Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes
Abstract
:1. Introduction
2. Results
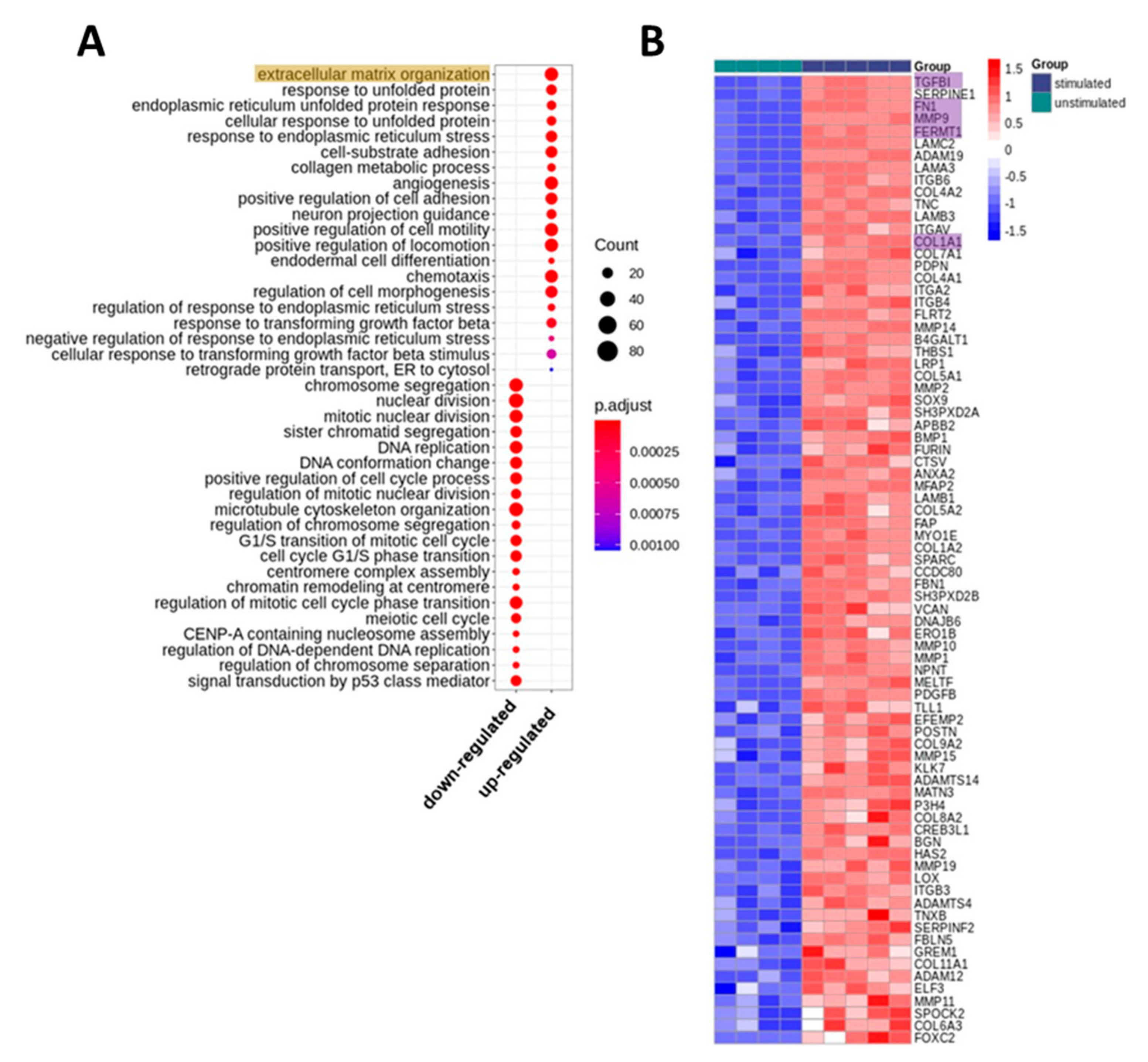
2.1. Whole Transcriptome Analyses of PRGF-Stimulated Human Keratinocytes Revealed Induction of Factors Involved in Extracellular Matrix Organization
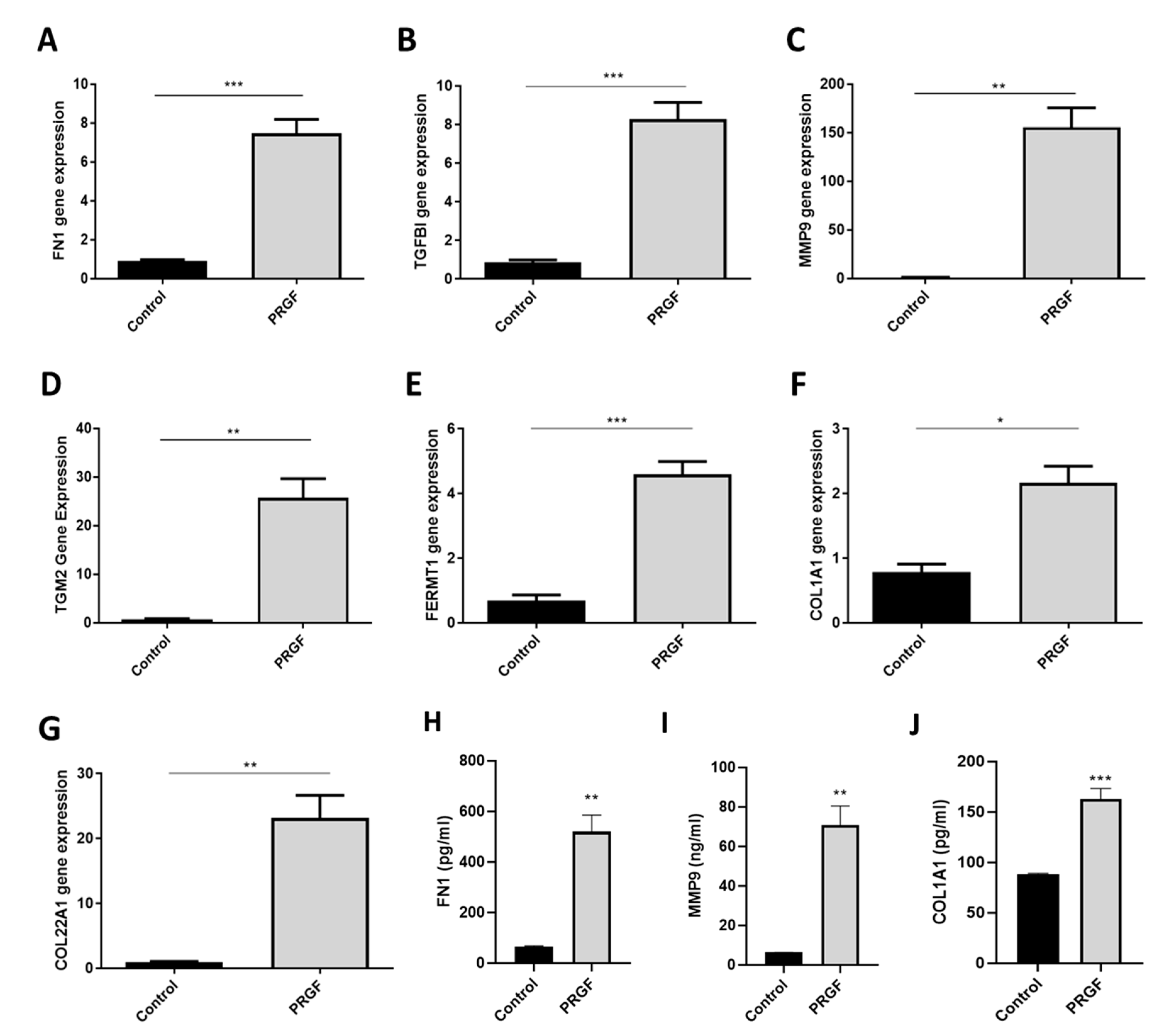
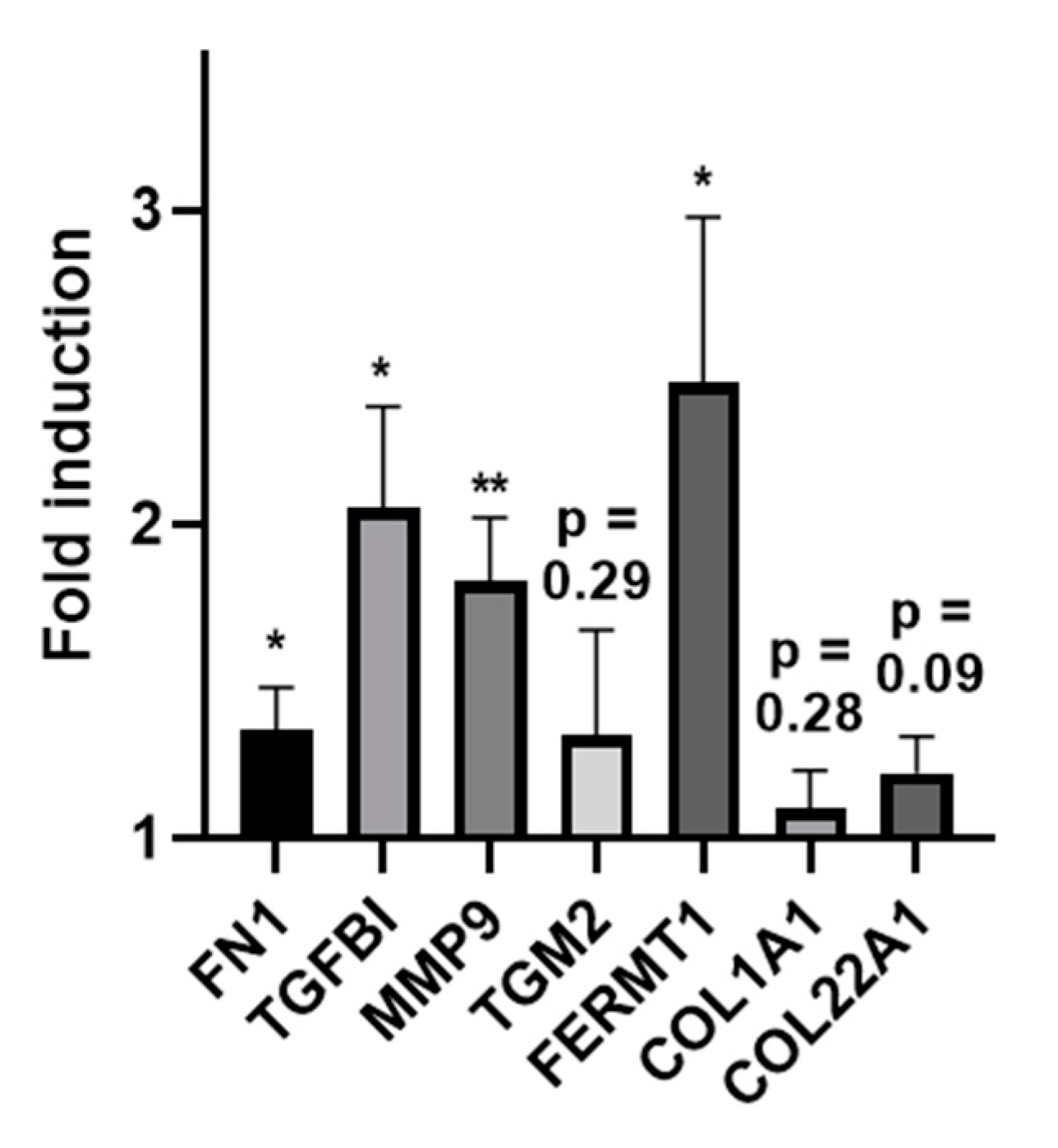
2.2. Real-time PCR Confirmed PRGF-Mediated Induction of Selected ECM-Associated Factors in Human Primary Keratinocytes
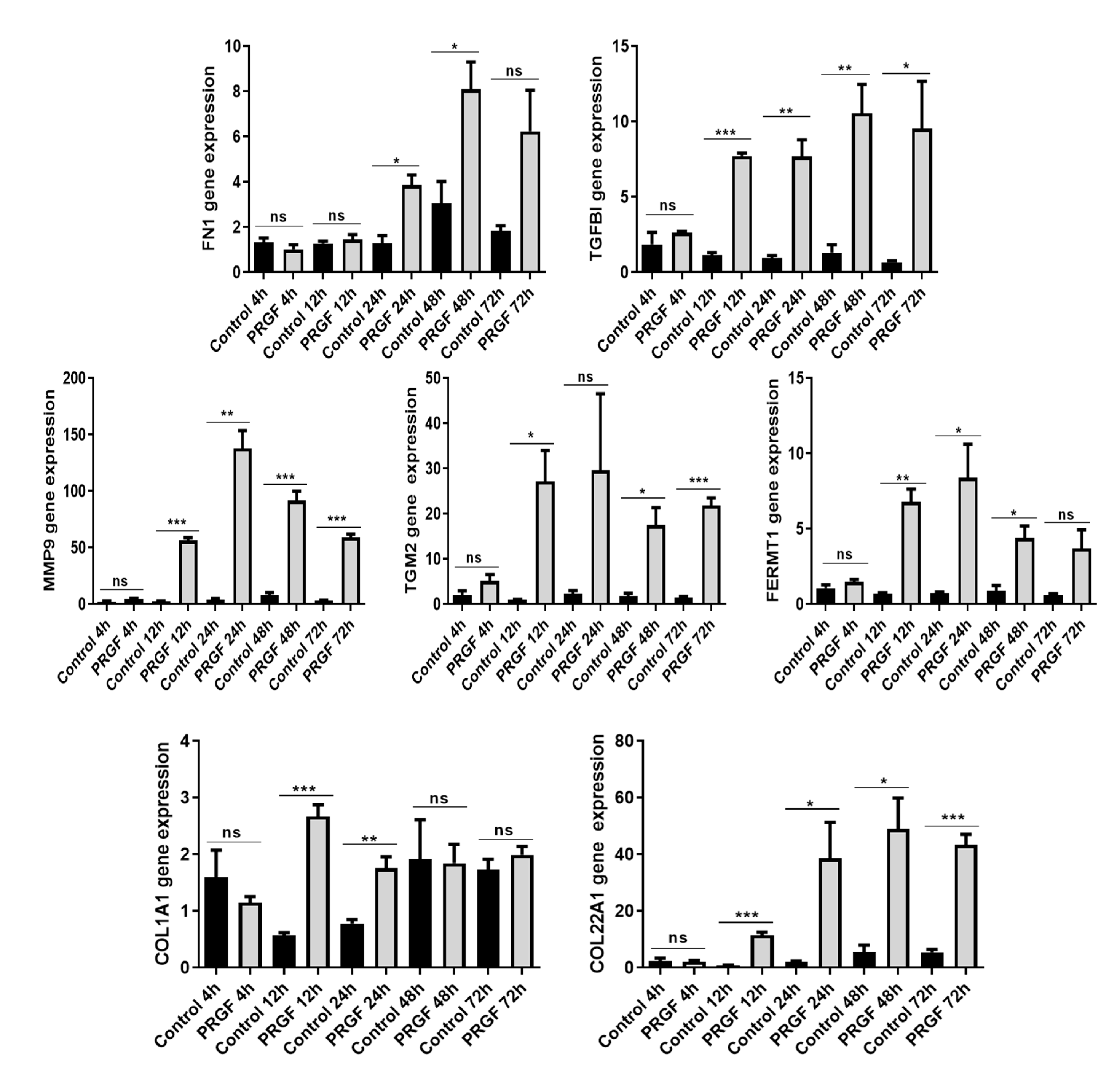
2.3. Time-Dependent Induction of ECM-Related Genes in PRGF-Treated Keratinocytes
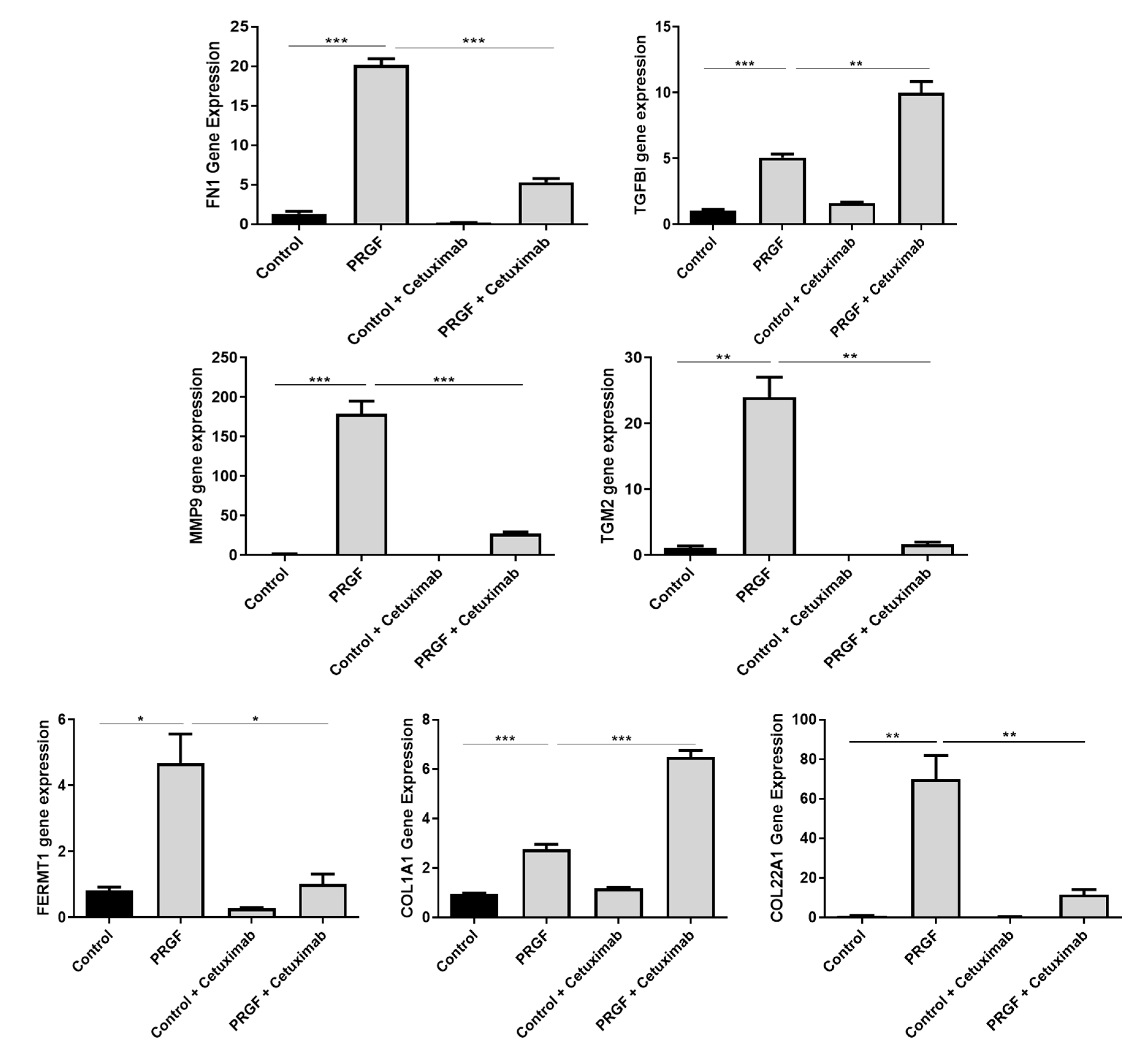
2.4. Blockade of the Epidermal Growth Factor Receptor (EGFR) Influences PRGF-Mediated Induction of ECM-Related Factors in Keratinocytes
2.5. Increased Expression of ECM-Related Genes In Ex Vivo Skin Explants
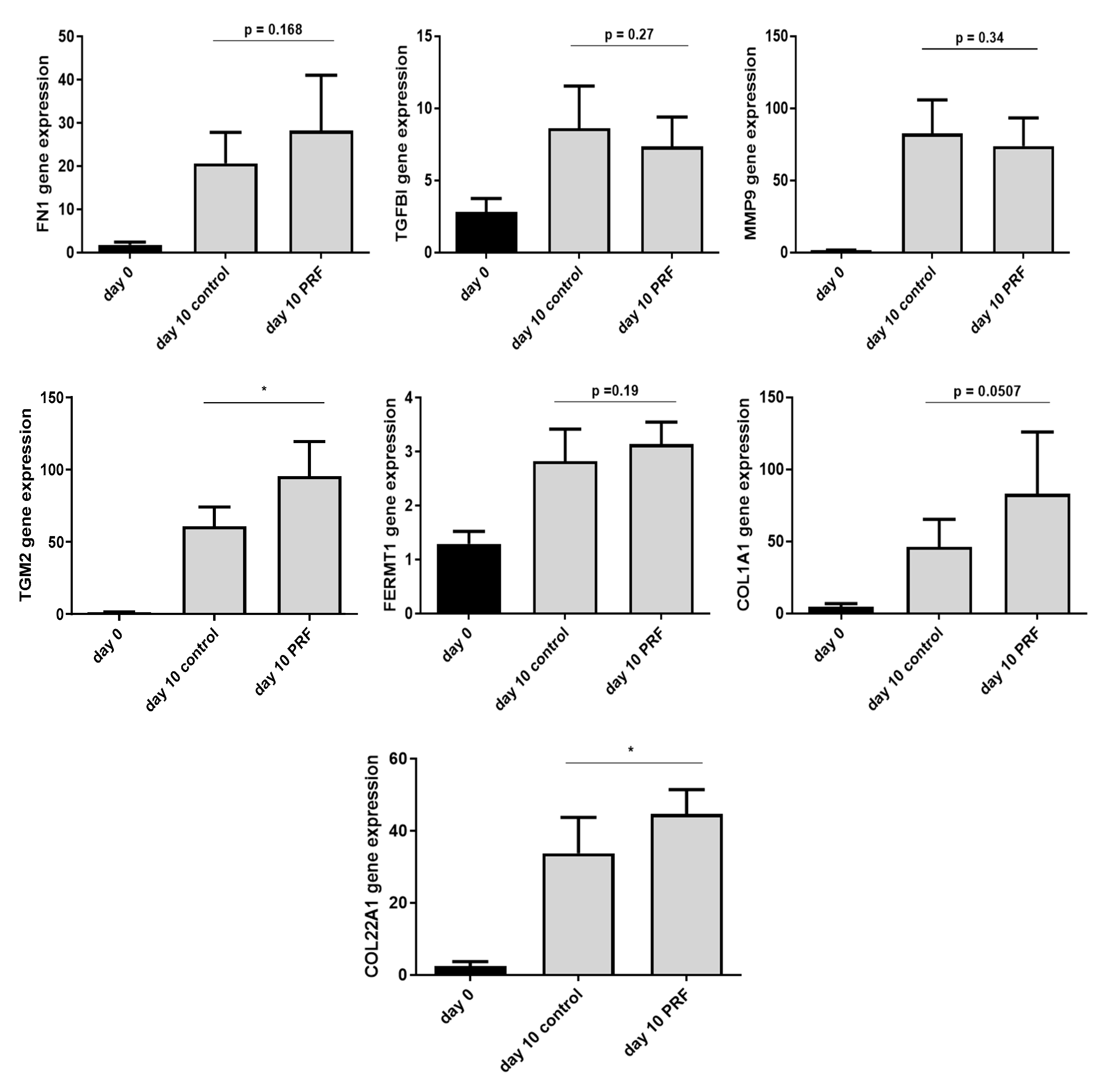
2.6. Increased Expression of ECM-Related Genes in Experimentally Generated Skin Wounds In Vivo and after Vivostat PRF® Treatment
3. Discussion
3.1. TGFBI
3.2. FN1
3.3. MMP9
3.4. TGM2
3.5. FERMT1
3.6. Collagens COL1A1 and COL22A1
4. Materials and Methods
4.1. Preparation of PRGF
4.2. Culture and Stimulation of Primary Human Keratinocytes
4.3. Whole Transcriptome Sequencing (RNA-Seq)
4.4. Real-Time PCR
4.5. ELISA
4.5.1. Expression Analysis of ECM-Related Genes In Ex Vivo Skin Explants
4.5.2. Expression Analysis of ECM-Related Genes in Experimentally Generated Skin Wounds and after PRF Treatment In Vivo
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair Regen. 2016, 24, 434–442. [Google Scholar] [CrossRef]
- Pelka, R. The economic situation of chronic wounds. Krankenpfl. J. 1997, 35, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purwins, S.; Herberger, K.; Debus, E.S.; Rustenbach, S.J.; Pelzer, P.; Rabe, E.; Schäfer, E.; Stadler, R.; Augustin, M. Cost-of-illness of chronic leg ulcers in Germany. Int. Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Joret, M.O.; Dean, A.; Cao, C.; Stewart, J.; Bhamidipaty, V. The financial burden of surgical and endovascular treatment of diabetic foot wounds. J. Vascular Surg. 2016, 64, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körber, A.; Klode, J.; Al-Benna, S.; Wax, C.; Schadendorf, D.; Steinstraesser, L.; Dissemond, J. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J. Ger. Soc. Dermatol. 2011, 9, 116–121. [Google Scholar] [CrossRef]
