Telocytes in the Normal and Pathological Peripheral Nervous System
Abstract
1. General Characteristics of Telocytes and Terminological Introduction
2. TCs in the Normal Peripheral Nervous System
2.1. TCs (TCs/CD34+SCs) in Nerves
2.2. TCs (TCs/CD34+SCs) in Sensory Nerve Endings
2.3. TCs in Ganglia
2.4. TCs (TCs/CD34+SCs) in the Autonomic Nervous System of the Digestive Tract
3. TCs (TCs/CD34+SCs) in the Pathologic Peripheral Nervous System (PNS)
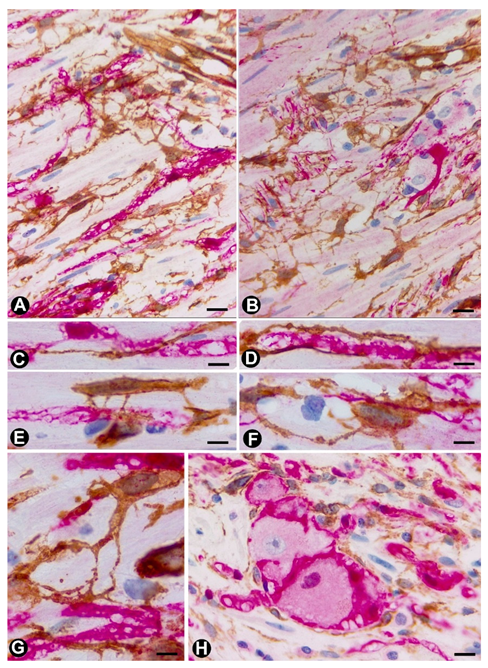
3.1. TCs (TCs/CD34+SCs) in Hyperplastic Neurogenic Processes
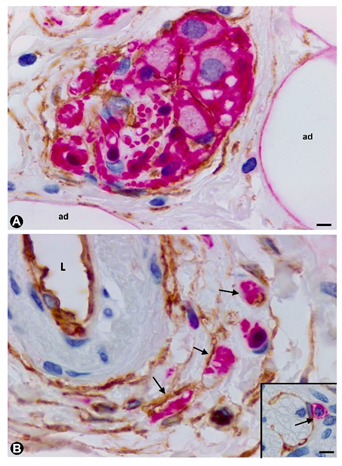
3.1.1. TCs/CD34+SCs in Hyperplastic Neurogenic Processes of the Appendix
3.1.2. TCs/CD34+SCs in Neurogenic Hyperplasia of Gallbladder
3.2. TCs/CD34+SCs in Tumours and of the Peripheral Nervous System
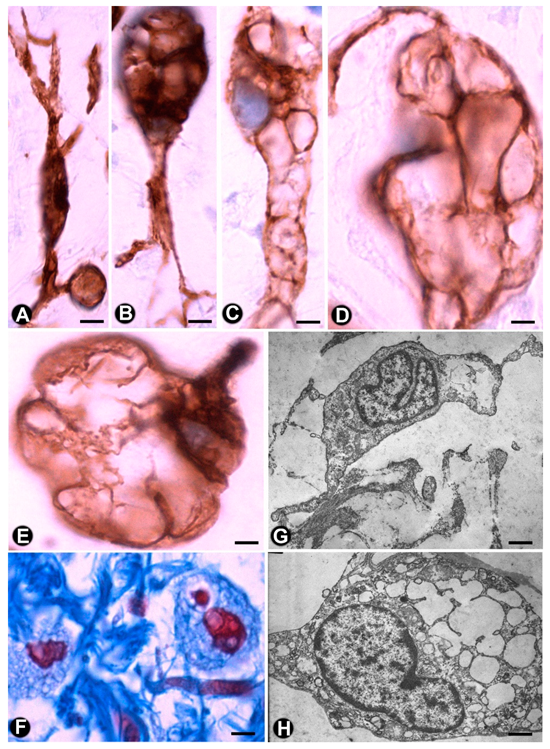
3.2.1. TCs/CD34+SCs in Neurofibromas
3.2.2. TCs/CD34+SCs in Schwannomas
3.2.3. TCs/CD34+SCs in Granular Cell Tumour
3.2.4. TCs/CD34+SCs in Nerve Sheath Myxoma
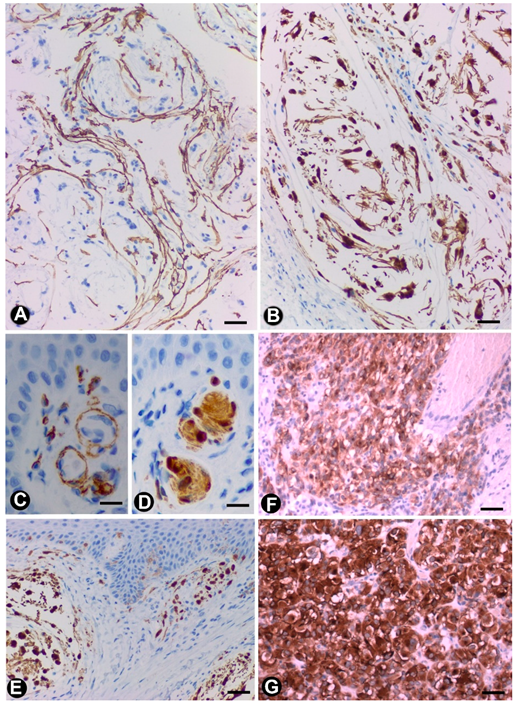
3.2.5. TCs/CD34+SCs in Gastrointestinal Stromal Tumours (GISTs)
3.3. TCs/CD34+SCs in Tumour-invaded Nerves
3.4. TCs/CD34+SCs in Other Pathologic Processes of the Peripheral Nervous System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popescu, L.M.; Faussone-Pellegrini, M.S. Telocytes—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Popescu, L.M. Telocytes. BioMol. Concepts 2011, 2, 481–489. [Google Scholar] [CrossRef]
- Popescu, L.M. Telocytes—A novel type of interstitial cells. In Recent Researches in Modern Medicine—HISTEM; Braisant, O., Wakamatsu, H., Kang, I., Allegaert, K., Lenbury, Y., Wacholtz, A., Eds.; WSEAS Press: Cambridge, UK, 2011; pp. 424–432. [Google Scholar]
- Cretoiu, D.; Radu, B.M.; Banciu, A.; Banciu, D.D.; Cretoiu, S.M. Telocytes heterogeneity: From cellular morphology to functional evidence. Semin. Cell Dev. Biol. 2017, 64, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Faussone-Pellegrini, M.S. The telocyte subtypes. Adv. Exp. Med. Biol. 2016, 913, 115–126. [Google Scholar] [PubMed]
- Cretoiu, D.; Xu, J.; Xiao, J.; Cretoiu, S.M. Telocytes and Their Extracellular Vesicles-Evidence and Hypotheses. Int. J. Mol. Sci. 2016, 17, 1322. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Roatesi, S.; Bica, I.; Plesca, C.; Stefan, A.; Bajenaru, O.; Condrat, C.E.; Cretoiu, S.M. Simulation and modeling of telocytes behavior in signaling and intercellular communication processes. Int. J. Mol. Sci. 2020, 21, 2615. [Google Scholar] [CrossRef]
- Cretoiu, S.M.; Popescu, L.M. Telocytes revisited. Biomol. Concepts 2014, 5, 353–369. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Uptake and intracytoplasmic storage of pigmented particles by human CD34+ stromal cells/telocytes: Endocytic property of telocytes. J. Cell. Mol. Med. 2014, 18, 2478–2487. [Google Scholar]
- Mandache, E.; Popescu, L.M.; Gherghiceanu, M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: Shed vesicles as intermediates. J. Cell. Mol. Med. 2007, 11, 1175–1184. [Google Scholar] [CrossRef]
- Nicolescu, M.I.; Popescu, L.M. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012, 41, 949–956. [Google Scholar] [CrossRef]
