Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal
Abstract
1. Introduction
2. Results and Discussions
2.1. Physical Characterisation of the Obtained Silica Hybrids
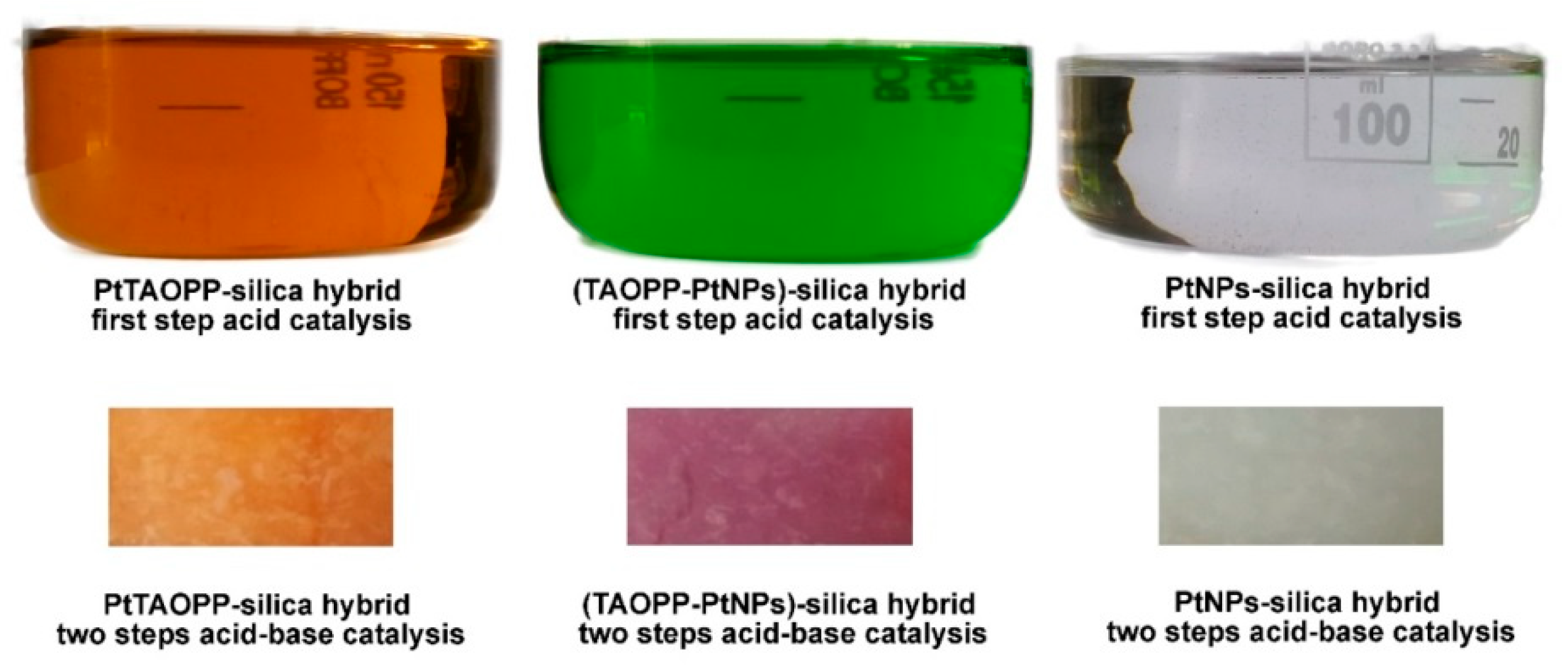
2.1.1. UV-Vis Spectroscopy of Sol Samples, during the Synthesis of Hybrid Materials
2.1.2. UV-Vis Spectroscopy of Solid Samples
2.1.3. Fluorescence Spectra of Solid Samples
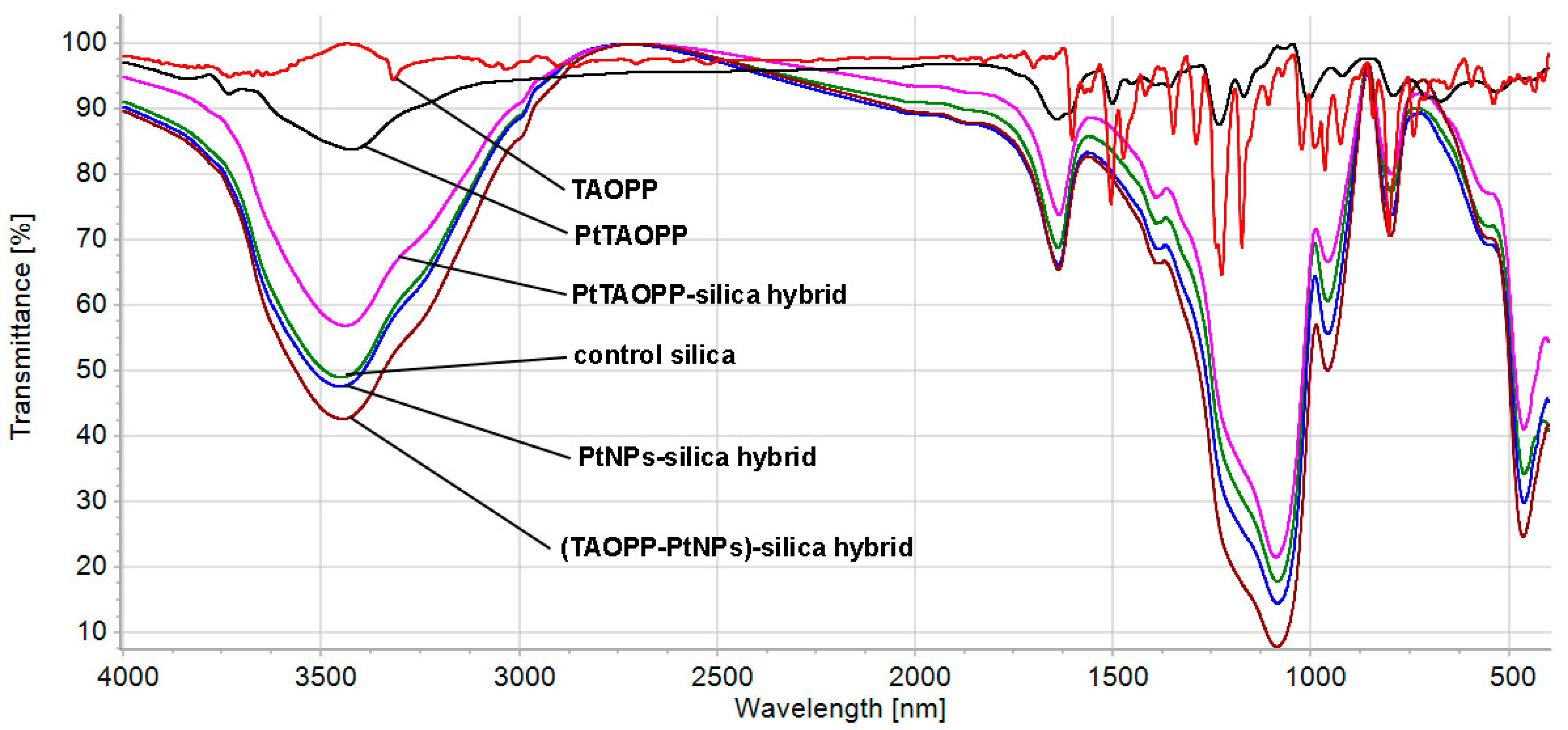
2.1.4. The FT-IR Spectroscopy
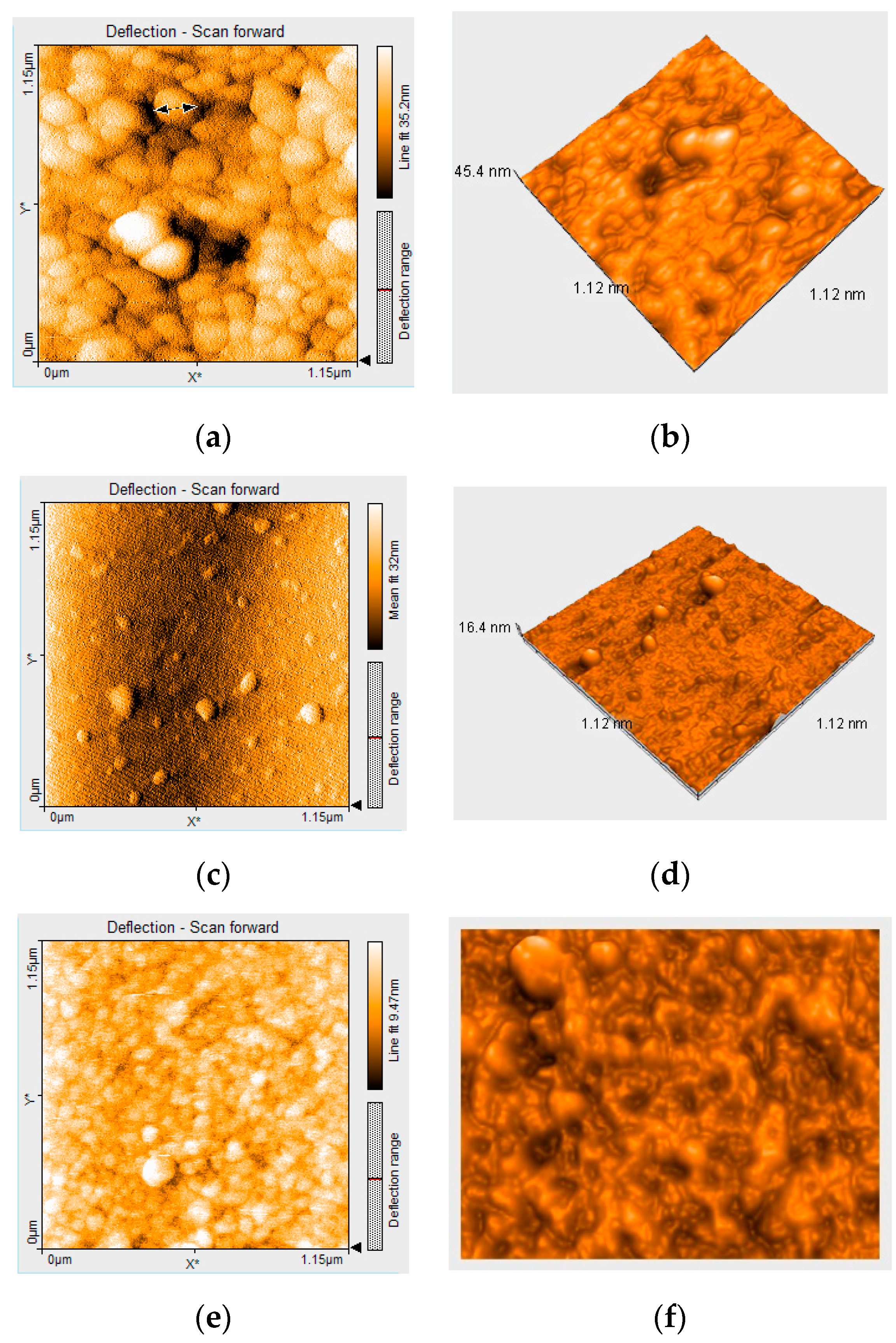