- Green, J.; Jester, R.; McKinley, R.; Pooler, A. The impact of chronic venous leg ulcers: A systematic review. J. Wound Care 2014, 23, 601–612. [Google Scholar] [CrossRef]
- Furtado, K.; Pina, E.; Moffatt, C.J.; Franks, P.J. Leg ulceration in Portugal: Quality of life. Int. Wound J. 2007, 5, 34–39. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. j. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Ågren, M.S.; Rasmussen, K.; Pakkenberg, B.; Jørgensen, B. Growth factor and proteinase profile of Vivostat® platelet-rich fibrin linked to tissue repair. Vox Sang. 2014, 107, 37–43. [Google Scholar] [CrossRef]
- Anitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb. Haemost. 2004, 91, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Steenvoorde, P.; van Doorn, L.P.; Naves, C.; Oskam, J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J. Wound Care 2008, 17, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Hersant, B.; Bosc, R.; Meningaud, J.-P. The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair Regen.Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2015, 23, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implant Res. 2006, 17, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Kakudo, N.; Minakata, T.; Mitsui, T.; Kushida, S.; Notodihardjo, F.Z.; Kusumoto, K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast. Reconstr. Surg. 2008, 122, 1352–1360. [Google Scholar] [CrossRef]
- Baeyens, W.; Glineur, R.; Evrard, L. The use of platelet concentrates: Platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in bone reconstruction prior to dental implant surgery. Rev. Médicale Brux. 2010, 31, 521–527. [Google Scholar]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: A review of the literature. J. Bone Jt. Surg. Br. Vol. 2009, 91, 987–996. [Google Scholar] [CrossRef]
- Alsousou, J.; Ali, A.; Willett, K.; Harrison, P. The role of platelet-rich plasma in tissue regeneration. Platelets 2013, 24, 173–182. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Dohan Ehrenfest, D.M.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2018, 29, 468–475. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, A.; Lammel, J.; Rademacher, F.; Groß, J.; Siggelkow, M.; Lippross, S.; Klüter, T.; Varoga, D.; Tohidnezhad, M.; Pufe, T.; et al. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp. Dermatol. 2016, 25. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer, A.; Lammel, J.; Lippross, S.; Klüter, T.; Behrendt, P.; Tohidnezhad, M.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. Platelet-released growth factors induce psoriasin in keratinocytes: Implications for the cutaneous barrier. Ann. Anat. Anat. Anz. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.; Tohidnezhad, M.; Lammel, J.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Jahr, H.; Cremer, J.; Rademacher, F.; et al. Platelet-Released Growth Factors Induce Differentiation of Primary Keratinocytes. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Bayer, A.; Tohidnezhad, M.; Berndt, R.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Jahr, H.; Cremer, J.; Rademacher, F.; et al. Platelet-released growth factors inhibit proliferation of primary keratinocytes in vitro. Ann. Anat. 2018, 215. [Google Scholar] [CrossRef]
- Somani, A.; Rai, R. Comparison of Efficacy of Autologous Platelet-rich Fibrin versus Saline Dressing in Chronic Venous Leg Ulcers: A Randomised Controlled Trial. J. Cutan. Aesthetic Surg. 2017, 10, 8–12. [Google Scholar] [CrossRef]
- Moneib, H.A.; Youssef, S.S.; Aly, D.G.; Rizk, M.A.; Abdelhakeem, Y.I. Autologous platelet-rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: A comparative study. J. Cosmet. Dermatol. 2017. [Google Scholar] [CrossRef]
- Skonier, J.; Bennett, K.; Rothwell, V.; Kosowski, S.; Plowman, G.; Wallace, P.; Edelhoff, S.; Disteche, C.; Neubauer, M.; Marquardt, H.; et al. beta ig-h3: A transforming growth factor-beta-responsive gene encoding a secreted protein that inhibits cell attachment in vitro and suppresses the growth of CHO cells in nude mice. DNA Cell Biol. 1994, 13, 571–584. [Google Scholar] [CrossRef]
- Bae, J.-S.; Lee, S.-H.; Kim, J.-E.; Choi, J.-Y.; Park, R.-W.; Park, J.Y.; Park, H.-S.; Sohn, Y.-S.; Lee, D.-S.; Lee, B.E.; et al. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002, 294, 940–948. [Google Scholar] [CrossRef]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Transforming Growth Factor-Beta-Induced Protein (TGFBI)/(βig-H3): A Matrix Protein with Dual Functions in Ovarian Cancer. Int. J. Mol. Sci. 2012, 13, 10461–10477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitkenhead, M.; Wang, S.