- Nicolescu, M.I.; Bucur, A.; Dinca, O.; Rusu, M.C.; Popescu, L.M. Telocytes in parotid glands. Anat. Rec. (Hoboken) 2012, 295, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Gherghiceanu, M.; Cretoiu, D.; Radu, E. The connective connection: Interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J. Cell. Mol. Med. 2005, 9, 714–730. [Google Scholar] [CrossRef]
- Popescu, L.M.; Nicolescu, M.I. Telocytes and stem cells. In Resident Stem Cells and Regenerative Therapy; Coeli dos Santos Goldenberg, R., Campos de Carvalho, A.C., Eds.; Academic Press; Elsevier: Oxford, UK, 2013; pp. 205–231. [Google Scholar]
- Richter, M.; Kostin, S. The failing human heart is characterized by decreased numbers of telocytes as result of apoptosis and altered extracellular matrix composition. J. Cell. Mol. Med. 2015, 19, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Smythies, J. Intercellular Signaling in Cancer-the SMT and TOFT Hypotheses, Exosomes, Telocytes and Metastases: Is the Messenger in the Message? J. Cancer 2015, 6, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Sáez, F.J.; Díaz-Flores, L., Jr.; Madrid, J.F. Telocytes in neuromuscular spindles. J. Cell. Mol. Med. 2013, 17, 457–465. [Google Scholar]
- Díaz-Flores, L.; Gutiérrez, R.; Díaz-Flores, L., Jr.; Goméz, M.G.; Sáez, F.J.; Madrid, J.F. Behaviour of telocytes during physiopathological activation. Semin. Cell Dev. Biol. 2016, 55, 50–61. [Google Scholar] [CrossRef]
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.S. Telocytes in human term placenta: Morphology and phenotype. Cells Tissues Organs 2010, 192, 325–339. [Google Scholar] [CrossRef]
- Bani, D.; Formigli, L.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J. Cell. Mol. Med. 2010, 14, 2531–2538. [Google Scholar] [CrossRef]
- Popescu, L.M. The tandem: Telocytes-stem cells. Int. J. Biol. Biomed. Eng. 2011, 5, 83–92. [Google Scholar]
- Popescu, L.M.; Gherghiceanu, M.; Suciu, L.C.; Manole, C.G.; Hinescu, M.E. Telocytes and putative stem cells in the lungs: Electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011, 345, 391–403. [Google Scholar] [CrossRef]
- Popescu, L.M.; Manole, E.; Serboiu, C.S.; Manole, C.G.; Suciu, L.C.; Gherghiceanu, M.; Popescu, B.O. Identification of telocytes in skeletal muscle interstitium: Implication for muscle regeneration. J. Cell. Mol. Med. 2011, 15, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Hinescu, M.E.; Popescu, L.M.; Gherghiceanu, M.; Faussone-Pellegrini, M.S. Interstitial Cajal-like cells in rat mesentery: An ultrastructural and immunohistochemical approach. J. Cell. Mol. Med. 2008, 12, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Pieri, L.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J. Cell. Mol. Med. 2008, 12, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, M.; Qian, M.; Wang, L.; Cismasiu, V.B.; Bai, C.; Popescu, L.M.; Wang, X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J. Cell. Mol. Med. 2013, 17, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Cretoiu, D.; Shen, Z.J.; Yang, X.J. An in vitro investigation of telocytes-educated macrophages: Morphology, heterocellular junctions, apoptosis and invasion analysis. J. Transl. Med. 2018, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Nistri, S. New insights into the morphogenic role of stromal cells and their relevance for regenerative medicine. Lessons from the heart. J. Cell. Mol. Med. 2014, 18, 363–370. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Popescu, L.M. Heterocellular communication in the heart: Electron tomography of telocyte-myocyte junctions. J. Cell. Mol. Med. 2011, 15, 1005–1011. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhu, P.; Sun, H.; Mou, Y.; Duan, C.; Yao, A.; Lv, S.; Wang, C. Distribution and characteristics of telocytes as nurse cells in the architectural organization of engineered heart tissues. Sci. China Life Sci. 2014, 57, 241–247. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, S.; Liu, J.; Yuan, Z.; Qi, X.; Qin, J.; Zheng, X.; Shen, X.; Yu, Y.; Qnin, T.J.; et al. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J. Cell. Mol. Med. 2013, 17, 123–133. [Google Scholar] [CrossRef]
- Zheng, Y.; Cretoiu, D.; Yan, G.; Cretoiu, S.M.; Popescu, L.M.; Fang, H.; Wang, X. Protein profiling of human lung telocytes and microvascular endothelial cells using iTRAQ quantitative proteomics. J. Cell. Mol. Med. 2014, 18, 1035–1059. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Traini, C.; Manetti, M.; Ibba-Manneschi, L.; Faussone-Pellegrini, M.S. Telocytes express PDGFRα in the human gastrointestinal tract. J. Cell. Mol. Med. 2013, 17, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Evangelista, S. Neurokinin receptors in the gastrointestinal muscle wall: Cell distribution and possible roles. BioMol. Concepts. 2013, 4, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, M.G.; Traini, C.; Guasti, D.; Del Popolo, G.; Faussone-Pellegrini, M.S. Telocytes subtypes in human urinary bladder. J. Cell. Mol. Med. 2014, 18, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Ceafalan, L.; Gherghiceanu, M.; Popescu, L.M.; Simionescu, O. Telocytes in human skin-are they involved in skin regeneration? J. Cell. Mol. Med. 2012, 16, 1405–1420. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; Lizartza, K.; Goméz, M.G.; García, M.P.; Sáez, F.J.; Díaz-Flores, L., Jr.; Madrid, J.F. Behavior of in situ human native adipose tissue CD34+ stromal/progenitor cells during different stages of repair. Tissue-resident CD34+ stromal cells as a source of myofibroblasts. Anat. Rec. (Hoboken) 2015, 298, 917–930. [Google Scholar]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González, M.; Sáez, F.J.; Aparicio, F.; Díaz-Flores, L., Jr.; Madrid, J.F. Human resident CD34+ stromal cells/telocytes have progenitor capacity and are a source of αSMA+ cells during repair. Histol. Histopathol. 2015, 30, 615–627. [Google Scholar]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González, M.; Díaz-Flores, L., Jr.; Madrid, J.F. Telocytes as a source of progenitor cells in regeneration and repair through granulation tissue. Curr. Stem Cell Res. Ther. 2016, 11, 395–403. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Bani, D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J. Cell. Mol. Med. 2010, 14, 1061–1063. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Popescu, L.M. Cardiac telocytes-their junctions and functional implications. Cell Tissue Res. 2012, 348, 265–279. [Google Scholar] [CrossRef]
- Vannucchi, M.G.; Bani, D.; Faussone-Pellegrini, M.S. Telocytes contribute as cell progenitors and differentiation inductors in tissue regeneration. Curr. Stem Cell Res. Ther. 2016, 11, 383–389. [Google Scholar] [CrossRef]
