A Flowable Placental Formulation Prevents Bleomycin-Induced Dermal Fibrosis in Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Procurement and Ethics Statement
2.2. Tissue Processing and Cryopreservation
2.3. Assessment of FPF Cell Viability
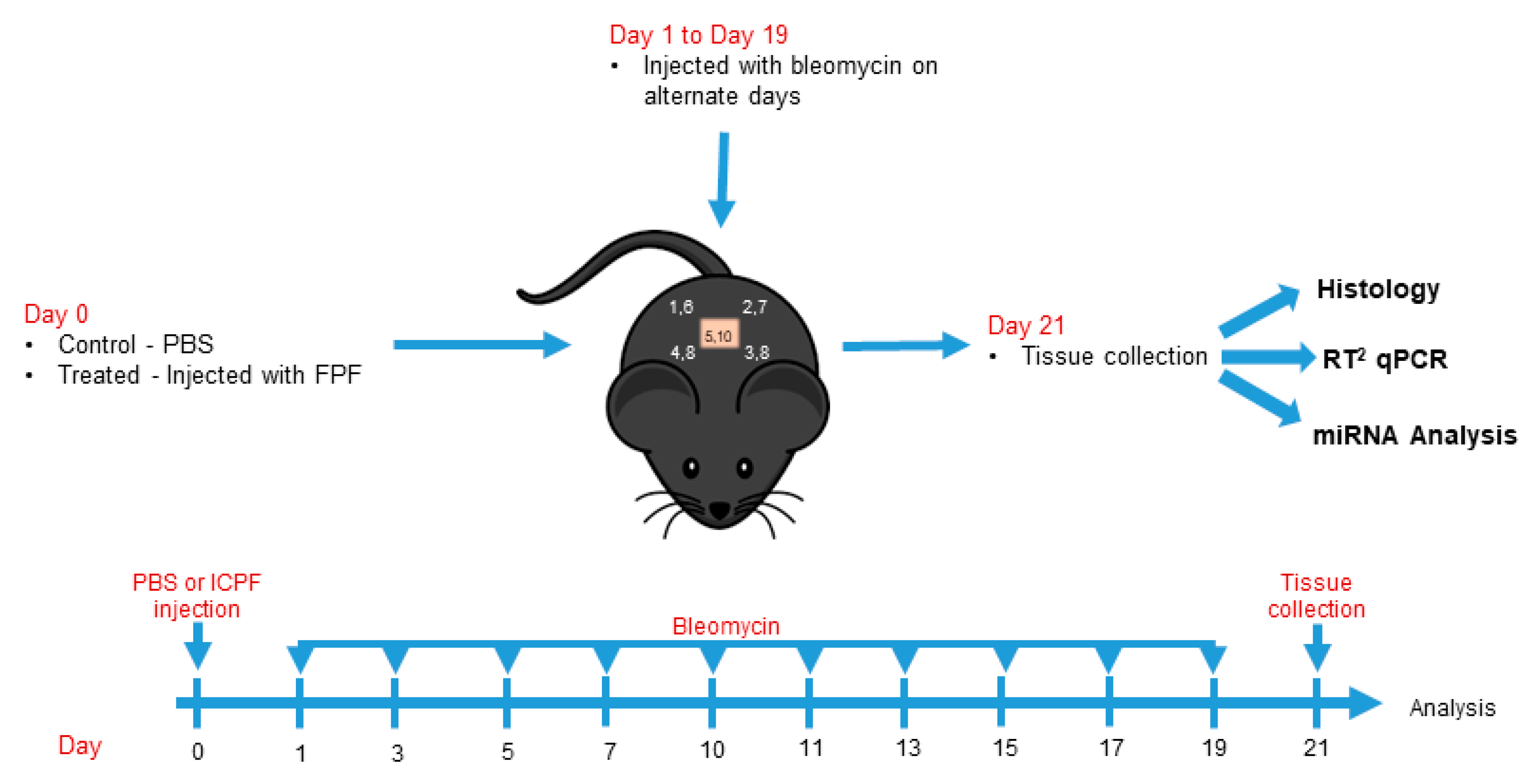
2.4. In Vivo Testing
2.4.1. Animals
2.4.2. Dermal fibrosis model
2.5. Histological and Immunohistochemical Assessment
2.6. MicroRNA Array and Quantitative RT-PCR Analysis
2.6.1. Sample Preparation
2.6.2. Library Preparation and Sequencing (QIAseq miR Tissue)
2.6.3. RT2 qPCR
2.7. Statistical Ana Lysis
3. Results
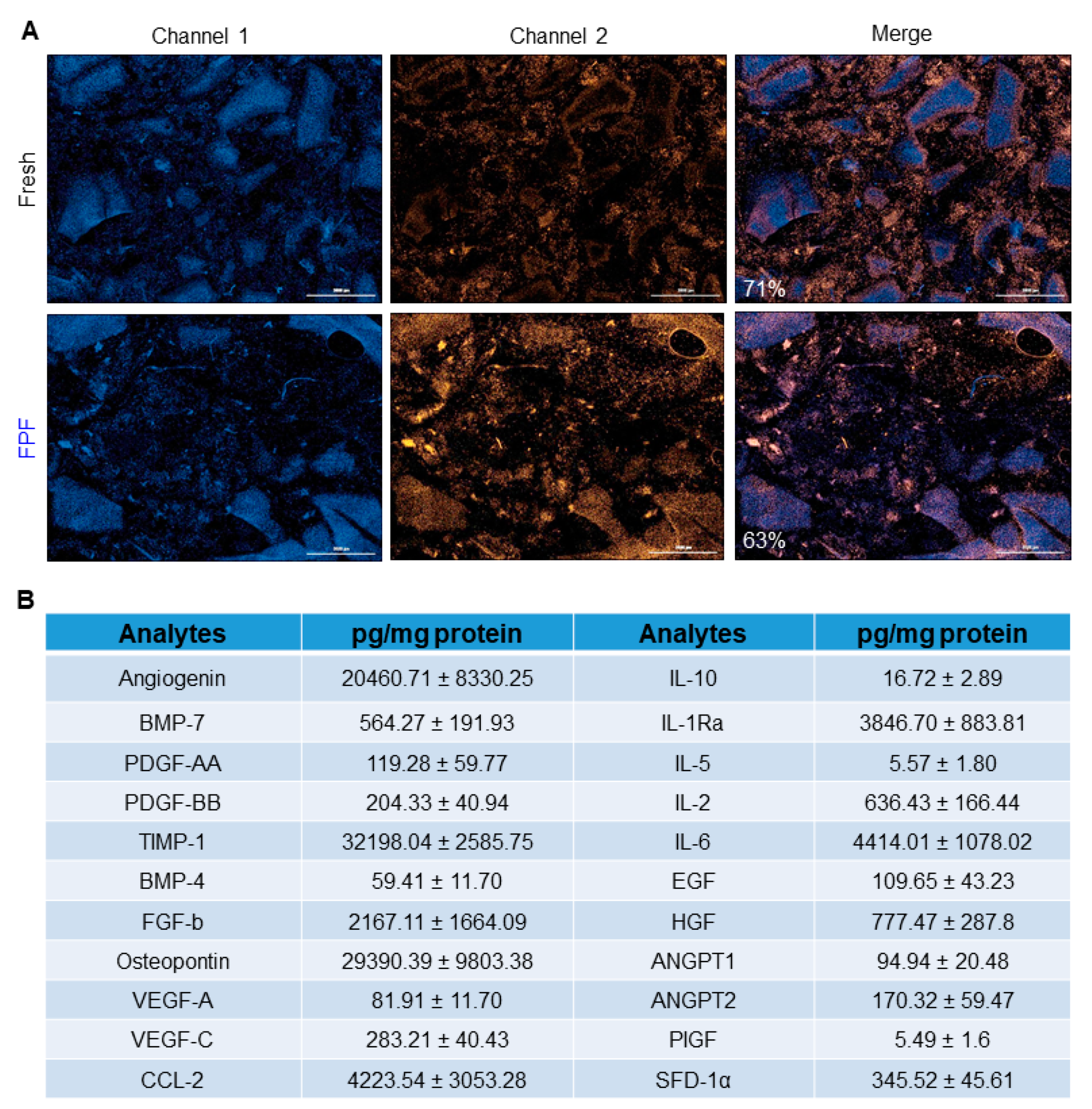
3.1. Characterization of FPF
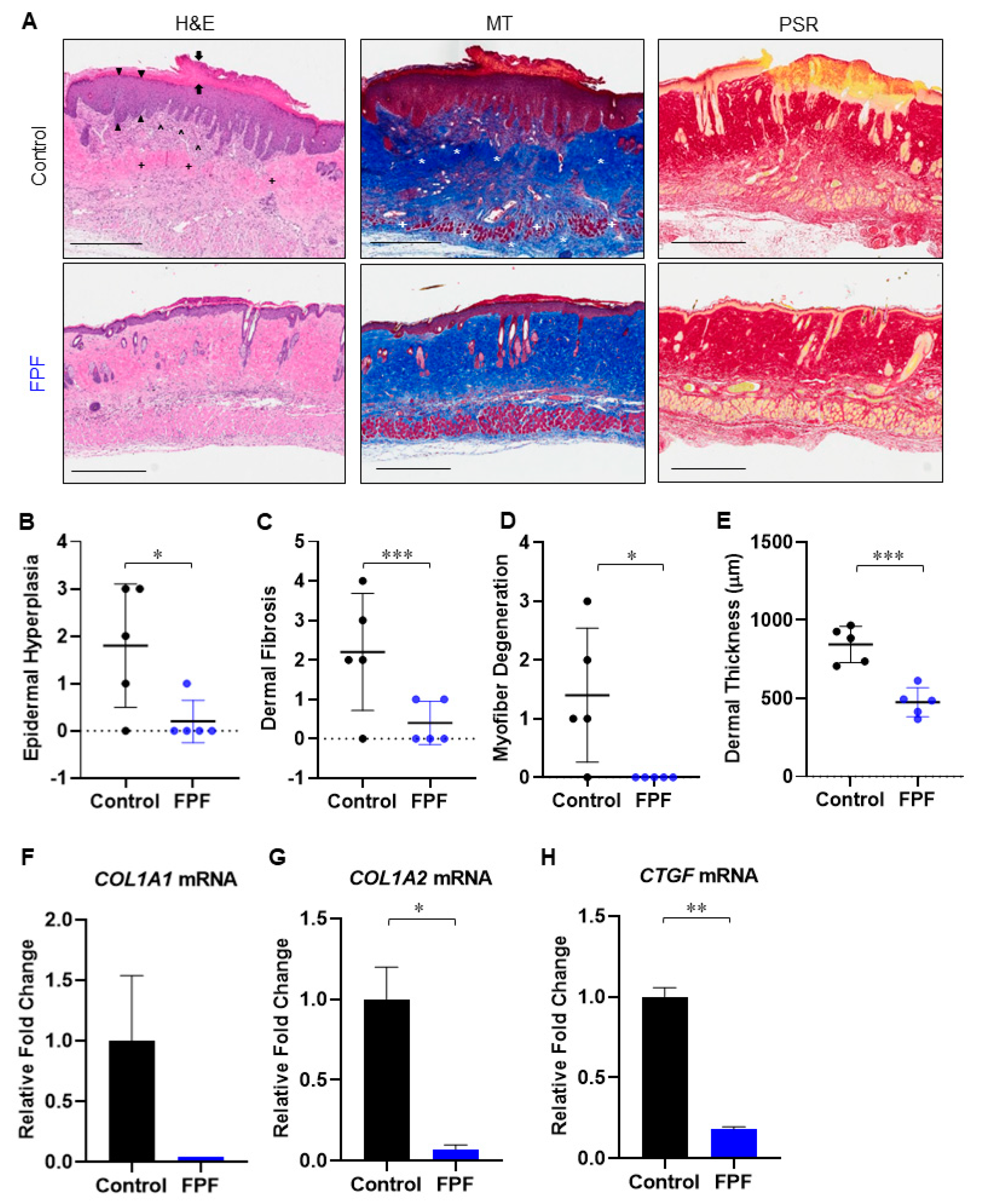
3.2. FPF Reduces Bleomycin-Induced Dermal Fibrosis in Aged Mouse Skin
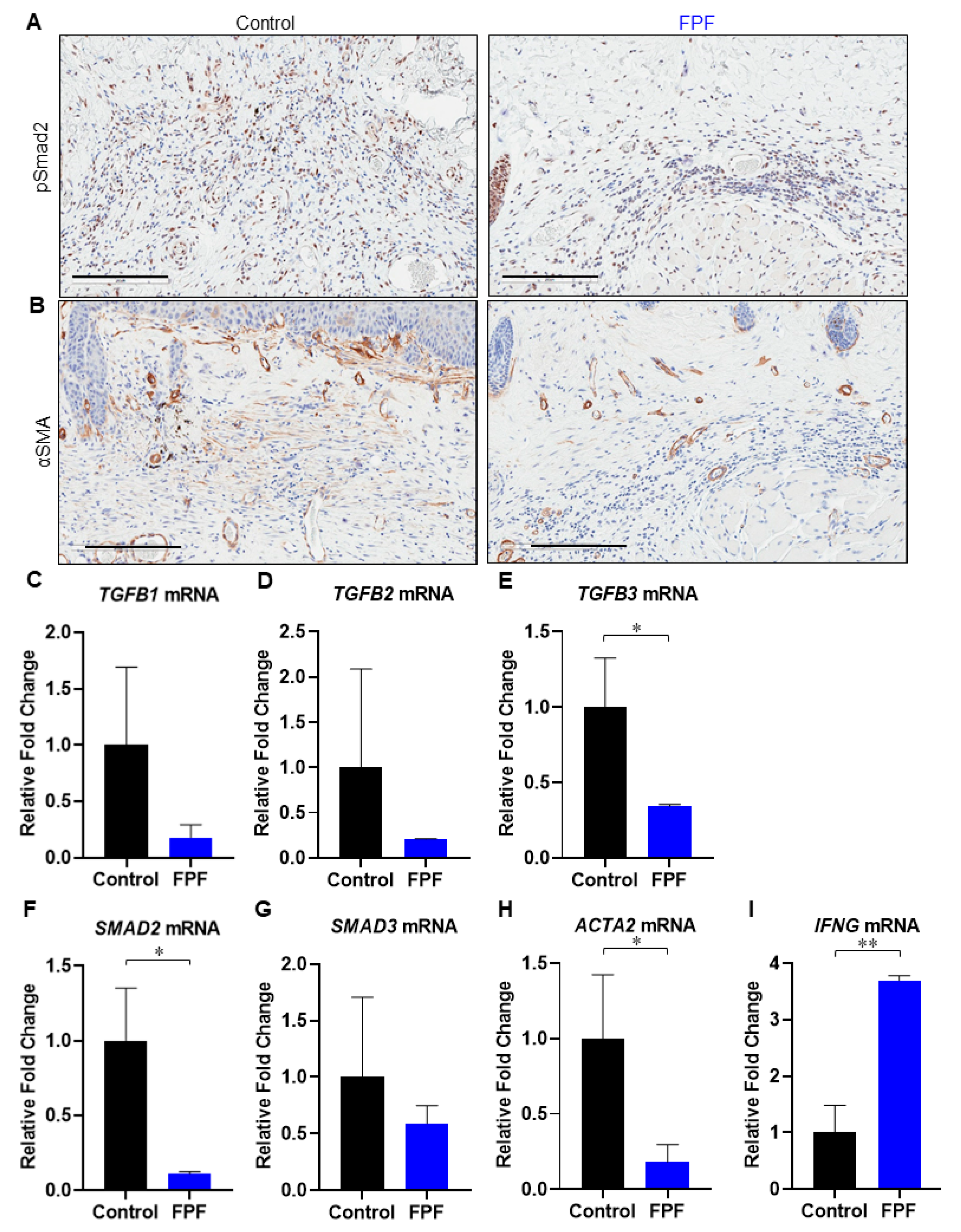
3.3. FPF Treatment Downregulates the TGF-β Pathway
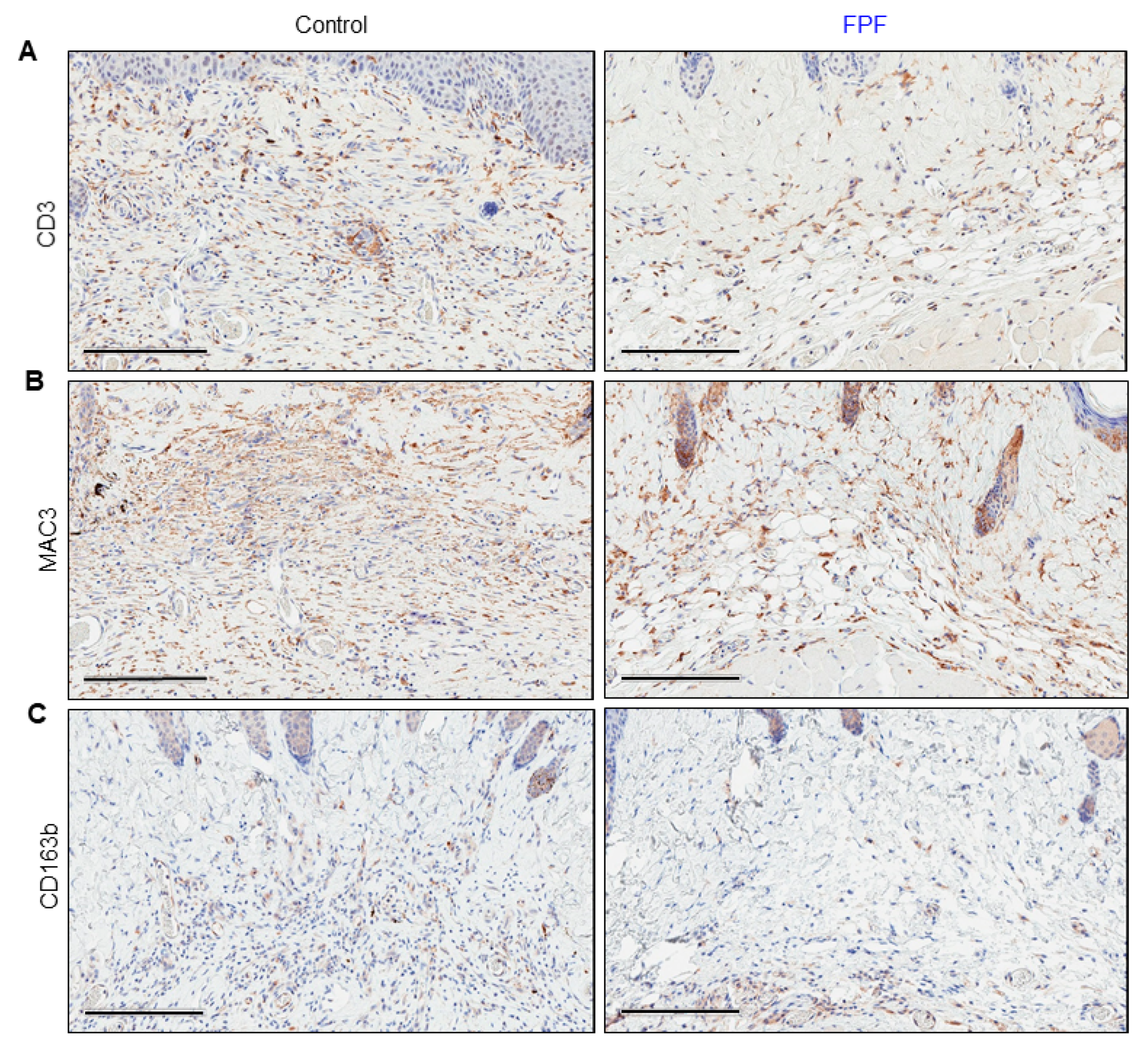
3.4. Infiltration of Inflammatory Cells Is Diminished in FPF-Treated Mice
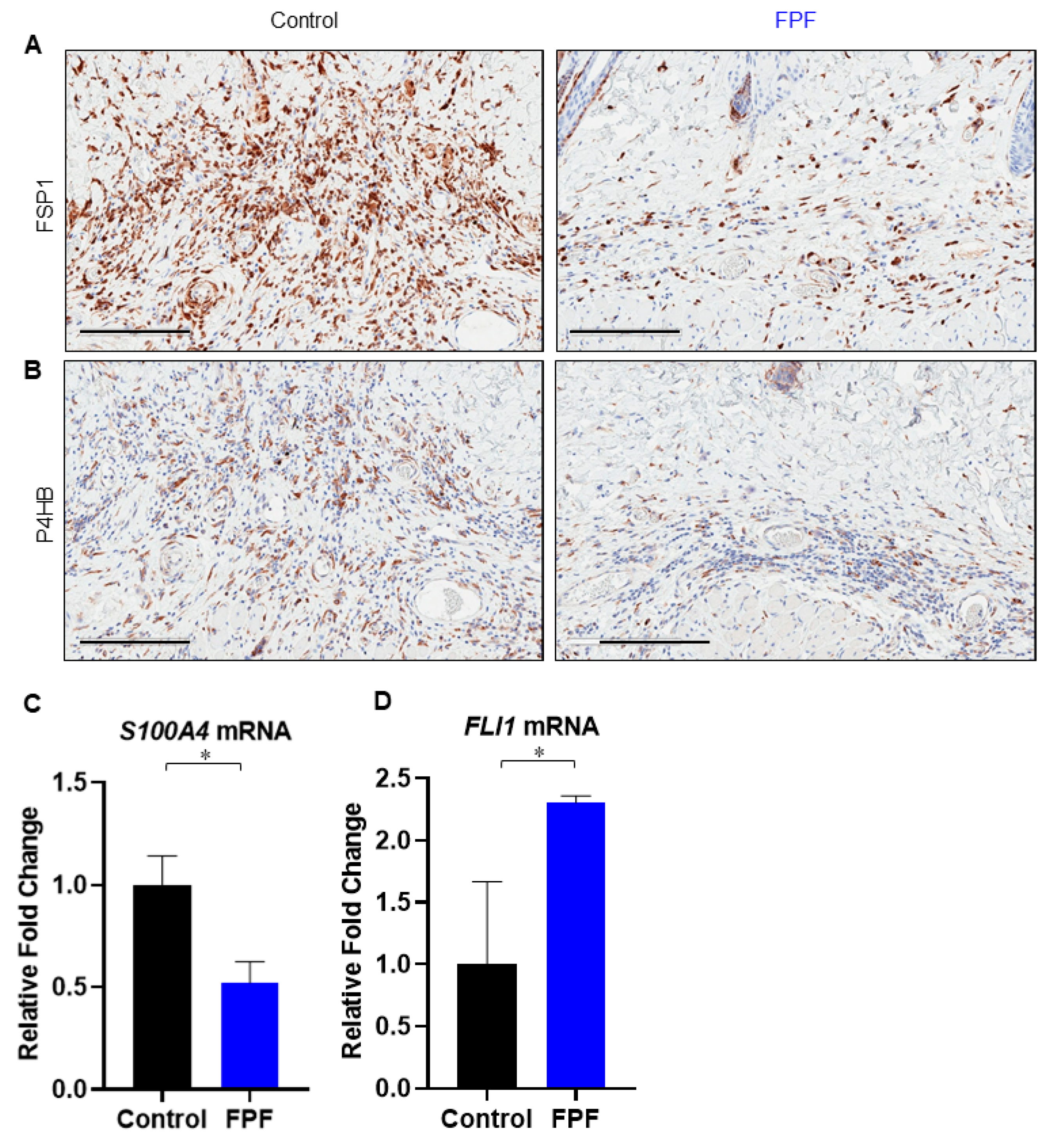
3.5. FPF Decreases Fibroblast and Endothelial to Mesenchymal Transition in the Dermis
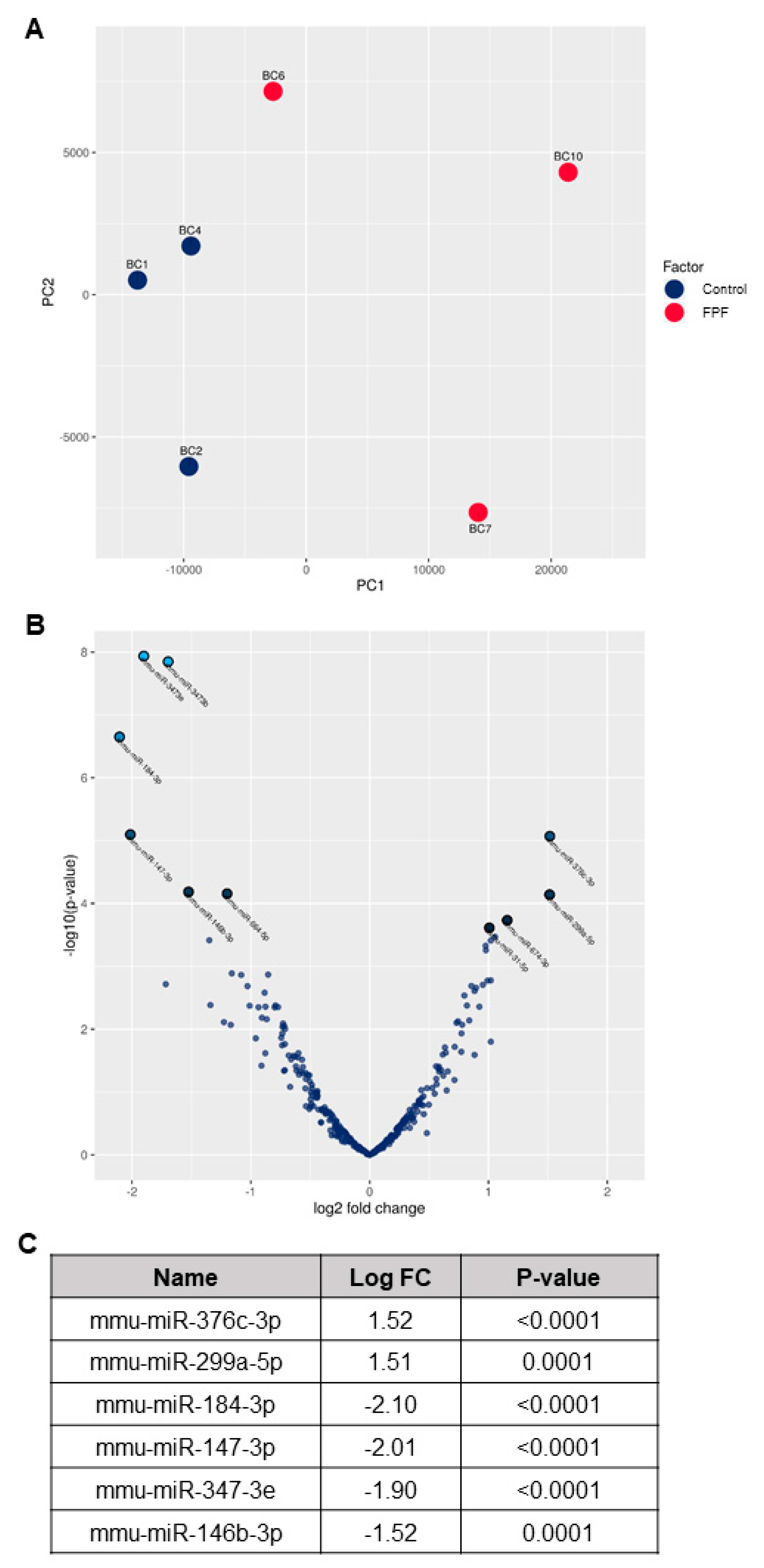
3.6. FPF Treatment Improves Transcriptional Dysregulation in Fibrosis Disease Pathogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Brantley, J.N.; Verla, T.D. Use of Placental Membranes for the Treatment of Chronic Diabetic Foot Ulcers. Adv. Wound Care 2015, 4, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Caruso, M.; De Munari, S.; Vertua, E.; De, D.; Manuelpillai, U.; Parolini, O. Human Amniotic Membrane-Derived Mesenchymal and Epithelial Cells Exert Different Effects on Monocyte-Derived Dendritic Cell Differentiation and Function. Cell Transplant. 2015, 24, 1733–1752. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, N.; Randolph, M.; Redmond, R. The clinical applications of human amnion in plastic surgery. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Koo, J.B.; Kim, H.Y.; Seo, J.W.; Lee, E.J.; Kim, W.R.; Cho, J.Y.; Hahm, K.B.; Hong, S.P.; Kim, D.H.; et al. Umbilical cord/placenta-derived mesenchymal stem cells inhibit fibrogenic activation in human intestinal myofibroblasts via inhibition of myocardin-related transcription factor A. Stem Cell Res. Ther. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-C.; Fu, T.-W.; Chen, Y.-M.A.; Ko, T.-L.; Chen, T.-H.; Shih, Y.-H.; Hung, S.-C.; Fu, Y. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transplant. 2009, 15, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Usunier, B.; Benderitter, M.; Tamarat, R.; Chapel, A. Management of Fibrosis: The Mesenchymal Stromal Cells Breakthrough. Stem Cells Int. 2014, 2014, 340257. [Google Scholar] [CrossRef]
- Bank, R.; Mia, M.M. Paracrine Factors of Human Amniotic Fluid-Derived Mesenchymal Stem Cells Show Strong Anti-Fibrotic Properties by Inhibiting Myofibroblast Differentiation and Collagen Synthesis. J. Stem Cell Res. Ther. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; Li, D.-Q.; Ma, X. Suppression of transforming growth factor-beta isoforms, TGF-b receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J. Cell Physiol. 1999, 179, 325–335. [Google Scholar] [CrossRef]
- Madhusoodanan, J. Matrix mimics shape cell studies. Nature 2019, 566, 563–565. [Google Scholar] [CrossRef]
- Rubio, G.A.; Elliot, S.; Wikramanayake, T.C.; Xia, X.; Pereira-Simon, S.; Thaller, S.R.; Glinos, G.D.; Jozic, I.; Hirt, P.; Pastar, I.; et al. Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J. Cell. Physiol. 2018, 233, 5503–5512. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Parolini, O.; Huppertz, B.; Lang, I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr. Stem Cell Res. Ther. 2013, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Cargnoni, A.; Ressel, L.; Rossi, D.; Poli, A.; Arienti, D.; Lombardi, G.; Parolini, O. Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosis. Cytotherapy 2011, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Coksaygan, T.; Hoffman, T.; Moorman, M.; Lerch, A.; Kuang, J.-Q.; Sathyamoorthy, M.; Danilkovitch, A. Viable cryopreserved umbilical tissue (vCUT) reduces post-operative adhesions in a rabbit abdominal adhesion model. Bioact. Mater. 2019, 4, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; Difede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER). Chest 2017, 151, 971–981. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Zeng, C.; Wang, W.E. Functionally Improved Mesenchymal Stem Cells to Better Treat Myocardial Infarction. Stem Cells Int. 2018, 2018, 7045245. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.-H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Haldar, D.; Henderson, N.C.; Hirschfield, G.M.; Newsome, P.N. Mesenchymal stromal cells and liver fibrosis: A complicated relationship. FASEB J. 2016, 30, 3905–3928. [Google Scholar] [CrossRef]
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501. [Google Scholar] [CrossRef]
- Lu, Q.; El-hashash, A.H.K. Cell-based therapy for idiopathic pulmonary fibrosis. Stem Cell Investig 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Jacob, V.; Johnson, N.; Lerch, M.A.; Jones, M.B.; Dhall, S.; Sathyamoorthy, M.; Danilkovitch, A. Structural and functional equivalency between lyopreserved and cryopreserved chorions with viable cells. Adv. Wound Care 2020, 1–14. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Uveges, T.E.; Jacobstein, D.A.; Danilkovitch, A. Retention of Endogenous Viable Cells Enhances the Anti-Inflammatory Activity of Cryopreserved Amnion. Adv. Wound Care 2015, 4, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human Umbilical Cord Mesenchymal Stem Cells Reduce Fibrosis of Bleomycin-Induced Lung Injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Takeda, K.; Okamoto, A.; Matsuo, S.-I.; Isobe, K.-I. Induction of Autoimmunity in a Bleomycin-Induced Murine Model of Experimental Systemic Sclerosis: An Important Role for CD4+ T Cells. J. Investig. Dermatol. 2009, 129, 1688–1695. [Google Scholar] [CrossRef]