-J.; Nakatsu, M.N.; Mestas, J.; Heard, C.; Hughes, C.C.W. Identification of Endothelial Cell Genes Expressed in an in Vitro Model of Angiogenesis: Induction of ESM-1, βig-h3, and NrCAM. Microvasc. Res. 2002, 63, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Maeng, Y.-S.; Lee, G.-H.; Lee, B.; Choi, S.-I.; Kim, T.; Kim, E.K. Role of TGFBIp in Wound Healing and Mucin Expression in Corneal Epithelial Cells. Yonsei Med. J. 2017, 58, 423. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kwak, T.; Butmarc, J.; Kim, T.-A.; Yufit, T.; Carson, P.; Kim, S.-J.; Falanga, V. Fibroblasts from non-healing human chronic wounds show decreased expression of βig-h3, a TGF-β inducible protein. J. Dermatol. Sci. 2008, 50, 15–23. [Google Scholar] [CrossRef]
- Thapa, N.; Lee, B.-H.; Kim, I.-S. TGFBIp/βig-h3 protein: A versatile matrix molecule induced by TGF-β. Int. J. Biochem. Cell Biol. 2007, 39, 2183–2194. [Google Scholar] [CrossRef]
- Etich, J.; Koch, M.; Wagener, R.; Zaucke, F.; Fabri, M.; Brachvogel, B. Gene Expression Profiling of the Extracellular Matrix Signature in Macrophages of Different Activation Status: Relevance for Skin Wound Healing. Int. J. Mol. Sci. 2019, 20, 5086. [Google Scholar] [CrossRef] [Green Version]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. In Advances in Experimental Medicine and Biology; Kluwer: Alphen aan den Rijn, The Netherlands, 2014; Volume 802, pp. 31–47. [Google Scholar]
- Stoffels, J.M.J.; Zhao, C.; Baron, W. Fibronectin in tissue regeneration: Timely disassembly of the scaffold is necessary to complete the build. Cell. Mol. Life Sci. 2013, 70, 4243–4253. [Google Scholar] [CrossRef]
- Larsen, M.; Artym, V.V.; Green, J.A.; Yamada, K.M. The matrix reorganized: Extracellular matrix remodeling and integrin signaling. Curr. Opin. Cell Biol. 2006, 18, 463–471. [Google Scholar] [CrossRef]
- Larivière, B.; Rouleau, M.; Picard, S.; Beaulieu, A.D. Human plasma fibronectin potentiates the mitogenic activity of platelet-derived growth factor and complements its wound healing effects. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair 2003, 11, 79–89. [Google Scholar] [CrossRef]
- Brotchie, H.; Wakefield, D. Fibronectin: Structure, Function and Significance in Wound Healing. Australas. J. Dermatol. 1990, 31, 47–56. [Google Scholar] [CrossRef]