- Manetti, M.; Tani, A.; Rosa, I.; Chellini, F.; Squecco, R.; Idrizaj, E.; Zecchi-Orlandini, S.; Ibba-Manneschi, L.; Sassoli, C. Morphological evidence for telocytes as stromal cells supporting satellite cell activation in eccentric contraction-induced skeletal muscle injury. Sci. Rep. 2019, 9, 14515. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Toma, J.S.; Johnston, A.P.W.; Steadman, P.E.; Yuzwa, S.A.; Mahmud, N.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Mesenchymal Precursor Cells in Adult Nerves Contribute to Mammalian Tissue Repair and Regeneration. Cell Stem Cell 2019, 24, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Richard, L.; Védrenne, N.; Vallat, J.M.; Funalot, B. Characterization of Endoneurial Fibroblast-like Cells from Human and Rat Peripheral Nerves. J. Histochem. Cytochem. 2014, 62, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Tani, T.; Shimada, T.; Ishizawa, K.; Shimada, S.; Sano, T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod. Pathol. 2003, 16, 293–298. [Google Scholar] [CrossRef]
- Khalifa, M.A.; Montgomery, E.A.; Ismiil, N.; Azumi, N. What are the CD34+ cells in benign peripheral nerve sheath tumors? Double immunostaining study of CD34 and S-100 protein. Am. J. Clin. Pathol. 2000, 114, 123–126. [Google Scholar] [CrossRef]
- Weiss, S.W.; Nickoloff, B.J. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am. J. Surg. Pathol. 1993, 17, 1039–1045. [Google Scholar] [CrossRef]
- Mirancea, N. Telocyte-a particular cell phenotype. Infrastructure, relationships and putative functions. Rom. J. Morphol. Embryol. 2016, 57, 7–21. [Google Scholar]
- Richard, L.; Topilko, P.; Magy, L.; Decouvelaere, A.V.; Charnay, P.; Funalot, B.; Vallat, J.M. Endoneurial fibroblast-like cells. J. Neuropathol. Exp. Neurol. 2012, 71, 938–947. [Google Scholar] [CrossRef]
- Schubert, T.; Friede, R.L. The role of endoneurial fibroblasts in myelin degradation. J. Neuropathol. Exp. Neurol. 1981, 40, 134–154. [Google Scholar] [CrossRef]
- Joseph, N.M.; Mukouyama, Y.S.; Mosher, J.T.; Jaegle, M.; Crone, S.A.; Dormand, E.L.; Lee, K.F.; Meijer, D.; Anderson, D.J.; Morrison, S.J. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development 2004, 131, 5599–5612. [Google Scholar] [CrossRef]
- Bosco, C.; Díaz, E.; Gutiérrez, R.; González, J.; Pérez, J. Ganglionar nervous cells and telocytes in the pancreas of Octodon degus: Extra and intrapancreatic ganglionar cells and telocytes in the degus. Auton. Neurosci. 2013, 177, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Cantarero Carmona, I.; Luesma Bartolomé, M.J.; Junquera Escribano, C. Identification of telocytes in the lamina propria of rat duodenum: Transmission electron microscopy. J. Cell. Mol. Med. 2011, 15, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Faussone-Pellegrini, M.S.; Gherghiceanu, M. Telocyte’s contacts. Semin. Cell Dev. Biol. 2016, 55, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, T.; De Vos, R.; Van Der Aa, F.; Joniau, S.; van den Oord, J.; Roskams, T.; De Ridder, D. Identification of telocytes in the upper lamina propria of the human urinary tract. J. Cell. Mol. Med. 2012, 16, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Gherghiceanu, M.; Manole, C.G.; Popescu, L.M. Telocytes in endocardium: Electron microscope evidence. J. Cell. Mol. Med. 2010, 14, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Luesma, M.J.; Gherghiceanu, M.; Popescu, L.M. Telocytes and stem cells in limbus and uvea of mouse eye. Version 2. J. Cell. Mol. Med. 2013, 17, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; Ibba-Manneschi, L.; Manetti, M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv. Exp. Med. Biol. 2017, 998, 237–254. [Google Scholar]
