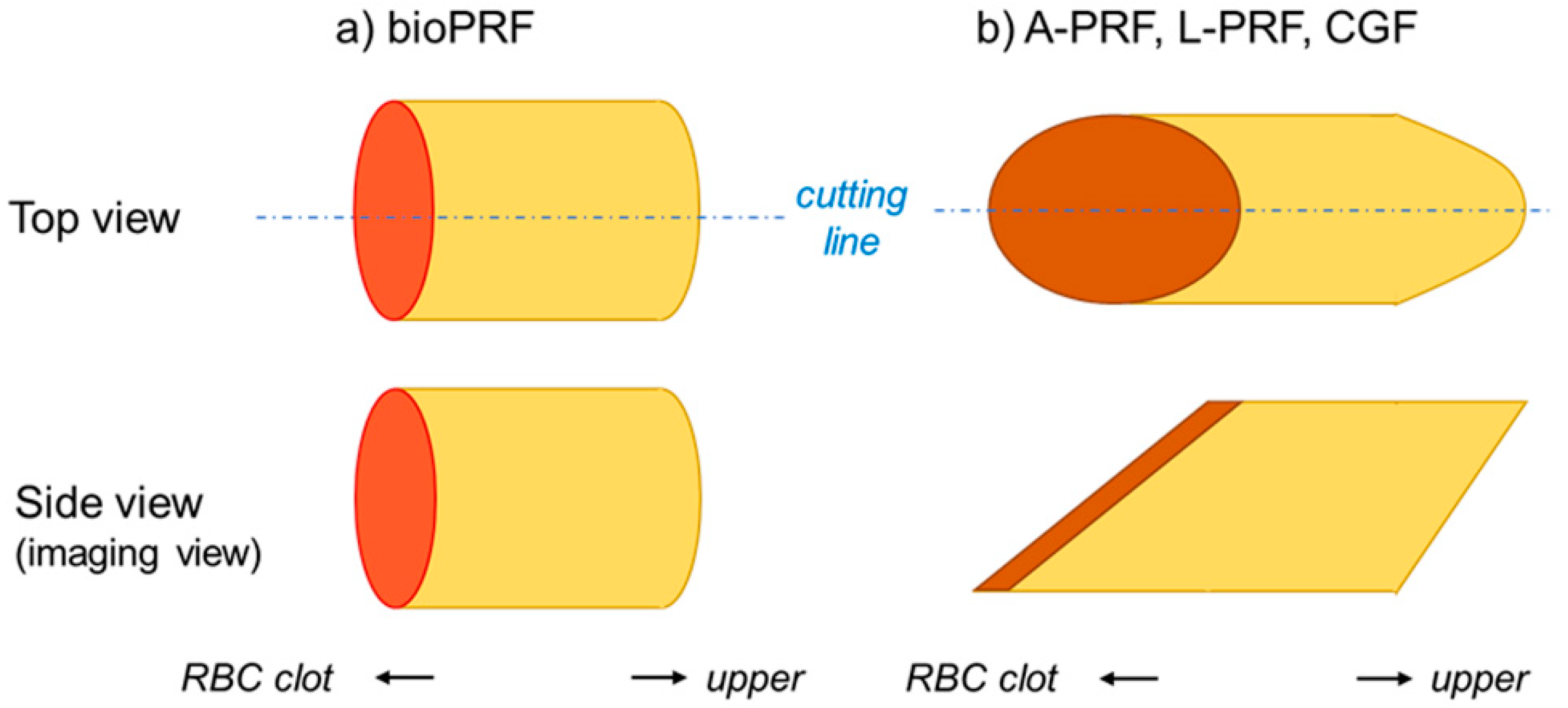
3.2. How Are Platelets Distributed in PRF Matrices?
3.3. What Does the Difference in Platelet Distribution Imply?
3.4. What Does the Platelet Distribution Influence?
3.5. Clinical Relevance of These Findings
4. Materials and Methods
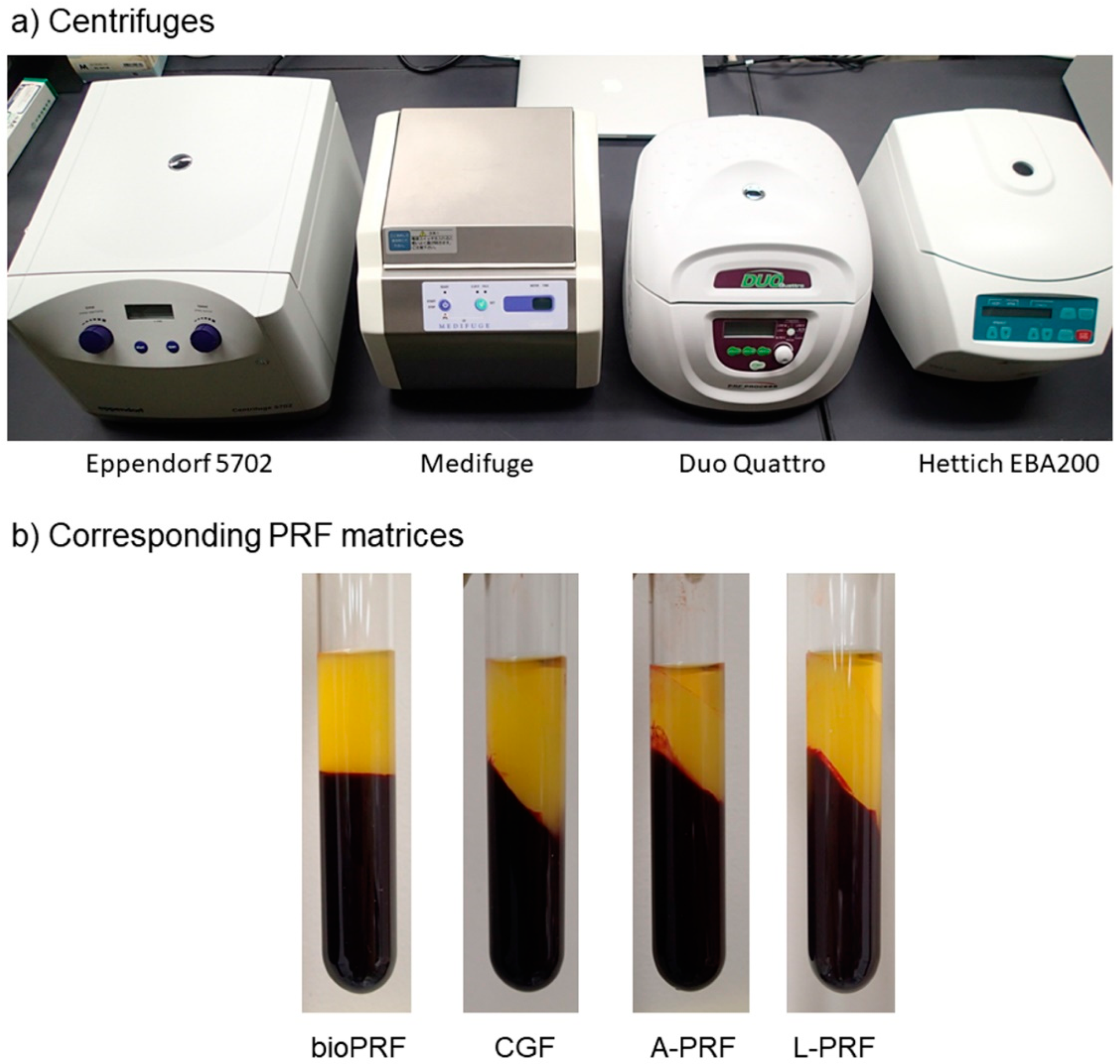
4.1. Preparation of PRF Matrices
4.2. NIR Imaging of Platelet Distribution in PRF Matrices
4.3. Immunohistochemical Examination
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PRF | platelet-rich fibrin |
| A-PRF | advanced platelet-rich fibrin |
| CGF | concentrated growth factors |
| L-PRF | leukocyte- and platelet-rich fibrin |
| NIR | near infrared |
| SEM | scanning electron microcopy |
| 3D | three dimensional |
| 0.1T-PBS | PBS containing 0.1% Tween 20 |
| BA-TPBS | 2% Block Ace solution dissolved in 0.1% T-PBS |
| DAB | 3,3′-diaminobenzidine |
| ANOVA | analysis of variance |
| TGFβ1 | transforming growth factor β1 |
| PDGF-BB | platelet derived growth factor-BB |
| VEGF | vascular endothelial growth factor |
| RBC | red blood cell |
References
- Kawase, T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: Basic principles and concepts underlying recent advances. Odontology 2015, 103, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Isobe, K.; Suzuki, T.; Kawabata, H.; Nakamura, M.; Tsukioka, T.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; et al. An Evaluation of the Accuracy of the Subtraction Method Used for Determining Platelet Counts in Advanced Platelet-Rich Fibrin and Concentrated Growth Factor Preparations. Dent. J. 2017, 5, 7. [Google Scholar] [CrossRef]
- Kitamura, Y.; Watanabe, T.; Nakamura, M.; Isobe, K.; Kawabata, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; Kawase, T. Platelet Counts in Insoluble Platelet-Rich Fibrin Clots: A Direct Method for Accurate Determination. Front. Bioeng. Biotechnol. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur. J. Trauma Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Kono, M.; Katagiri, H.