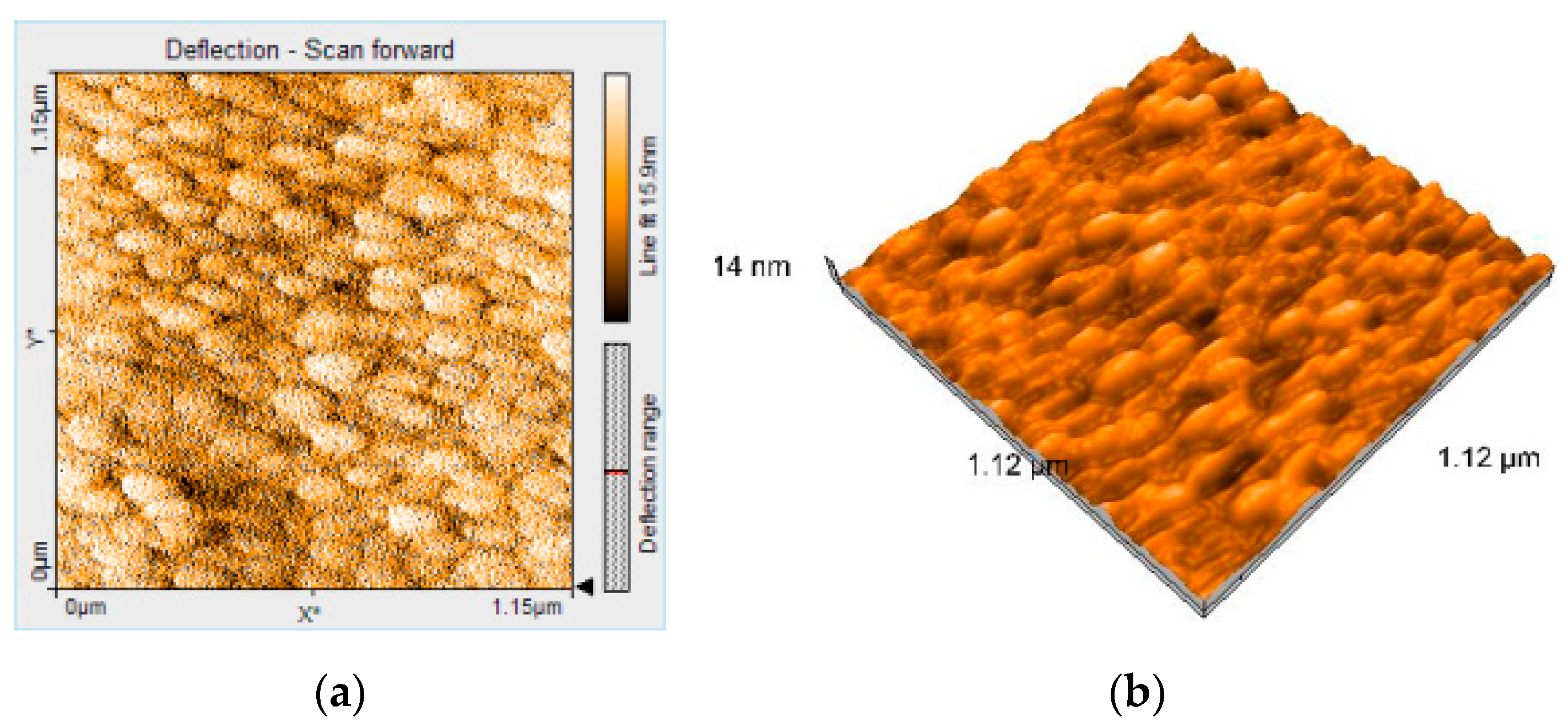
2.1.5. Atomic Force Microscopy (AFM) Analysis
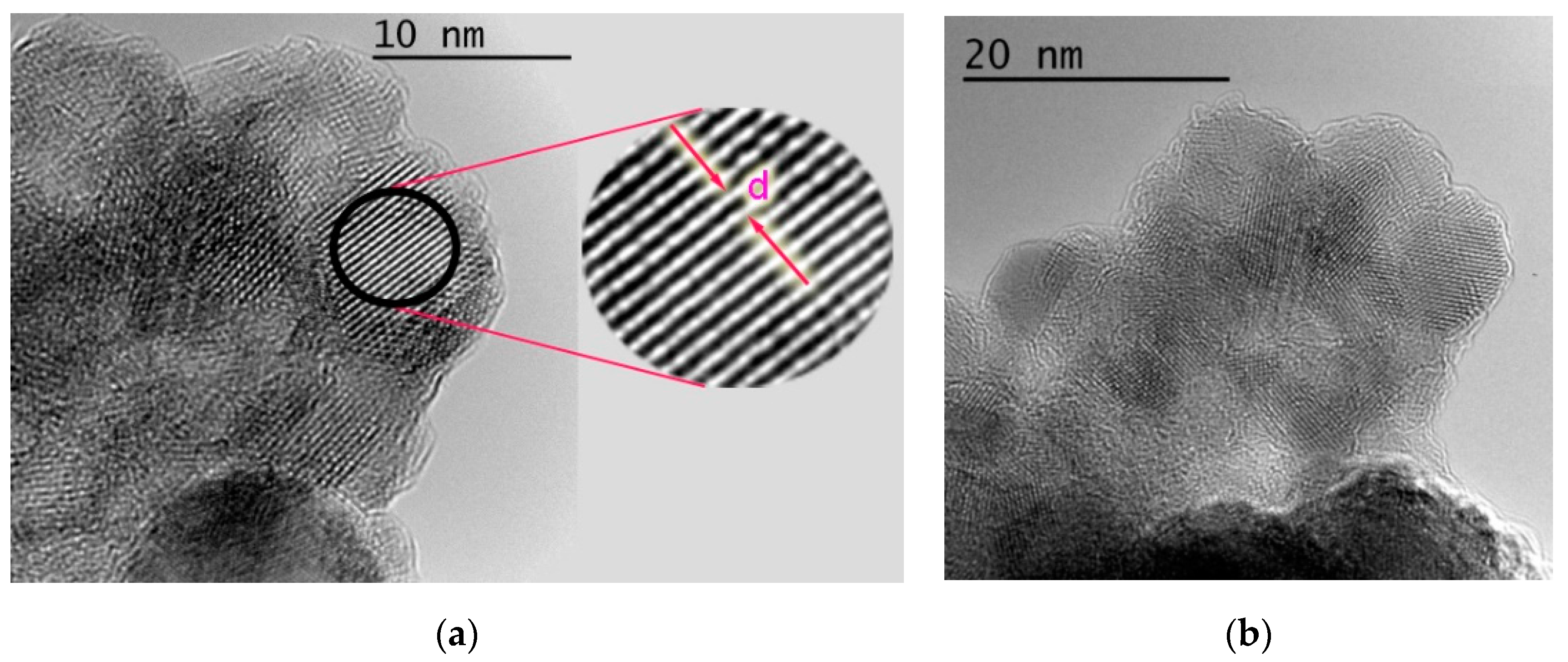
2.1.6. High Resolution Transmission Electron Microscopy (HRTEM)
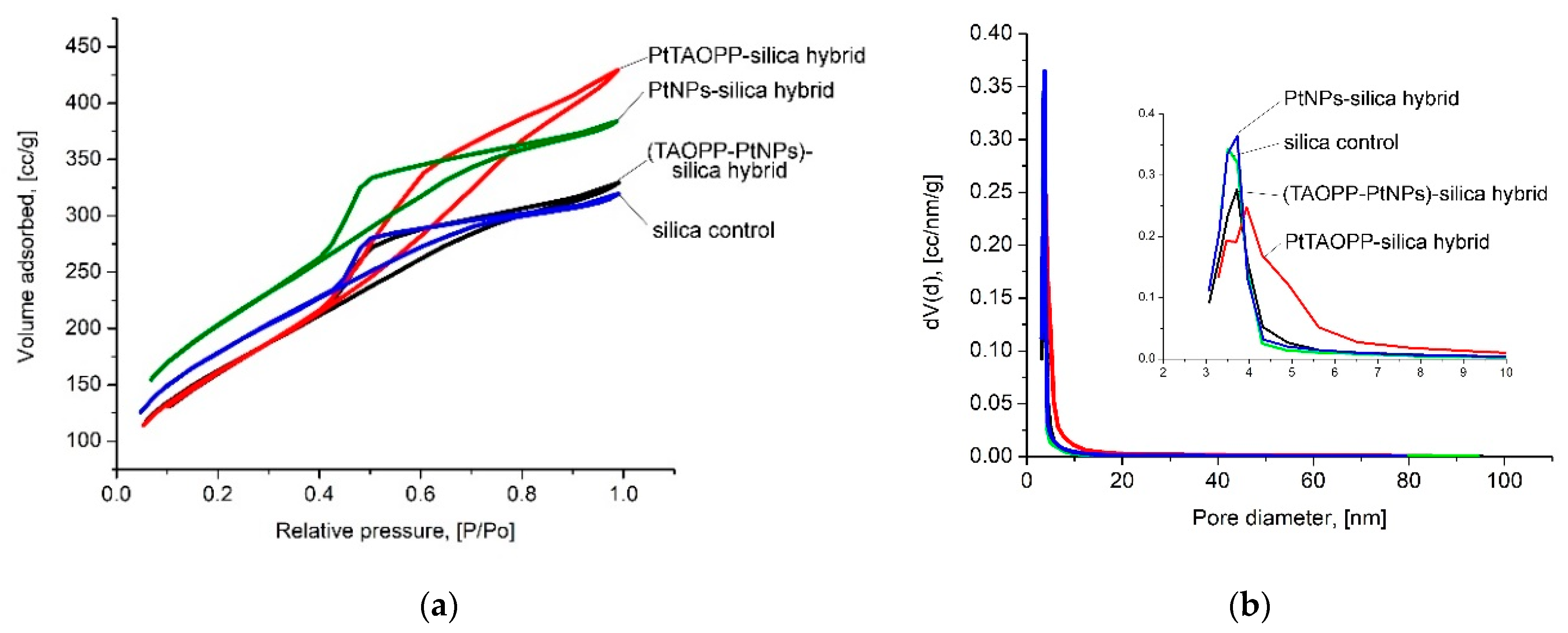
2.1.7. Morphological and Textural Analyses
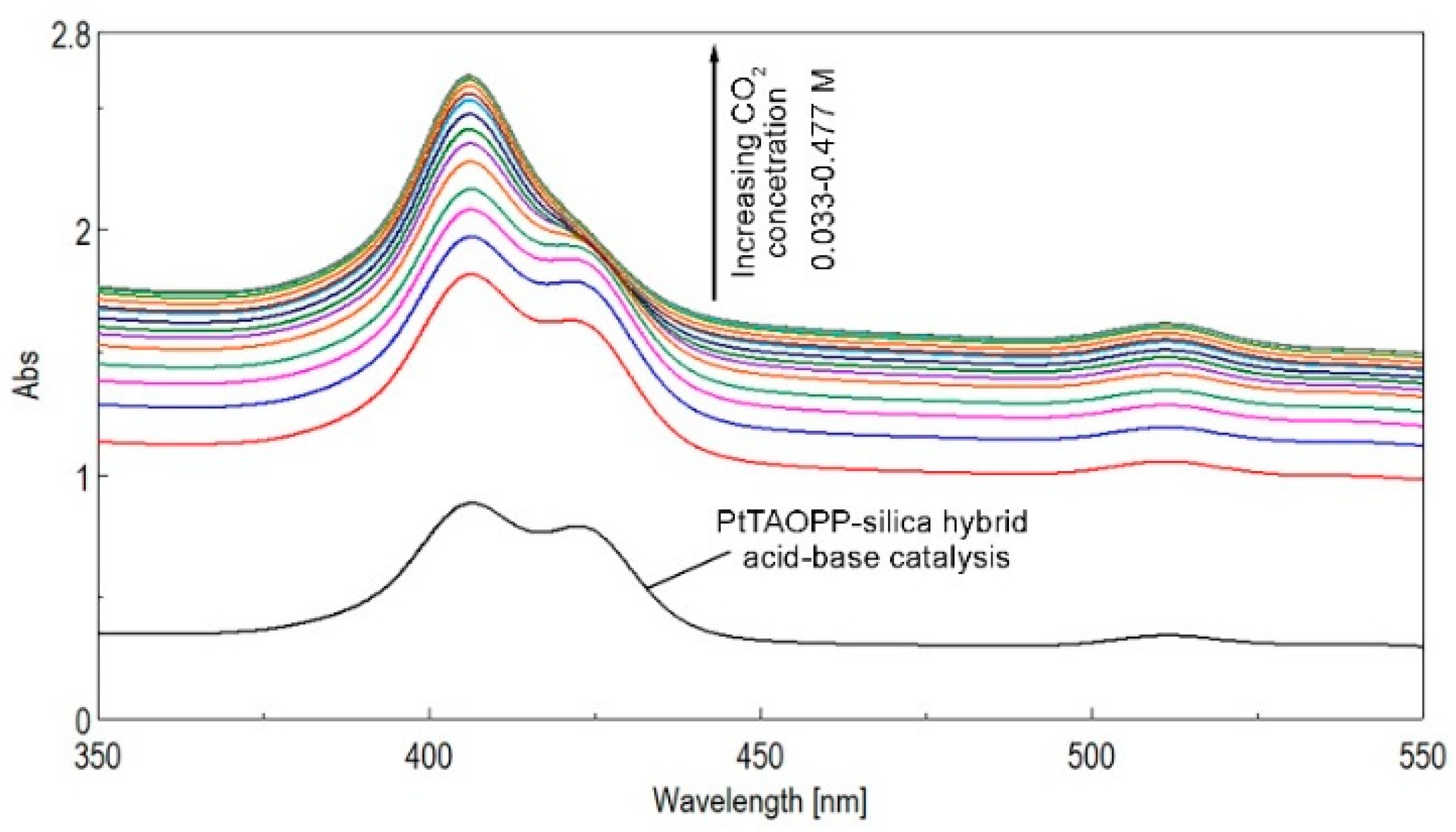
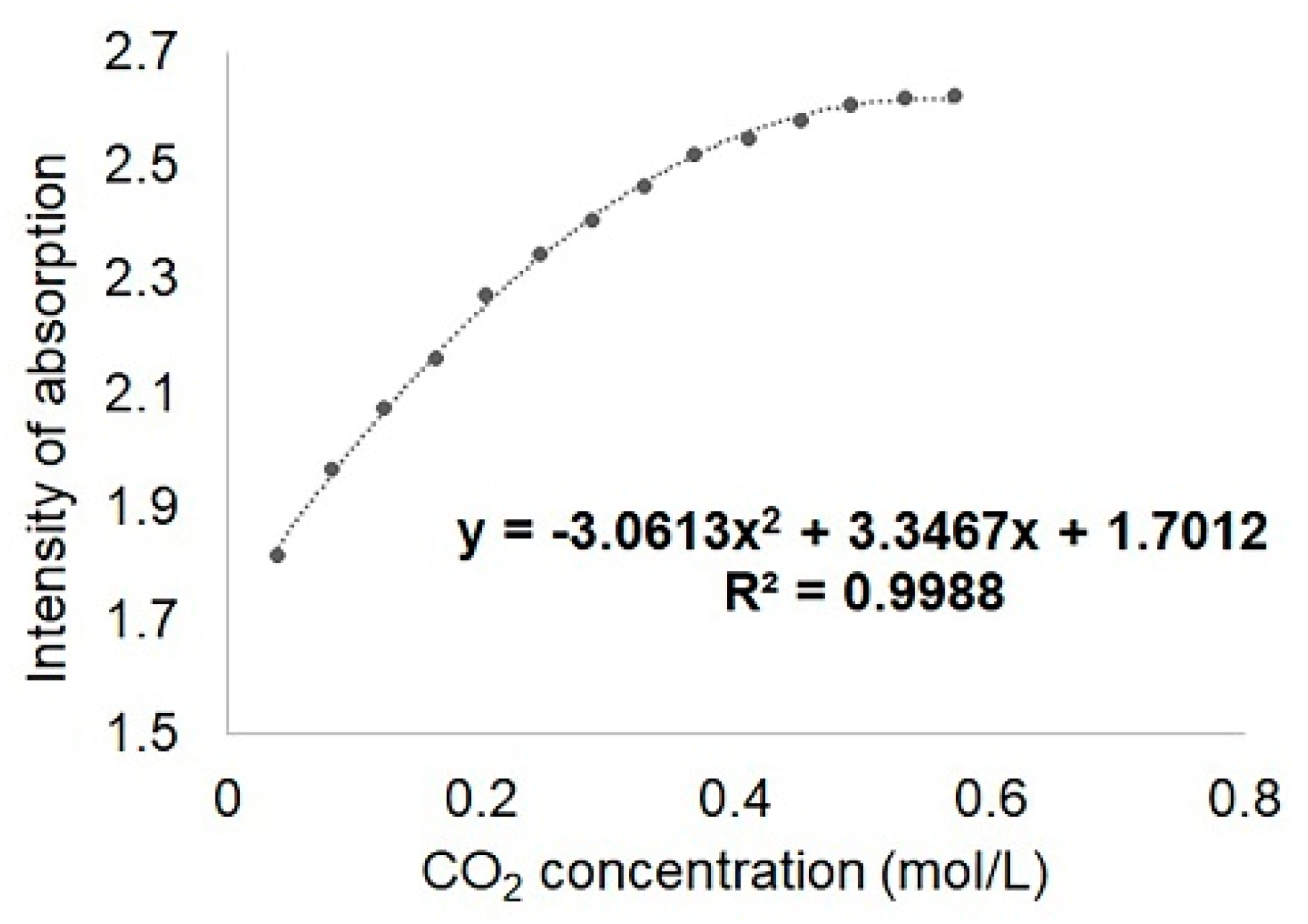
2.2. Silica Hybrid Materials Applied for CO2 Detection/Storage
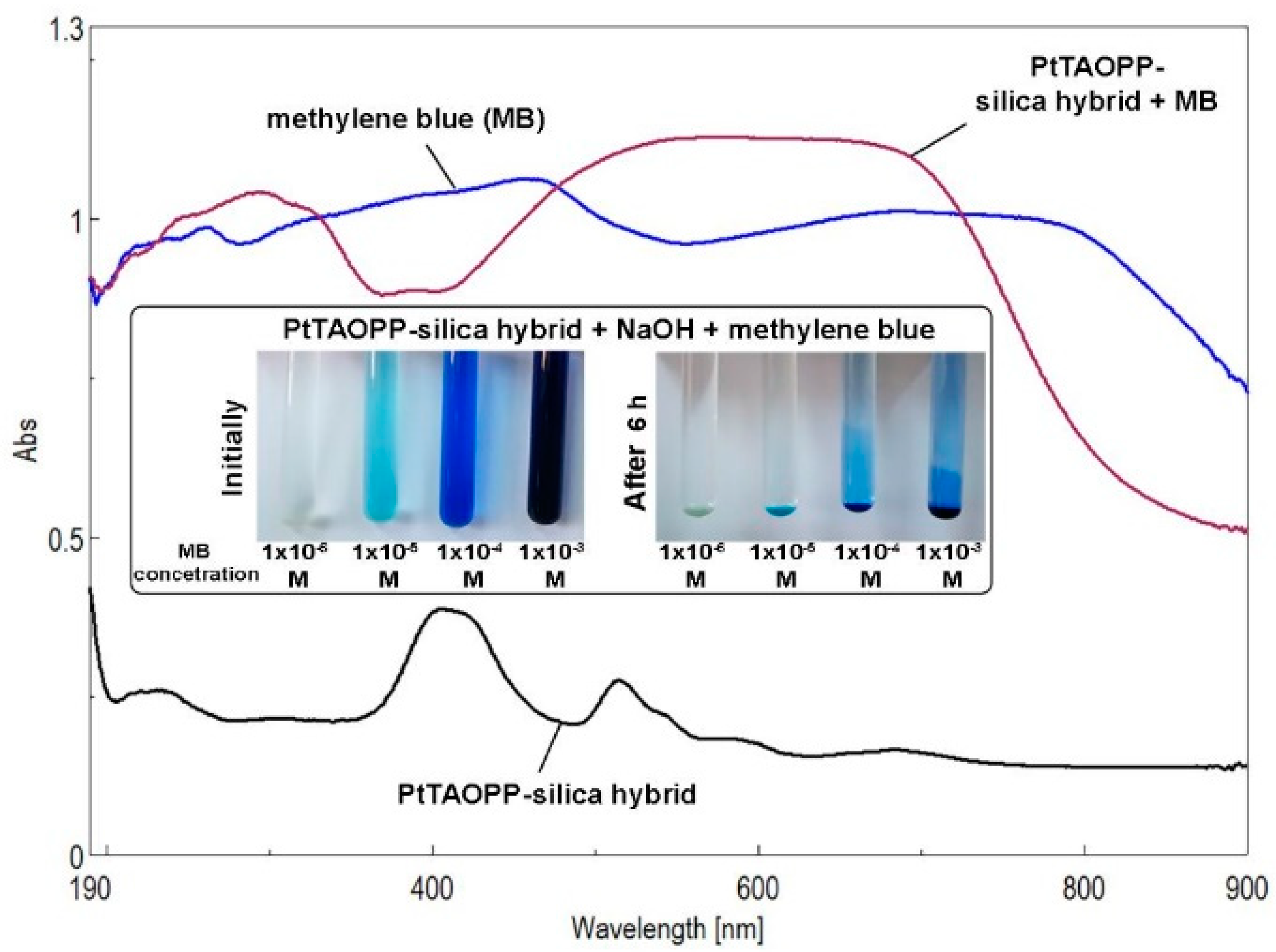