- Ruzehaji, N.; Avouac, J.; Elhai, M.; Fréchet, M.; Frantz, C.; Ruiz, B.; Distler, J.H.W.; Allanore, Y. Combined effect of genetic background and gender in a mouse model of bleomycin-induced skin fibrosis. Arthritis Res. 2015, 17, 145. [Google Scholar] [CrossRef]
- Johnson, A.; Gyurdieva, A.; Dhall, S.; Danilkovitch, A.; Duan-Arnold, Y. Understanding the Impact of Preservation Methods on the Integrity and Functionality of Placental Allografts. Ann. Plast. Surg. 2017, 79, 203–213. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Jacobstein, D.A.; Danilkovitch, A. Soluble Factors Released by Endogenous Viable Cells Enhance the Antioxidant and Chemoattractive Activities of Cryopreserved Amniotic Membrane. Adv. Wound Care 2015, 4, 329–338. [Google Scholar] [CrossRef]
- Akamatsu, T.; Arai, Y.; Kosugi, I.; Kawasaki, H.; Meguro, S.; Sakao, M.; Shibata, K.; Suda, T.; Chida, K.; Iwashita, T. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair 2013, 6, 15. [Google Scholar] [CrossRef]
- Granstein, R.D.; Flotte, T.J.; Amento, E.P. Interferons and Collagen Production. J. Investig. Dermatol. 1990, 95, S75–S80. [Google Scholar] [CrossRef]
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of metastasis-induced protein S100A4 in human non-tumor pathophysiologies. Cell Biosci. 2017, 7, 64. [Google Scholar] [CrossRef]
- Taniguchi, T.; Asano, Y.; Akamata, K.; Noda, S.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Trojanowska, M.; Sato, S. Fibrosis, vascular activation, and immune abnormalities resembling systemic sclerosis in bleomycin-treated Fli-1-haploinsufficient mice. Arthritis Rheumatol. 