- Ullah, S.; Yang, P.; Zhang, L.; Zhang, Q.; Liu, Y.; Chen, W.; Waqas, Y.; Le, Y.; Chen, B.; Chen, Q. Identification and characterization of telocytes in the uterus of the oviduct in the Chinese soft-shelled turtle, Pelodiscus sinensis: TEM evidence. J. Cell. Mol. Med. 2014, 18, 2385–2392. [Google Scholar] [CrossRef]
- Yang, P.; Ahmad, N.; Hunag, Y.; Ullah, S.; Zhang, Q.; Waqas, Y.; Liu, Y.; Li, Q.; Hu, L.; Chen, Q. Telocytes: Novel interstitial cells present in the testis parenchyma of the Chinese soft-shelled turtle Pelodiscus sinensis. J. Cell. Mol. Med. 2015, 19, 2888–2899. [Google Scholar] [CrossRef]
- García-Piqueras, J.; Cobo, R.; Cárcaba, L.; García-Mesa, Y.; Feito, J.; Cobo, J.; García-Suárez, O.; Vega, J.A. The capsule of human Meissner corpuscles: Immunohistochemical evidence. J. Anat. 2020, 236, 854–861. [Google Scholar]
- García-Piqueras, J.; García-Suárez, O.; Rodríguez-González, M.C.; Cobo, J.L.; Cabo, R.; Vega, J.A.; Feito, J. Endoneurial-CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann. Anat. 2017, 211, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Cretoiu, D.; Vrapciu, A.D.; Hostiuc, S.; Dermengiu, D.; Manoiu, V.S.; Cretoiu, S.M.; Mirancea, N. Telocytes of the human adult trigeminal ganglion. Cell Biol. Toxicol. 2016, 32, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Mănoiu, V.S.; Creţoiu, D.; Creţoiu, S.M.; Vrapciu, A.D. Stromal cells/telocytes and endothelial progenitors in the perivascular niches of the trigeminal ganglion. Ann. Anat. 2018, 218, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Cretoiu, D.; Cretoiu, S.M.; Simionescu, A.A.; Popescu, L.M. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol. Histopathol. 2012, 27, 1067–1078. [Google Scholar]
- Hagger, R.; Gharaie, S.; Finlayson, C.; Kumar, D. Distribution of the interstitial cells of Cajal in the human anorectum. J. Auton. Nerv. Syst. 1998, 73, 75–79. [Google Scholar] [CrossRef]
- Milia, A.F.; Ruffo, M.; Manetti, M.; Rosa, I.; Conte, D.; Fazi, M.; Messerini, L.; Ibba-Manneschi, L. Telocytes in Crohn’s disease. J. Cell. Mol. Med. 2013, 17, 1525–1536. [Google Scholar] [CrossRef]
- Vanderwinden, J.M.; Rumessen, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, S.N. CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab. Investig. 1999, 79, 59–65. [Google Scholar]
- Vanderwinden, J.M.; Rumessen, J.J.; De Laet, M.H.; Vanderhaeghen, J.J.; Schiffmann, S.N. CD34 immunoreactivity and interstitial cells of Cajal in the human and mouse gastrointestinal tract. Cell Tissue Res. 2000, 302, 145–153. [Google Scholar] [CrossRef]
- Veress, B.; Ohlsson, B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J. Cell. Mol. Med. 2020, 24, 3399–3406. [Google Scholar] [CrossRef]
- Zani, B.C.; Sanches, B.D.A.; Maldarine, J.S.; Biancardi, M.F.; Santos, F.C.A.; Barquilha, C.N.; Zucão, M.I.; Baraldi, C.M.B.; Felisbino, S.L.; Góes, R.M.; et al. Telocytes role during the postnatal development of the Mongolian gerbil jejunum. Exp. Mol. Pathol. 2018, 105, 130–138. [Google Scholar] [CrossRef]