2.3. Testing of PtTAOPP-Silica Hybrid Material and of Silica Control for Methylene Blue Removal from Wastewaters
2.3.1. Method Applied for the Adsorption of Methylene Blue (MB) from Wastewaters
2.3.2. Results of the Kinetic Studies for the Adsorption Process of MB by Silica Control and by PtTAOPP-Silica Hybrid
3. Materials and Methods
3.1. Reagents
3.2. Apparatus
3.3. In Situ Two Steps Acid/Base Catalyzed Sol-Gel Method for Obtaining Silica Hybrids
- For the obtaining of the PtTAOPP-silica hybrid, 10 mL of a solution of 2.1 × 10−6 M PtTAOPP in THF (molar ratio: TEOS:PtTAOPP = 25,000:1) was added slowly under stirring.
- For the obtaining of the PtNPs-silica hybrid, 5 mL of 4.2 × 10−6 M Pt colloidal solution was added to respect the same molar ratio regarding TEOS (TEOS:PtNPs = 25,000:1).
- For the obtaining of the (TAOPP-PtNPs)-silica hybrid, a mixture composed from 10 mL of a solution of 2.1 × 10−6 M PtTAOPP in THF and 5 mL of 4.2 × 10−6 M Pt colloidal solution was added, maintaining the same molar ratio: TEOS:PtNPs:TAOPP = 25,000:1:1.