2015, 67, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Czuwara-Ladykowska, J.; Moussa, O.; Markiewicz, M.; Smith, E.; Silver, R.M.; Jablonska, S.; Blaszczyk, M.; Watson, D.K.; Trojanowska, M. Persistent Down-Regulation of Fli1, a Suppressor of Collagen Transcription, in Fibrotic Scleroderma Skin. Am. J. Pathol. 2003, 163, 571–581. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Kulp, G.A.; Kraft, R.; Finnerty, C.C.; Mlcak, R.; Lee, J.O.; Herndon, D.N. Intensive Insulin Therapy in Severely Burned Pediatric Patients. Am. J. Respir. Crit. Care Med. 2010, 182, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pandit, K.; Milosevic, J.; Kaminski, N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 2011, 157, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Cargnoni, A.; Magatti, M.; Pianta, S.; Parolini, O. The Long Path of Human Placenta, and Its Derivatives, in Regenerative Medicine. Front. Bioeng. Biotechnol. 2015, 3, 423. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Moodley, Y.P.; Borlongan, C.V.; Parolini, O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32, S320–S325. [Google Scholar] [CrossRef]
- Chase, D.M.; Gallicchio, V.S. The effect of mesenchymal stem cells and exosomes to treat idiopathic pulmonary fibrosis. J. Stem Cell Res. Ther. 2019, 5, 48–59. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Dhall, S.; Singal, A.; Sathyamoorthy, M.; Danilkovitch, A.; Kohn, J. Endogenous viable cells in lyopreserved amnion retain differentiation potential and anti-fibrotic activity in vitro. Acta Biomater. 2019, 94, 330–339. [Google Scholar] [CrossRef]
- Sant’anna, L.B.; Cargnoni, A.; Ressel, L.; Vanosi, G.; Parolini, O. Amniotic Membrane Application Reduces Liver Fibrosis in a Bile Duct Ligation Rat Model. Cell Transplant. 2011, 20, 441–453. [Google Scholar] [CrossRef]