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Histological comparison of Platelet rich fibrin clots prepared by fixed-angle versus horizontal centrifugation. Platelets 2020, 1–7. [Google Scholar]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scari, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; Mourao, C.; Sculean, A.; Fujioka Kobayashi, M.; Zhang, Y. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Aizawa, H.; Sato, A.; Tsujino, T.; Isobe, K.; Kitamura, Y.; Watanabe, T.; Okudera, H.; Mourao, C.F.; Kawase, T. Concentrated growth factor matrices prepared using silica-coated plastic tubes are distinguishable from those prepared using glass tubes in platelet distribution: Application of a novel near-infrared imaging-based, quantitative technique. Front. Bioeng. Biotechnol. 2020, 8, 600. [Google Scholar] [CrossRef]
- Tsujino, T.; Masuki, H.; Nakamura, M.; Isobe, K.; Kawabata, H.; Aizawa, H.; Watanabe, T.; Kitamura, Y.; Okudera, H.; Okuda, K.; et al. Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes. J. Funct. Biomater. 2019, 10, 43. [Google Scholar] [CrossRef]
- Aggarwal, A.; Singhal, N. Evaluation of content and distribution of platelets in platelet rich fibrin at various centrifugation time periods: A light microscopic study. Int. J. Dent. Med. Res. 2015, 1, 61–64. [Google Scholar]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Eren, G.; Gurkan, A.; Atmaca, H.; Donmez, A.; Atilla, G. Effect of centrifugation time on growth factor and MMP release of an experimental platelet-rich fibrin-type product. Platelets 2016, 27, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J. Glass surface and blood coagulation. Nature 1956, 178, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Suzuki, M.; Tsukioka, T.; Isobe, K.; Tsujino, T.; Watanabe, T.; Watanabe, T.; Okudera, H.; Nakata, K.; Tanaka, T.; et al. Spectrophotometric determination of platelet counts in platelet-rich plasma. Int. J. Implant Dent. 2018, 4, 29. [Google Scholar] [CrossRef]