- Rømert, P.; Mikkelsen, H.B. c-kit immunoreactive interstitial cells of Cajal in the human small and large intestine. Histochem. Cell Biol. 1998, 109, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Esterson, Y.B.; Esterson, A.Y.; Grimaldi, G.M.; Pellerito, J.S.; Warshawsky, R.J. Appendiceal ganglioneuroma in neurofibromatosis type 2. Clin. Imaging 2017, 45, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Güller, U.; Oertli, D.; Terracciano, L.; Harder, F. Neurogene Appendicopathie: Ein häufiges, fast unbekanntes Krankheitsbild. Auswertung von 816 Appendices und Literaturübersicht. Chirurg 2001, 72, 684–689. [Google Scholar]
- Masson, P. Carcinoids (Argentaffin-Cell Tumors) and Nerve Hyperplasia of the Appendicular Mucosa. Am. J. Pathol. 1928, 4, 181–212. [Google Scholar]
- Michalany, J.; Galindo, W. Classification of neuromas of the appendix. Beitr. Pathol. 1973, 150, 213–228. [Google Scholar] [CrossRef]
- Merck, C.; Kindblom, L.G. Neurofibromatosis of the appendix in von Recklinghausen’s disease. A report of a case. Acta Pathol. Microbiol. Scand. A 1975, 83, 623–627. [Google Scholar]
- Olsen, B.S.; Holck, S. Neurogenous hyperplasia leading to appendiceal obliteration: An immunohistochemical study of 237 cases. Histopathology 1987, 11, 843–849. [Google Scholar] [CrossRef]
- Sesia, S.B.; Mayr, J.; Bruder, E.; Haecker, F.M. Neurogenic appendicopathy: Clinical, macroscopic, and histopathological presentation in pediatric patients. Eur. J. Pediatr. Surg. 2013, 23, 238–242. [Google Scholar]
- van Eeden, S.; Offerhaus, G.J.; Peterse, H.L.; Dingemans, K.P.; Blaauwgeers, H.L. Gangliocytic paraganglioma of the appendix. Histopathology 2000, 36, 47–49. [Google Scholar] [CrossRef]
- Hennig, R.; Zanli, J.; Osman, T.; Esposito, I.; Berhane, T.; Vetrhus, M.; Søndenaa, K.; Büchler, M.W.; Friess, H. Association between gallstone-evoked pain, inflammation and proliferation of nerves in the gallbladder: A possible explanation for clinical differences. Scand. J. Gastroenterol. 2007, 42, 878–884. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Henson, D.E. Adenomyomatous hyperplasia of the gallbladder with perineural invasion. Arch. Pathol. Lab. Med. 1995, 119, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Albores-Saavedra, J.; Keenportz, B.; Bejarano, P.A.; Alexander, A.A.; Henson, D.E. Adenomyomatous hyperplasia of the gallbladder with perineural invasion: Revisited. Am. J. Surg. Pathol. 2007, 31, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Picón-Coronel, G.; Chablé-Montero, F.; Angeles-Ángeles, A.; Albores-Saavedra, J. Multiple venous and arterial thromboses of the gallbladder causing acute cholecystitis. A previously undescribed complication of essential thrombocythemia. Ann. Hepatol. 2011, 10, 365–369. [Google Scholar] [CrossRef]
- Magro, G.; Amico, P.; Vecchio, G.M.; Caltabiano, R.; Castaing, M.; Kacerovska, D.; Kazakov, D.V.; Michal, M. Multinucleated floret-like giant cells in sporadic and NF1-associated neurofibromas: A clinicopathologic study of 94 cases. Virchows Arch. 2010, 456, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.M.; Antonescu, C.R.; Fletcher, C.D.M.; Kim, A.; Lazar, A.J.; Quezado, M.M.; Reilly, K.M.; Stemmer-Rachamimov, A.; Stewart, D.R.; Viskochil, D.; et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum. Pathol. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Ide, F.; Muramatsu, T.; Kikuchi, K.; Saito, I.; Kusama, K. Oral plexiform schwannoma with unusual epithelial induction. J. Cutan. Pathol. 2015, 42, 978–982. [Google Scholar] [CrossRef]