- Eskandarlou, M.; Azimi, M.; Rabiee, S.; Rabiee, M.A.S. The Healing Effect of Amniotic Membrane in Burn Patients. World J. Plast. Surg. 2016, 5, 39–44. [Google Scholar]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, O.; Pastar, I.; Vukelic, S.; Mahoney, M.G.; Brennan, N.; Krzyzanowska, A.; Golinko, M.; Brem, H.; Tomic-Canic, M. Deregulation of keratinocyte differentiation and activation: A hallmark of venous ulcers. J. Cell. Mol. Med. 2008, 12, 2675–2690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiao, H.; Stewart, T.L.; Shankowsky, H.A.; Scott, P.G.; Tredget, E.E. Increased Severity of Bleomycin-Induced Skin Fibrosis in Mice with Leukocyte-Specific Protein 1 Deficiency. J. Investig. Dermatol. 2008, 128, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Stawski, L.; Han, R.; Bujor, A.M.; Trojanowska, M. Angiotensin II induces skin fibrosis: A novel mouse model of dermal fibrosis. Arthritis Res. Ther. 2012, 14, R194. [Google Scholar] [CrossRef] [PubMed]
- Sonnylal, S.; Shi-Wen, X.; Leoni, P.; Naff, K.; Van Pelt, C.S.; Nakamura, H.; Leask, A.; Abraham, D.; Bou-Gharios, G.; De Crombrugghe, B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010, 62, 1523–1532. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Blom, I.E.; Goldschmeding, R.; Leask, A. Gene regulation of connective tissue growth factor: New targets for antifibrotic therapy? Matrix Boil. 2002, 21, 473–482. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.; et al. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis Find the latest version: Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef]
- Asano, Y. Epigenetic suppression of Fli1, a potential predisposing factor in the pathogenesis of systemic sclerosis. Int. J. Biochem. Cell Boil. 2015, 67, 86–91. [Google Scholar] [CrossRef]
- Asano, Y. What can we learn from Fli1-deficient mice, new animal models of systemic sclerosis? J. Scleroderma Relat. Disord. 2018, 3, 6–13. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Iwata, Y.; Komura, K.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. CD19 Regulates Skin and Lung Fibrosis via Toll-Like Receptor Signaling in a Model of Bleomycin-Induced Scleroderma. Am. J. Pathol. 