- Tsujino, T.; Isobe, K.; Kawabata, H.; Aizawa, H.; Yamaguchi, S.; Kitamura, Y.; Masuki, H.; Watanabe, T.; Okudera, H.; Nakata, K.; et al. Spectrophotometric Determination of the Aggregation Activity of Platelets in Platelet-Rich Plasma for Better Quality Control. Dent. J. (Basel). 2019, 7, 61. [Google Scholar] [CrossRef]
- Takahashi, A.; Tsujino, T.; Yamaguchi, S.; Isobe, K.; Watanabe, T.; Kitamura, Y.; Okuda, K.; Nakata, K.; Kawase, T. Distribution of platelets, TGFβ1, PDGF-BB, VEGF, MMP9 and fibronectin in advanced platelet-rich fibrin (A-PRF) and concentrated growth factors (CGF) matrices. J. Invest. Clin. Dent. 2019, 10, e12458. [Google Scholar]
- Masuki, H.; Isobe, K.; Kawabata, H.; Tsujino, T.; Yamaguchi, S.; Watanabe, T.; Sato, A.; Aizawa, H.; Mourao, C.F.; Kawase, T. Acute cytotoxic effects of silica microparticles used for coating of plastic blood-collection tubes on human periosteal cells. Odontology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Takahashi, A.; Yamaguchi, S.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Tanaka, T.; Nakata, K.; Kawase, T. Evidence for Contamination of Silica Microparticles in Advanced Platelet-Rich Fibrin Matrices Prepared Using Silica-Coated Plastic Tubes. Biomedicines 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
| Title | Bio-PRF | CGF | A-PRF | L-PRF |
|---|---|---|---|---|
| Vacuum tubes (manufacturer) | Plain plastic tube (Nipro) | Plain plastic tube (Nipro) | Plain plastic tube (Nipro) | Plain plastic tube (Nipro) |
| 2nd tubes (manufacturer) | Plain glass tube (Nichiden-Rika Glass) | Plain glass tube (Nichiden-Rika Glass) | Plain glass tube (Nichiden-Rika Glass) | Plain glass tube (Nichiden-Rika Glass) |
| Centrifuges (manufacture) | #5702 (Eppendorf) | Medifuge (Silfradent) | Duo Quattro (Process for PRF) | EBA200 (Hettich) |
| Rotor types | Swing | Angle | Angle | Angle |