- The control silica sample was identically synthesized, without the addition of any porphyrin derivatives or PtNPs. A transparent gel was the resulting product.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Figueira, F.; Cavaleiro, J.A.S.; Tomé, J.P.C. Silica nanoparticles functionalized with porphyrins and analogs for biomedical studies. J. Porphyr. Phthalocyanines 2011, 15, 517–533. [Google Scholar] [CrossRef]
- Osica, I.; Imamura, G.; Shiba, K.; Ji, Q.; Shrestha, L.K.; Hill, J.P.; Kurzydlowski, K.J.; Yoshikawa, G.; Ariga, K. Highly Networked Capsular Silica–Porphyrin Hybrid Nanostructures as Efficient Materials for Acetone Vapor Sensing. ACS Appl. Mater. Interfaces 2017, 9, 9945–9954. [Google Scholar] [CrossRef] [PubMed]
- Sebarchievici, I.; Taranu, B.-O.; Rus, S.F.; Fagadar-Cosma, E. Electrochemical behaviour and analytical applications of a manganese porphyrin—Silica hybrid film prepared by pulsed laser deposition. J. Electroanal. Chem. 2020, 865, 114127. [Google Scholar] [CrossRef]
- Radi, S.; Abiad, C.E.; Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2017. [Google Scholar] [CrossRef]
- Neves, C.M.B.; Rebelo, S.L.H.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Simões, M.M.Q. Second-Generation Manganese(III) Porphyrins Bearing 3,5-Dichloropyridyl Units: Innovative Homogeneous and Heterogeneous Catalysts for the Epoxidation of Alkenes. Catalysts 2019, 9, 967. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Xu, Y.; Wang, N.; Li, J. Preparation, characterization and catalytic oxidation properties of silica composites immobilized with cationic metalloporphyrins. J. Mater. Sci. 2018, 53, 14241–14249. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Halma, M.; Machado, G.S.; Ricci, G.P.; Ucoski, G.M.; Ciuffi, K.J.; Nakagaki, S. Preparation of catalysts based on iron(III) porphyrins heterogenized on silica obtained by the Sol-Gel process for hydroxylation and epoxidation reactions. J. Braz. Chem. Soc. 2010, 21, 1329–1340. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G.; Palade, A.; Lascu, A.; Creanga, I.; Birdeanu, M.; Cristescu, R.; Cernica, I. A Sensitive A3B Porphyrin Nanomaterial for CO2 Detection. Molecules 2014, 19, 21239–21252. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Li, B.; Li, Y.; Shi, Y.; Shi, T. Synthesis, characterization, and oxygen sensing properties of functionalized mesoporous silica SBA-15 and MCM-41 with a Pt(II)–porphyrin complex. Sens. Actuators B Chem. 2014, 190, 93–100. [Google Scholar] [CrossRef]
- Lascu, A.; Palade, A.; Fagadar-Cosma, G.; Creanga, I.; Ianasi, C.; Sebarchievici, I.; Birdeanu, M.; Fagadar-Cosma, E. Mesoporous manganese–porphyrin–silica hybrid nanomaterial sensitive to H2O2 fluorescent detection. Mater. Res. Bull. 2016, 74, 325–332. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Ye, K.; Zhang, P.; Wang, Y. Oxygen sensing materials based on mesoporous silica MCM-41 and Pt(II)–porphyrin complexes. J. Mater. Chem. 2005, 15, 3181–3186. [Google Scholar] [CrossRef]
- Hu, M.; Kang, W.; Zhong, Z.; Cheng, B.; Xing, W. Porphyrin-Functionalized Hierarchical Porous Silica Nanofiber Membrane for Rapid HCl Gas Detection. Ind. Eng. Chem. Res. 2018. [Google Scholar] [CrossRef]
- Tao, S.; Li, G.; Zhu, H. Metalloporphyrins as sensing elements for the rapid detection of trace TNT vapor. J. Mater. Chem. 2006, 16, 4521. [Google Scholar] [CrossRef]
- Johnson, B.J.; Anderson, N.E.; Charles, P.T.; Malanoski, A.P.; Melde, B.J.; Nasir, M.; Deschamps, J.R. Porphyrin-Embedded Silicate Materials for Detection of Hydrocarbon Solvents. Sensors 2011, 11, 886–904. [Google Scholar] [CrossRef]
- Jayakumar, S.; Li, H.; Tao, L.; Li, C.; Liu, L.; Chen, J.; Yang, Q. Cationic Zn-Porphyrin Immobilized in Mesoporous Silicas as Bifunctional Catalyst for CO2 Cycloaddition Reaction under Cocatalyst Free Conditions. ACS Sustain. Chem. Eng. 2018, 6, 9237–9245. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Sun, C.; Drage, T.C.; Snape, C.E. Capturing CO2 from ambient air using a polyethyleneimine–silica adsorbent in fluidized beds. Chem. Eng. Sci. 2014, 116, 306–316. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 adsorption on branched polyethyleneimine-impregnated mesoporous silica SBA-15. Appl. Surf. Sci. 2010, 256, 5323–5328. [Google Scholar] [CrossRef]