- Clark, R.A. Potential roles of fibronectin in cutaneous wound repair. Arch. Dermatol. 1988, 124, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Igisu, K. The role of fibronectin in the process of wound healing. Thromb. Res. 1986, 44, 455–465. [Google Scholar] [CrossRef]
- Grinnell, F. Fibronectin and wound healing. J. Cell. Biochem. 1984, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.B. Fibronectin in acute and chronic wounds. J. ET Nurs. Off. Publ. Int. Assoc. Enteros. Ther. 1992, 19, 166–170. [Google Scholar]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.L.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix Metalloproteinase Gelatinase B (MMP-9) Coordinates and Effects Epithelial Regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salo, T.; Mäkelä, M.; Kylmäniemi, M.; Autio-Harmainen, H.; Larjava, H. Expression of matrix metalloproteinase-2 and-9 during early human wound healing. Lab. Investig. J. Tech. Methods Pathol. 1994, 70, 176–182. [Google Scholar]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef]
- Nurminskaya, M.V.; Belkin, A.M. Cellular functions of tissue transglutaminase. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 294, pp. 1–97. [Google Scholar]
- Greenberg, C.; Greenberg, C.S. TGM2 and implications for human disease: Role of alternative splicing. Front. Biosci. 2013, 18, 504. [Google Scholar] [CrossRef] [Green Version]
- Odii, B.O.; Coussons, P. Biological Functionalities of Transglutaminase 2 and the Possibility of Its Compensation by Other Members of the Transglutaminase Family. Sci. World J. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.S.; Park, H.H. Structural aspects of transglutaminase 2: Functional, structural, and regulatory diversity. Apoptosis 2017, 22, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Verderio, E.A.M.; Johnson, T.; Griffin, M. Tissue transglutaminase in normal and abnormal wound healing: Review article. Amino Acids 2004, 26, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Grenard, P.; Aeschlimann, P.; Langley, M.; Blain, E.; Errington, R.; Kipling, D.; Thomas, D.; Aeschlimann, D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J. Cell Sci. 2004, 117, 3389–3403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)-a wound response enzyme. Front. Biosci. 