2008, 172, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Higashi-Kuwata, N.; Makino, T.; Inoue, Y.; Takeya, M.; Ihn, H. Alternatively activated macrophages (M2 macrophages) in the skin of patient with localized scleroderma. Exp. Dermatol. 2009, 18, 727–729. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.C. MicroRNAs in fibrosis: Opportunities and challenges. Arthritis Res. 2016, 18, 11. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, H.; Hu, Y.; Li, Q.; Hu, Z.; Ma, T.; Mao, X. Burkholderia pseudomallei-derived miR-3473 enhances NF-κB via targeting TRAF3 and is associated with different inflammatory responses compared to Burkholderia thailandensis in murine macrophages. BMC Microbiol. 2016, 16, 283. [Google Scholar] [CrossRef]
- Park, M.-J.; Moon, S.-J.; Lee, E.-J.; Jung, K.-A.; Kim, E.-K.; Kim, D.-S.; Lee, J.-H.; Kwok, S.-K.; Min, J.-K.; Park, S.-H.; et al. IL-1-IL-17 Signaling Axis Contributes to Fibrosis and Inflammation in Two Different Murine Models of Systemic Sclerosis. Front. Immunol. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Riva, F.; Bonavita, E.; Barbati, E.; Muzio, M.; Mantovani, A.; Garlanda, C. TIR8/SIGIRR is an Interleukin-1 Receptor/Toll Like Receptor Family Member with Regulatory Functions in Inflammation and Immunity. Front. Immunol. 2012, 3, 1–13. [Google Scholar] [CrossRef]
- He, X.; Pu, G.; Tang, R.; Zhang, D.; Pan, W. Activation of Nuclear Factor Kappa B in the Hepatic Stellate Cells of Mice with Schistosomiasis Japonica. PLoS ONE 2014, 9, e104323. [Google Scholar] [CrossRef]
- Chen, B. The miRNA-184 drives renal fibrosis by targeting HIF1AN in vitro and in vivo. Int. Urol. Nephrol. 2018, 51, 543–550. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; He, J.; Zhao, W. Influence of erythropoietin on microvesicles derived from mesenchymal stem cells protecting renal function of chronic kidney disease. Stem Cell Res. Ther. 2015, 6, 100. [Google Scholar] [CrossRef]