| Rotor angulation | Horizontal | 33° | 41.3° | 33° |
| Centrifugal force (time) | 700× g (8 min) | 692× g (2 min) 547× g (4 min) 592× g (4 min) 885× g (3 min) | 200× g (14 min*) | 400× g (12 min) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aizawa, H.; Tsujino, T.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Sato, A.; Yamaguchi, S.; Okudera, H.; Okuda, K.; Kawase, T. Quantitative Near-Infrared Imaging of Platelets in Platelet-Rich Fibrin (PRF) Matrices: Comparative Analysis of Bio-PRF, Leukocyte-Rich PRF, Advanced-PRF and Concentrated Growth Factors. Int. J. Mol. Sci. 2020, 21, 4426. https://doi.org/10.3390/ijms21124426
Aizawa H, Tsujino T, Watanabe T, Isobe K, Kitamura Y, Sato A, Yamaguchi S, Okudera H, Okuda K, Kawase T. Quantitative Near-Infrared Imaging of Platelets in Platelet-Rich Fibrin (PRF) Matrices: Comparative Analysis of Bio-PRF, Leukocyte-Rich PRF, Advanced-PRF and Concentrated Growth Factors. International Journal of Molecular Sciences. 2020; 21(12):4426. https://doi.org/10.3390/ijms21124426
Chicago/Turabian StyleAizawa, Hachidai, Tetsuhiro Tsujino, Taisuke Watanabe, Kazushige Isobe, Yutaka Kitamura, Atsushi Sato, Sadahiro Yamaguchi, Hajime Okudera, Kazuhiro Okuda, and Tomoyuki Kawase. 2020. "Quantitative Near-Infrared Imaging of Platelets in Platelet-Rich Fibrin (PRF) Matrices: Comparative Analysis of Bio-PRF, Leukocyte-Rich PRF, Advanced-PRF and Concentrated Growth Factors" International Journal of Molecular Sciences 21, no. 12: 4426. https://doi.org/10.3390/ijms21124426
APA StyleAizawa, H., Tsujino, T., Watanabe, T., Isobe, K., Kitamura, Y., Sato, A., Yamaguchi, S., Okudera, H., Okuda, K., & Kawase, T. (2020). Quantitative Near-Infrared Imaging of Platelets in Platelet-Rich Fibrin (PRF) Matrices: Comparative Analysis of Bio-PRF, Leukocyte-Rich PRF, Advanced-PRF and Concentrated Growth Factors. International Journal of Molecular Sciences, 21(12), 4426. https://doi.org/10.3390/ijms21124426