2006, 11, 867. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Perez, M.; Lee, E.-S.; Kojima, S.; Griffin, M. The functional relationship between transglutaminase 2 and transforming growth factor β1 in the regulation of angiogenesis and endothelial–mesenchymal transition. Cell Death Dis. 2017, 8, e3032. [Google Scholar] [CrossRef] [Green Version]
- Kárpáti, S.; Sárdy, M.; Németh, K.; Mayer, B.; Smyth, N.; Paulsson, M.; Traupe, H. Transglutaminases in autoimmune and inherited skin diseases: The phenomena of epitope spreading and functional compensation. Exp. Dermatol. 2018, 27, 807–814. [Google Scholar] [CrossRef]
- Akimov, S.S.; Krylov, D.; Fleischman, L.F.; Belkin, A.M. Tissue Transglutaminase Is an Integrin-Binding Adhesion Coreceptor for Fibronectin. J. Cell Biol. 2000, 148, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xu, X.; Bai, L.; Chen, W.; Lin, Y. Epidermal Growth Factor Receptor-mediated Tissue Transglutaminase Overexpression Couples Acquired Tumor Necrosis Factor-related Apoptosis-inducing Ligand Resistance and Migration through c-FLIP and MMP-9 Proteins in Lung Cancer Cells. J. Biol. Chem. 2011, 286, 21164–21172. [Google Scholar] [CrossRef] [Green Version]
- Rognoni, E.; Ruppert, R.; Fässler, R. The kindlin family: Functions, signaling properties and implications for human disease. J. Cell Sci. 2016, 129, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Michael, M.; Begum, R.; Chan, G.K.; Whitewood, A.J.; Matthews, D.R.; Goult, B.T.; McGrath, J.A.; Parsons, M. Kindlin-1 Regulates Epidermal Growth Factor Receptor Signaling. J. Investig. Dermatol. 2019, 139, 369–379. [Google Scholar] [CrossRef]
- Rognoni, E.; Widmaier, M.; Jakobson, M.; Ruppert, R.; Ussar, S.; Katsougkri, D.; Böttcher, R.T.; Lai-Cheong, J.E.; Rifkin, D.B.; McGrath, J.A.; et al. Kindlin-1 controls Wnt and TGF-β availability to regulate cutaneous stem cell proliferation. Nat. Med. 2014, 20, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ussar, S.; Moser, M.; Widmaier, M.; Rognoni, E.; Harrer, C.; Genzel-Boroviczeny, O.; Fässler, R. Loss of Kindlin-1 Causes Skin Atrophy and Lethal Neonatal Intestinal Epithelial Dysfunction. PLoS Genet. 2008, 4, e1000289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Sun, L.; Zhu, N.; Qi, F. Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int. J. Mol. Med. 2017, 39, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Luo, C.; Yin, Z.; Li, P.; Wang, S.; Chen, J.; He, Q.; Zhou, J. Downregulation of let-7b promotes COL1A1 and COL1A2 expression in dermis and skin fibroblasts during heat wound repair. Mol. Med. Rep. 2016, 13, 2683–2688. [Google Scholar] [CrossRef] [PubMed]