- Palade, A.; Creanga, I.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid silica-porphyrin nanomaterials sensitive to gas detection. New Front. Chem. 2014, 23, 53–60. [Google Scholar]
- Wiśniewska, M.; Wawrzkiewicz, M.; Polska-Adach, E.; Fijałkowska, G.; Goncharuk, O. Nanosized silica–titanium oxide as a potential adsorbent for C.I. Acid Yellow 219 dye removal from textile baths and wastewaters. Appl. Nanosci. 2018, 8, 867–876. [Google Scholar] [CrossRef]
- Farrukh, A.; Akram, A.; Ghaffar, A.; Tuncel, E.; Oluz, Z.; Duran, H.; Rehman, H.; Yameen, B. Surface-functionalized silica gel adsorbents for efficient remediation of cationic dyes. Pure Appl. Chem. 2014, 86, 1177–1188. [Google Scholar] [CrossRef]
- Farias, R.S.; Buarque, H.L.; Cruz, M.R.; Cardoso, L.M.F.; Gondim, T.A.; Paulo, V.R. Adsorption of congo red dye from aqueous solution onto amino-functionalized silica gel. Eng. Sanit. Ambient. 2018, 23, 1053–1060. [Google Scholar] [CrossRef]
- Messina, P.V.; Schulz, P.C. Adsorption of reactive dyes on titania–silica mesoporous materials. J. Colloid Interface Sci. 2006, 299, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Chang, K.-P.; Ou, H.-D.; Chiang, Y.-C.; Wang, C.-F. Adsorption of cationic dyes onto mesoporous silica. Microporous Mesoporous Mater. 2011, 141, 102–109. [Google Scholar] [CrossRef]
- Gholamrezapor, E.; Eslami, A. Sensitization of magnetic TiO2 with copper(II) tetrahydroxylphenyl porphyrin for photodegradation of methylene blue by visible LED light. J. Mater. Sci. Mater. Electron. 2019. [Google Scholar] [CrossRef]
- Wan, J.M.; Wu, Z.Z.; Wang, H.G.; Zheng, X.M. Visible-Light Photocatalytic Degradation of Methylene Blue with Porphyrin-Sensitized TiO2. Adv. Mater. Res. 2012, 441, 544–548. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.-Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, J.; Zhang, D.; Qi, D.; Jiang, J. Porphyrin-Alkaline Earth MOFs with the Highest Adsorption Capacity for Methylene Blue. Chem. Eur. J. 2016, 22, 6345–6352. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Dudás, Z.; Birdeanu, M.; Almásy, L. Hybrid organic – silica nanomaterials based on novel A3B mixed substituted porphyrin. Mater. Chem. Phys. 2014, 148, 143–152. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Vlascici, D.; Fagadar-Cosma, G.; Vasile, M.; Bazylak, G. Novel nanomaterials based on 5,10,15,20-tetrakis(3,4-dimethoxyphenyl)-21H,23H-porphyrin entrapped in silica matrices. Mater. Res. Bull. 2009, 44, 2186–2193. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Dascalu, D.; Fagadar-Cosma, G.; Gavrila, R. FT-IR, fluorescence and electronic spectra for monitoring the aggregation process of tetra-pyridylporphyrine entrapped in silica matrices. Optoelectron. Adv. Mater. 2008, 2, 437–444. [Google Scholar]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid silica-porphyrin materials with tailored pore sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Ramakrishna, B.; Kumar, A.R.; Veeraiah, N.; Venkatesan, R. Enhanced stokes shift of S2→S0 emission and structural investigations of Sn(IV) Porphyrins doped hybrid borate glasses. J. Alloys Compd. 2012, 513, 318–323. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, B. A cationic porphyrin-based self-assembled film for mercury ion detection. Tetrahedron Lett. 2008, 49, 2311–2315. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Tripathi, V.S.; Lakshminarayana, G.; Nogami, M. Optical oxygen sensors based on platinum porphyrin dyes encapsulated in ORMOSILS. Sens. Actuators B Chem. 2010, 147, 741–747. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, K.; Yue, B.; He, H.; Dickinson, C. HRTEM of negative replicas of mesoporous silica. Stud. Surf. Sci. Catal. 2004, 154, 924–930. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Vásquez-Agustín, M.A.; Morales-Sánchez, A.; Aceves-Mijares, M. Emission Mechanisms of Si Nanocrystals and Defects in SiO2 Materials. J. Nanomater. 2014, 1–17. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Hatch, C.D.; Tumminello, P.R.; Cassingham, M.A.; Greenaway, A.L.; Meredith, R.; Christie, M.J. Technical note: Frenkel, Halsey and Hill analysis of water on clay minerals: Toward closure between cloud condensation nuclei activity and water adsorption. Atmos. Chem. Phys. 2019, 19, 13581–13589. [Google Scholar] [CrossRef]
- Son, W.-J.; Choi, J.-S.; Ahn, W.-S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 2008, 113, 31–40. [Google Scholar] [CrossRef]