- Sergheraert, J.; Zachar, D.; Furon, V.; Khonsari, R.H.; Ortonne, N.; Mauprivez, C. Oral plexiform schwannoma: A case report and relevant immunohistochemical investigation. SAGE Open Med. Case Rep. 2019, 7, 2050313X19838184. [Google Scholar] [CrossRef]
- Laskin, W.B.; Fetsch, J.F.; Miettinen, M. The “neurothekeoma”: Immunohistochemical analysis distinguishes the true nerve sheath myxoma from its mimics. Hum. Pathol. 2000, 31, 1230–1241. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Laskin, W.B.; Hallman, J.R.; Lupton, G.P.; Miettinen, M. Neurothekeoma: An analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am. J. Surg. Pathol. 2007, 31, 1103–1114. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Laskin, W.B.; Miettinen, M. Nerve sheath myxoma: A clinicopathologic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate. Am. J. Surg. Pathol. 2005, 29, 1615–1624. [Google Scholar] [CrossRef]
- Antonescu, C.R. Gastrointestinal stromal tumor (GIST) pathogenesis, familial GIST, and animal models. Semin. Diagn. Pathol. 2006, 23, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, C.R. Targeted therapy of cancer: New roles for pathologists in identifying GISTs and other sarcomas. Mod. Pathol. 2008, 21, S31–S36. [Google Scholar] [CrossRef] [PubMed]
- Min, K.W. Gastrointestinal stromal tumor: An ultrastructural investigation on regional differences with considerations on their histogenesis. Ultrastruct. Pathol. 2010, 34, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.T.; Rubin, B.P. Gastrointestinal stromal tumor: Advances in diagnosis and management. Arch. Pathol. Lab. Med. 2011, 135, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Sircar, K.; Hewlett, B.R.; Huizinga, J.D.; Chorneyko, K.; Berezin, I.; Riddell, R.H. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am. J. Surg. Pathol. 1999, 23, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ibba-Manneschi, L.; Rosa, I.; Manetti, M. Telocytes in chronic inflammatory and fibrotic diseases. Adv. Exp. Med. Biol. 2016, 913, 51–76. [Google Scholar] [PubMed]
- Ibba-Manneschi, L.; Rosa, I.; Manetti, M. Telocyte implications in human pathology: An overview. Semin. Cell Dev. Biol. 2016, 55, 62–69. [Google Scholar] [CrossRef]
- Manley, P.N.; Abu-Abed, S.; Kirsch, R.; Hawrysh, A.; Perrier, N.; Feilotter, H.; Pollett, A.; Riddell, R.H.; Hookey, L.; Walia, J.S. Familial PDGFRA-mutation syndrome: Somatic and gastrointestinal phenotype. Hum. Pathol. 2018, 76, 52–57. [Google Scholar] [CrossRef]
- Ricci, R.; Giustiniani, M.C.; Gessi, M.; Lanza, P.; Castri, F.; Biondi, A.; Persiani, R.; Vecchio, F.M.; Risio, M. Telocytes are the physiological counterpart of inflammatory fibroid polyps and PDGFRA-mutant GISTs. J. Cell. Mol. Med. 2018, 22, 4856–4862. [Google Scholar] [CrossRef]
- Varga, I.; Polák, Š.; Kyselovič, J.; Kachlík, D.; Danišovič, Ľ.; Klein, M. Recently discovered interstitial cell population of telocytes: Distinguishing facts from fiction regarding their role in the pathogenesis of diverse diseases called “Telocytopathies”. Medicina (Kaunas) 2019, 55, 56. [Google Scholar] [CrossRef]