- Rosensteel, S.M.; Wilson, R.P.; White, S.L.; Ehrlich, H.P. COL1A1 oligodeoxynucleotides decoy: Biochemical and morphologic effects in an acute wound repair model. Exp. Mol. Pathol. 2010, 89, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Oliveira, J.; Guimarães, L.H.; Carvalho, E.M.; Blackwell, J.M.; Castellucci, L. Wound healing genes and susceptibility to cutaneous leishmaniasis in Brazil: Role of COL1A1. Infect. Genet. Evol. 2015, 30, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Schulze, J.; Hansen, U.; Ashwodt, T.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E.; Bruckner, P.; Bruckner-Tuderman, L. A Novel Marker of Tissue Junctions, Collagen XXII. J. Biol. Chem. 2004, 279, 22514–22521. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Frost, D.B.; Mlakar, L.; Heywood, J.; da Silveira, W.A.; Hardiman, G.; Feghali-Bostwick, C. A Human Skin Model Recapitulates Systemic Sclerosis Dermal Fibrosis and Identifies COL22A1 as a TGFβ Early Response Gene that Mediates Fibroblast to Myofibroblast Transition. Genes 2019, 10, 75. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Bahar-Shany, K.; Ravid, A.; Koren, R. Upregulation of MMP-9 production by TNFÎ ± in keratinocytes and its attenuation by vitamin D. J. Cell. Physiol. 2009. [Google Scholar] [CrossRef]
- Antonyak, M.A.; Li, B.; Regan, A.D.; Feng, Q.; Dusaban, S.S.; Cerione, R.A. Tissue Transglutaminase Is an Essential Participant in the Epidermal Growth Factor-stimulated Signaling Pathway Leading to Cancer Cell Migration and Invasion. J. Biol. Chem. 2009, 284, 17914–17925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenberger, B.M.; Gerber, P.A.; Holcmann, M.; Buhren, B.A.; Amberg, N.; Smolle, V.; Schrumpf, H.; Boelke, E.; Ansari, P.; Mackenzie, C.; et al. Epidermal EGFR Controls Cutaneous Host Defense and Prevents Inflammation. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Moro, L. Integrins induce activation of EGF receptor: Role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998, 17, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Griffith, L.; Wells, A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004, 12, 262–268. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work With High-Throughput Sequencing Data. Bioinformatics (Oxf. Engl.) 2015, 31. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. Cluster Profiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.G.O.; Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.A.; Simanski, M.; Rademacher, F.; Schröder, L.; Harder, J. The pattern recognition receptor NOD2 mediates Staphylococcus aureus-induced IL-17C expression in keratinocytes. J. Investig. Dermatol. 2014, 134, 374–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Transforming Growth Factor Beta Induced, TGFBI | ACCCAGAAGCCCTGAGAG | TGCAGCCCACCTCCAGTG |
| Fibronectin 1, FN1 | ACAACGTCATAGTGGAGGCA | CATCCGTAGGTTGGTTCAAG |
| Matrix Metalloproteinase 9, MMP9 | GACACGCACGACGTCTTCCA | CACTGCAGGATGTCATAGGTCA |
| Transglutaminase 2, TGM2 | CTCAACCTGGAGCCTTTCTC | AGGGCCCGCACCTTGATGA |
| Fermitin Family Member 1, FERMT1 | GATTCCAGTGACAACATGGAG | TCAAACTCGATGACCACCTG |
| Collagen Type I Alpha 1 Chain, COL1A1 | CTGGAAGAGTGGAGAGTACTG | GTCTCCATGTTGCAGAAGAC |
| Collagen Type XXII Alpha 1 Chain, COL22A1 | CAGGAGAGAAAGGAGTCCC | TGCCCCAGGCTGGCCTTTTC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayer, A.; Wijaya, B.; Möbus, L.; Rademacher, F.; Rodewald, M.; Tohidnezhad, M.; Pufe, T.; Drücke, D.; Gläser, R.; Harder, J.
Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes
. Int. J. Mol. Sci. 2020, 21, 4404.
https://doi.org/10.3390/ijms21124404
Bayer A, Wijaya B, Möbus L, Rademacher F, Rodewald M, Tohidnezhad M, Pufe T, Drücke D, Gläser R, Harder J.
Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes
. International Journal of Molecular Sciences. 2020; 21(12):4404.
https://doi.org/10.3390/ijms21124404
Bayer, Andreas, Bernard Wijaya, Lena Möbus, Franziska Rademacher, Meno Rodewald, Mersedeh Tohidnezhad, Thomas Pufe, Daniel Drücke, Regine Gläser, and Jürgen Harder.
2020. "Platelet-Released Growth Factors and Platelet-Rich Fibrin Induce Expression of Factors Involved in Extracellular Matrix Organization in Human Keratinocytes
" International Journal of Molecular Sciences 21, no. 12: 4404.
https://doi.org/10.3390/ijms21124404