- Zehavi, L.; Avraham, R.; Aviv, B.; Bar-Ilan, D.; Navon, R.; Sidi, Y.; Avni, D.; Leibowitz-Amit, R. Silencing of a large microRNA cluster on human chromosome 14q32 in melanoma: Biological effects of mir-376a and mir-376c on insulin growth factor 1 receptor. Mol. Cancer 2012, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Formosa, A.; Markert, E.K.; Lena, A.M.; Italiano, D.; Finazzi-Agrò, E.; Levine, A.J.; Bernardini, S.; Garabadgiu, A.V.; Melino, G.; Candi, E. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene 2013, 33, 5173–5182. [Google Scholar] [CrossRef] [PubMed]
- Nho, R.S. Alteration of Aging-Dependent MicroRNAs in Idiopathic Pulmonary Fibrosis. Drug Dev. Res. 2015, 76, 343–353. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhall, S.; Lerch, A.; Johnson, N.; Jacob, V.; Jones, B.; Park, M.S.; Sathyamoorthy, M. A Flowable Placental Formulation Prevents Bleomycin-Induced Dermal Fibrosis in Aged Mice. Int. J. Mol. Sci. 2020, 21, 4242. https://doi.org/10.3390/ijms21124242
Dhall S, Lerch A, Johnson N, Jacob V, Jones B, Park MS, Sathyamoorthy M. A Flowable Placental Formulation Prevents Bleomycin-Induced Dermal Fibrosis in Aged Mice. International Journal of Molecular Sciences. 2020; 21(12):4242. https://doi.org/10.3390/ijms21124242
Chicago/Turabian StyleDhall, Sandeep, Anne Lerch, Nicholas Johnson, Vimal Jacob, Brielle Jones, Min Sung Park, and Malathi Sathyamoorthy. 2020. "A Flowable Placental Formulation Prevents Bleomycin-Induced Dermal Fibrosis in Aged Mice" International Journal of Molecular Sciences 21, no. 12: 4242. https://doi.org/10.3390/ijms21124242
APA StyleDhall, S., Lerch, A., Johnson, N., Jacob, V., Jones, B., Park, M. S., & Sathyamoorthy, M. (2020). A Flowable Placental Formulation Prevents Bleomycin-Induced Dermal Fibrosis in Aged Mice. International Journal of Molecular Sciences, 21(12), 4242. https://doi.org/10.3390/ijms21124242