- Klein, D.; Martini, R. Myelin and macrophages in the PNS: An intimate relationship in trauma and disease. Brain Res. 2016, 1641, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Martini, R.; Willison, H. Neuroinflammation in the peripheral nerve: Cause, modulator, or bystander in peripheral neuropathies? Glia 2016, 64, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, L.; He, R.; Huang, W.; Wang, H.; Lai, X.; Zou, Z.; Sun, J.; Ke, Q.; Zheng, M.; et al. Modeling the Pathogenesis of Charcot-Marie-Tooth Disease Type 1A Using Patient-Specific iPSCs. Stem Cell Rep. 2018, 10, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Groh, J.; Basu, R.; Stanley, E.R.; Martini, R. Cell-Surface and Secreted Isoforms of CSF-1 Exert Opposing Roles in Macrophage-Mediated Neural Damage in Cx32-Deficient Mice. J. Neurosci. 2016, 36, 1890–1901. [Google Scholar] [CrossRef] [PubMed]
- Elbarrany, W.G.; Alasmari, W.A. A possible role of endoneurial fibroblast-like cells in the resolution of endoneurial oedema following nerve crush injury. Int. J. Morphol. 2019, 37, 141–148. [Google Scholar] [CrossRef]
- Lazard, Z.W.; Olmsted-Davis, E.A.; Salisbury, E.A.; Gugala, Z.; Sonnet, C.; Davis, E.L.; Beal, E., 2nd; Ubogu, E.E.; Davis, A.R. Osteoblasts have a neural origin in heterotopic ossification. Clin. Orthop. Relat. Res. 2015, 473, 2790–2806. [Google Scholar] [CrossRef]
- Ochiai, T.; Ito, K.; Shichino, H.; Chin, M.; Mugishima, H. Ultrastructural findings of cutaneous nerves in patients with Hunter’s syndrome following hematopoietic stem cell transplant. Med. Mol. Morphol. 2005, 38, 118–122. [Google Scholar] [CrossRef]
- Leuschner, U.; Usadel, K.H.; Schwedes, U.; Schöffling, K. Nervenfasern in Tubuluswänden des Hodens bei Patienten mit Klinefelter-Syndrom. Elektronenmikroskopische und elektronenmikroskopisch-enzymhistochemische Untersuchungen. Virchows Arch. A Pathol. Pathol. Anat. 1973, 358, 331–341. [Google Scholar] [CrossRef]
- Usadel, K.H.; Leuschner, U.; Schwedes, U.; Schade, C.; Schöffling, K. Über das Auftreten cholinerger Nervenfasern in den Tubuluswänden des Hodens bei primäreom Hypogonadismus (Klinefelter-Syndrom). Klin Wochenschr. 1973, 51, 1016–1023. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; Gayoso, S.; Gutiérrez, E.; Díaz-Flores, L., Jr.; Carrasco, J.L. Telocytes in the Normal and Pathological Peripheral Nervous System. Int. J. Mol. Sci. 2020, 21, 4320. https://doi.org/10.3390/ijms21124320
Díaz-Flores L, Gutiérrez R, García MP, Gayoso S, Gutiérrez E, Díaz-Flores L Jr., Carrasco JL. Telocytes in the Normal and Pathological Peripheral Nervous System. International Journal of Molecular Sciences. 2020; 21(12):4320. https://doi.org/10.3390/ijms21124320
Chicago/Turabian StyleDíaz-Flores, Lucio, Ricardo Gutiérrez, Mª Pino García, Sara Gayoso, Emma Gutiérrez, Lucio Díaz-Flores, Jr., and José Luis Carrasco. 2020. "Telocytes in the Normal and Pathological Peripheral Nervous System" International Journal of Molecular Sciences 21, no. 12: 4320. https://doi.org/10.3390/ijms21124320
APA StyleDíaz-Flores, L., Gutiérrez, R., García, M. P., Gayoso, S., Gutiérrez, E., Díaz-Flores, L., Jr., & Carrasco, J. L. (2020). Telocytes in the Normal and Pathological Peripheral Nervous System. International Journal of Molecular Sciences, 21(12), 4320. https://doi.org/10.3390/ijms21124320