- Aboudi, J.; Vafaeezadeh, M. Efficient and reversible CO2 capture by amine functionalized-silica gel confined task-specific ionic liquid system. J. Adv. Res. 2015, 6, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Aziz, B.; Hedin, N. Carbon dioxide adsorption on mesoporous silica surfaces containing amine-like motifs. Appl. Energy 2010, 87, 2907–2913. [Google Scholar] [CrossRef]
- Oliveira, R.J.; de Conto, J.F.; Oliveira, M.R.; Egues, S.M.S.; Borges, G.R.; Dariva, C.; Franceschi, E. CO2/CH4 adsorption at high-pressure using silica-APTES aerogel as adsorbent and near infrared as a monitoring technique. J. CO2 Util. 2019, 32, 232–240. [Google Scholar] [CrossRef]
- Mak, C.A.; Pericas, M.A.; Fagadar-Cosma, E. Functionalization of A3B-type porphyrin with Fe3O4 MNPs. Supramolecular assemblies, gas sensor and catalytic applications. Catal. Today 2018, 306, 268–275. [Google Scholar] [CrossRef]
- Borchert, N.B.; Kerry, J.P.; Papkovsky, D.B. A CO2 sensor based on Pt-porphyrin dye and FRET scheme for food packaging applications. Sens. Actuators B Chem. 2013, 176, 157–165. [Google Scholar] [CrossRef]
- Silva, J.D.A.E.; Domingos, V.F.; Marto, D.; Costa, L.D.; Marcos, M.; Silva, M.R.; Gil, J.M.; Sobral, A.J.F.N. Reversible sequestering of CO2 on a multiporous crystalline framework of 2-quinolyl-porphyrin. Tetrahedron Lett. 2013, 54, 2449–2451. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.-Z.; Li, X.; Xu, Y.-W.; Zhang, L.; Su, C.-Y. Catalytic Space Engineering of Porphyrin Metal-Organic Frameworks for Combined CO2 Capture and Conversion at a Low Concentration. ChemSusChem 2018, 11, 2340–2347. [Google Scholar] [CrossRef]
- Lai, N.; Zhu, Q.; Qiao, D.; Chen, K.; Tang, L.; Wang, D.; He, W.; Chen, Y.; Yu, T. CO2 Capture with Absorbents of Tertiary Amine Functionalized Nano–SiO2. Front. Chem. 2020, 8, 146. [Google Scholar] [CrossRef]
- Ngakou, S.C.; Anagho, G.S.; Ngomo, H.M. Non-linear Regression Analysis for the Adsorption Kinetics and Equilibrium Isotherm of Phenacetin onto Activated Carbons. Curr. J. Appl. Sci. Technol. 2019, 36, 1–18. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes Pigm. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Paşka, O.M.; Păcurariu, C.; Muntean, S.G. Kinetic and thermodynamic studies on methylene blue biosorption using corn-husk. RSC Adv. 2014, 4, 62621–62630. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A. A comparative study of dye removal using fly ash treated by different methods. Chemosphere 2005, 60, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Plesu, N.; Lascu, A.; Anghel, D.; Cazacu, M.; Ianasi, C.; Fagadar-Cosma, G.; Fratilescu, I.; Epuran, C. Novel Platinum-Porphyrin as Sensing Compound for Efficient Fluorescent and Electrochemical Detection of H2O2. Chemosensors 2020, 8, 29. [Google Scholar] [CrossRef]
- Yamashita, K.; Katsumata, N.; Tomita, S.; Fuwa, M.; Fujimaki, K.; Yoda, T.; Hirano, D.; Sugiura, K. Facile and practical synthesis of platinum(II) porphyrins under mild conditions. Chem. Lett. 2015, 44, 492–494. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, G.; Plesu, N.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric sensors for iodide and bromide based on Pt(II)-porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef]
- Wu, G.-W.; He, S.-B.; Peng, H.-P.; Deng, H.-H.; Liu, A.-L.; Lin, X.-H.; Xia, X.; Chen, W. Citrate-Capped Platinum Nanoparticle as a Smart Probe for Ultrasensitive Mercury Sensing. Anal. Chem. 2014, 86, 10955–10960. [Google Scholar] [CrossRef]
- Pomonis, P.J.; Tsaousi, E.T. Frenkel−Halsey−Hill Equation, Dimensionality of Adsorption, and Pore Anisotropy. Langmuir 2009, 25, 9986–9994. [Google Scholar] [CrossRef]
- Benstoem, F.; Becker, G.; Firk, J.; Kaless, M.; Wuest, D.; Pinnekamp, J.; Kruse, A. Elimination of micropollutants by activated carbon produced from fibers taken from wastewater screenings using hydrothermal carbonization. J. Environ. Manag. 2018, 211, 278–286. [Google Scholar] [CrossRef]
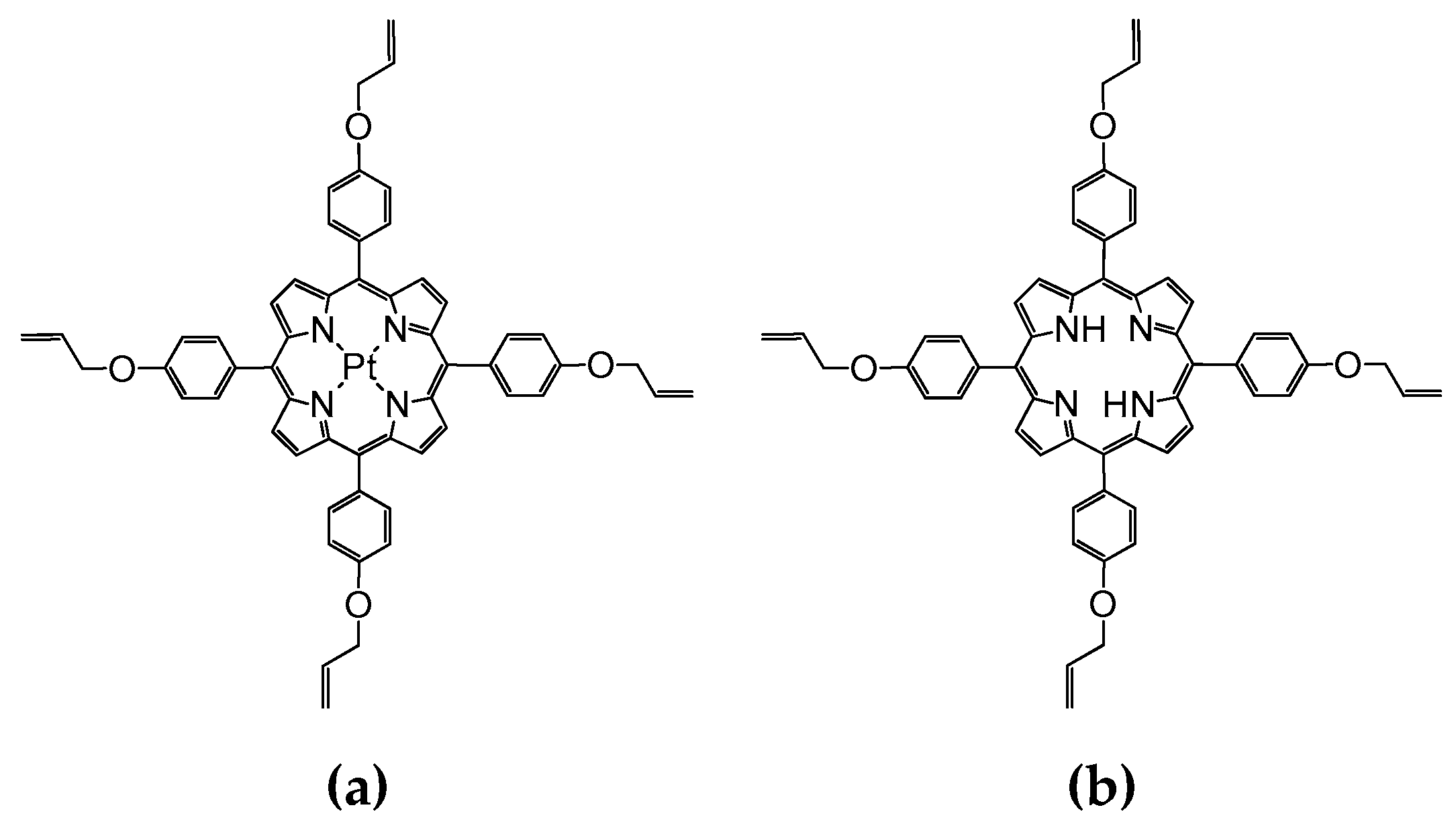
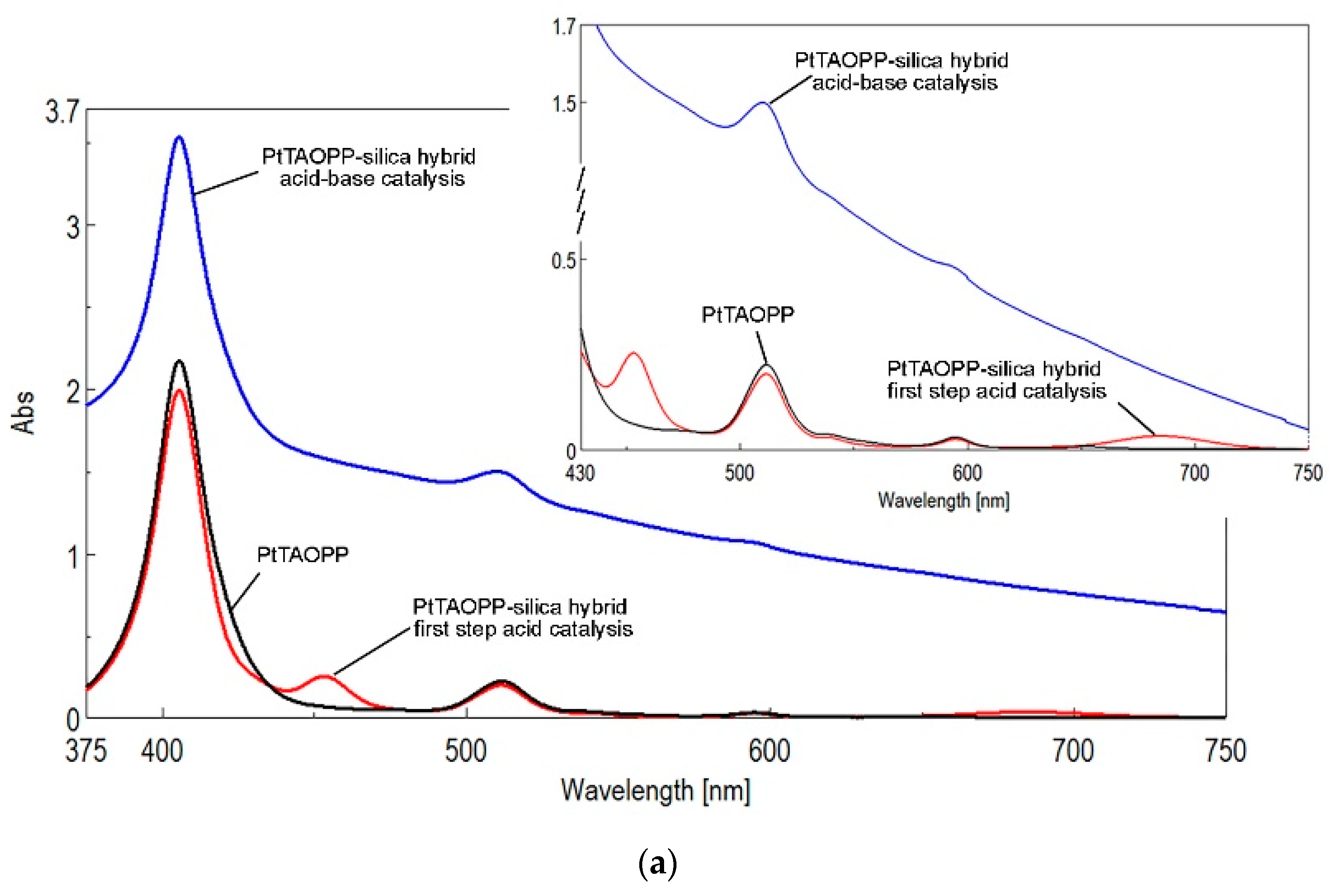
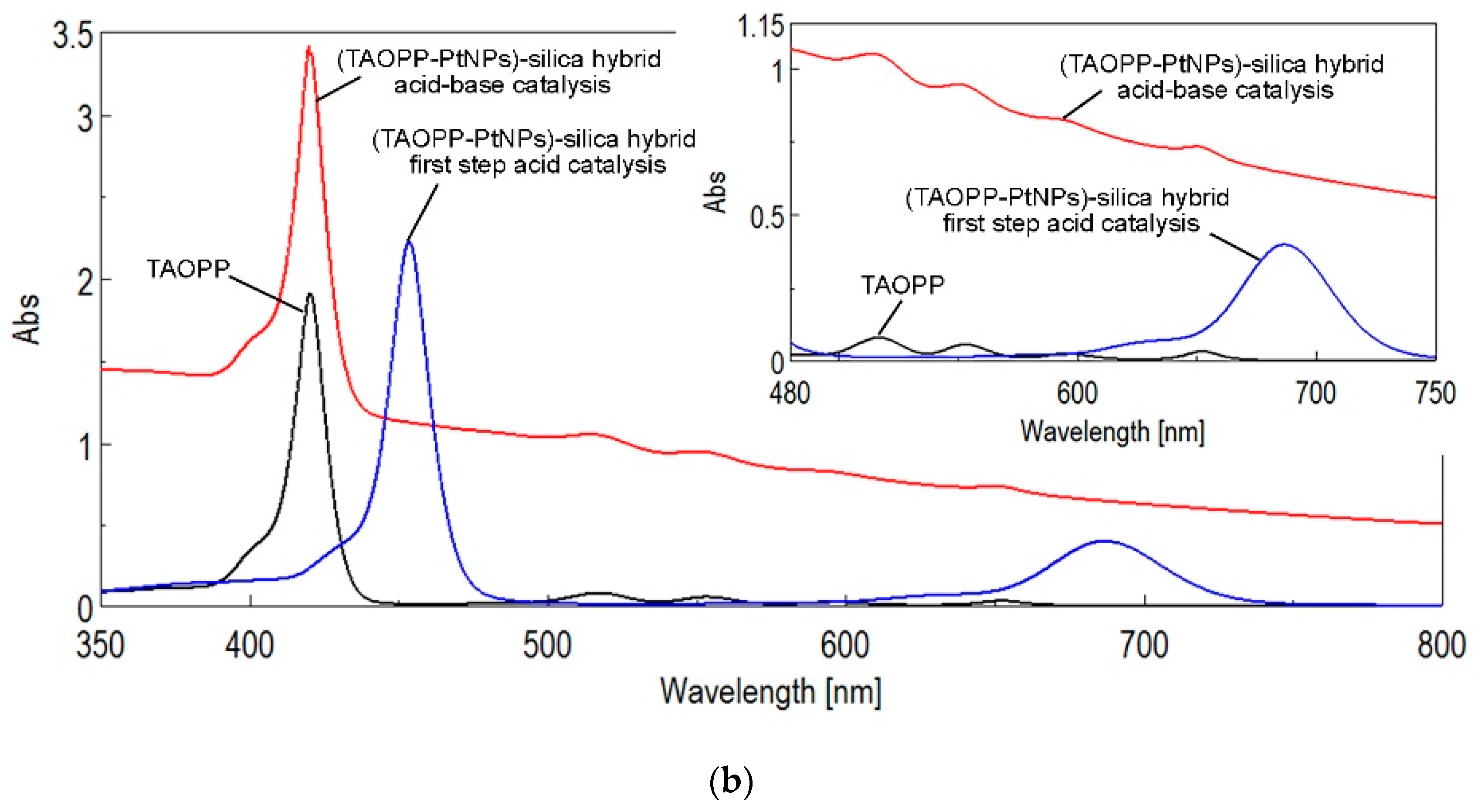

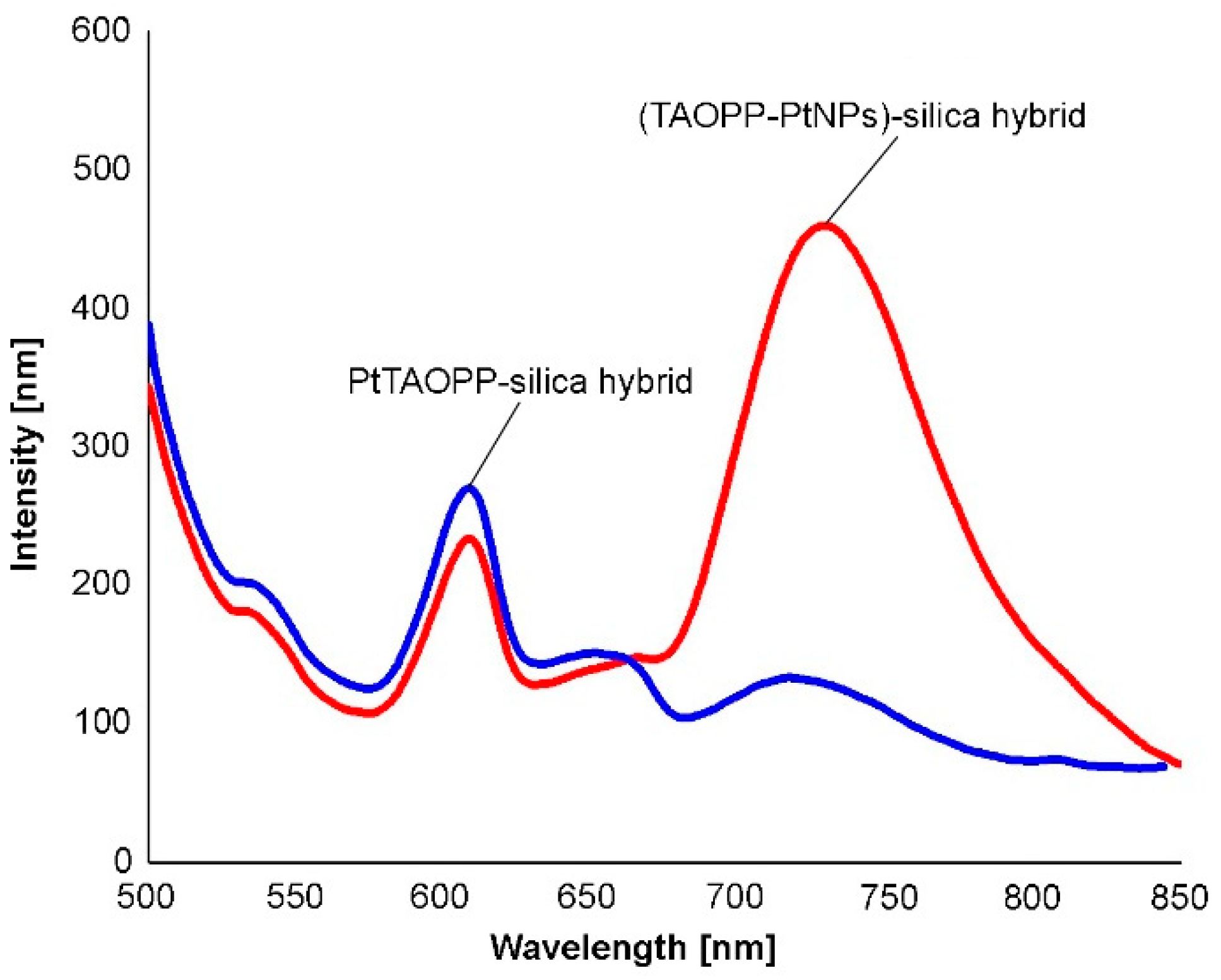
| Silica Base | Immobilized Component | Hybrid Abbreviation |
|---|---|---|
| Tetraethyl orthosilicate (TEOS) | Pt(II)-5,10,15,20-tetra-(4-allyloxy-phenyl)-porphyrin (PtTAOPP) | PtTAOPP-silica hybrid |
| TEOS | 5,10,15,20-tetra-(4-allyloxy-phenyl)-porphyrin (TAOPP) and Pt nanoparticles (PtNPs) | (TAOPP-PtNPs)-silica hybrid |
| TEOS | Platinum nanoparticles (PtNPs) | PtNPs-silica hybrid |
| TEOS | - | Silica control |
| Name | Specific Surface Area [m2/g] | Pore Size Diameter [nm] | Total Pore Volume [cc/g] | Frenkel-Halsey-Hill (FHH) Fractal Dimension |
|---|---|---|---|---|
| PtTAOPP-silica hybrid | 593 ± 15 | 4.12 | 0.66 | 2.20 |
| PtNPs-silica hybrid | 739 ± 19 | 3.73 | 0.59 | 2.45 |
| (TAOPP-PtNPs)-silica hybrid | 592 ± 15 | 3.71 | 0.51 | 2.41 |
| Control silica | 650 ± 17 | 3.51 | 0.49 | 2.50 |
| Silica- or Porphyrin- Based Materials | Pore Size Diameter (nm) | CO2 Adsorption (mmol/g) | Range of Detection (mM) | Ref. |
|---|---|---|---|---|
| Mesoporous silica obtained from TEOS and a triblock copolymer poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) impregnated with 50 wt % polyethyleneimine | 5.3 | 3.068 | - | [41] |
| Circulating Fluidized Bed method with mesoporous silica having BET surface area of 250 m2/g and pore volumes of 1.7 c3/g impregnated with 40 wt % polyethyleneimine | 20 | 1.65 | - | [16] |
| TEOS-based mesoporous silica impregnated with 70 wt % polyethyleneimine | - | 2.045 | - | [17] |
| Silica gel from sodium silicate activated with 2-[2-(3-Trimethoxysilylpropylamino)-ethylamino] ethylamine | 6 | 4.773 | - | [42] |
| Mesoporous silica gel from sodium silicate modified with propilamine | 67 | 2.3 | - | [43] |
| Silica aerogels starting from TEOS, modified with (3-aminopropyl) triethoxysilane | 1.27 | 2.87 ± 0.05 | - | [44] |
| TEOS-based silica matrix impregnated with 5,10,15,20-tetratolyl-21H,23H-porphyrin | 1.37–2.60 | 3.089 | 1.21–4.5 | [18] |
| TEOS based silica material impregnated with 5-(4-carboxyphenyl)-5,10,15-tris(4-phenoxyphenyl)-porphyrin and Fe3O4 magnetic nanoparticles | - | - | 3 × 10−2–0.20 | [45] |
| 5-(4-pyridyl)-10,15,20-tris(3,4-dimethoxyphenyl)-porphyrin | 200 | - | 49–306 | [8] |
| Colorimetric sensor film based on phosphorescent Pt(II) meso-Tetra(pentafluorophenyl)porphine, poly(isobutyl methacrylate), α-naphtholphthalein and cetyltrimethylammonium hydroxide | - | - | 1.51–30.3 (2 to 40% in ethanol and olive oil) | [46] |
| Porous crystalline framework based on 5, 10, 15, 20-tetra-(2-quinolyl)-21H, 23H-porphine (meso-tetra-2-quinolyl-porphyrin | 0.46 | 0.18 | - | [47] |
| Metal-organic framework built from Rh- tetrakis(4-carboxyphenyl)porphyrin and ZrCl4 | 1.85 | 44.2 | - | [48] |
| PtTAOPP-silica hybrid material | 4.12 | 25 ± 0.05 | 40–570 | This work |
| PtNPs-silica hybrid material | 3.73 | 11 ± 0.06 | - | This work |
| (TAOPP-PtNPs)-silica hybrid material | 3.71 | 16.3 ± 0.06 | - | This work |
| TEOS-based silica control | 3.51 | 21.6 ± 0.07 | - | This work |
| Equations | Parameters | Silica Control | PtTAOPP-Silica Hybrid | ||||
|---|---|---|---|---|---|---|---|
| 0.83 g/L | 1.66 g/L | 3.33 g/L | 0.83 g/L | 1.66 g/L | 3.33 g/L | ||
| Pseudo-first order | qe exp. [mg/g] | 6.921 | 3.611 | 4.803 | 7.261 | 3.656 | 4.803 |
| qe calc. [mg/g] | - | - | - | 15.26 ± 1.0 | 9.603 ± 0.9 | 14.429 ± 1.2 | |
| k1[min−1] | - | - | - | 0.0838 | 0.048 | 0.022 | |
| Pseudo-second order | qe calc. [mg/g] | 7.001 ± 1.1 | 3.868 ± 0.3 | 5.04 ± 0.5 | 7.31 ± 0.5 | 3.709 ± 0.1 | 4.853 ± 0.2 |
| k2[g × mg−1 × min−1] | 0.18 | 0.08 | 0.03 | 0.19 | 0.157 | 0.025 | |
| h [mg × g−1 × min−1] | 8.622 | 1.043 | 0.69 | 10.016 | 2.099 | 0.576 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. https://doi.org/10.3390/ijms21124262
Anghel D, Lascu A, Epuran C, Fratilescu I, Ianasi C, Birdeanu M, Fagadar-Cosma E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. International Journal of Molecular Sciences. 2020; 21(12):4262. https://doi.org/10.3390/ijms21124262
Chicago/Turabian StyleAnghel, Diana, Anca Lascu, Camelia Epuran, Ion Fratilescu, Catalin Ianasi, Mihaela Birdeanu, and Eugenia Fagadar-Cosma. 2020. "Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal" International Journal of Molecular Sciences 21, no. 12: 4262. https://doi.org/10.3390/ijms21124262
APA StyleAnghel, D., Lascu, A., Epuran, C., Fratilescu, I., Ianasi, C., Birdeanu, M., & Fagadar-Cosma, E. (2020). Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. International Journal of Molecular Sciences, 21(12), 4262. https://doi.org/10.3390/ijms21124262






