Transcriptome, Spliceosome and Editome Expression Patterns of the Porcine Endometrium in Response to a Single Subclinical Dose of Salmonella Enteritidis Lipopolysaccharide
Abstract
1. Introduction
2. Results
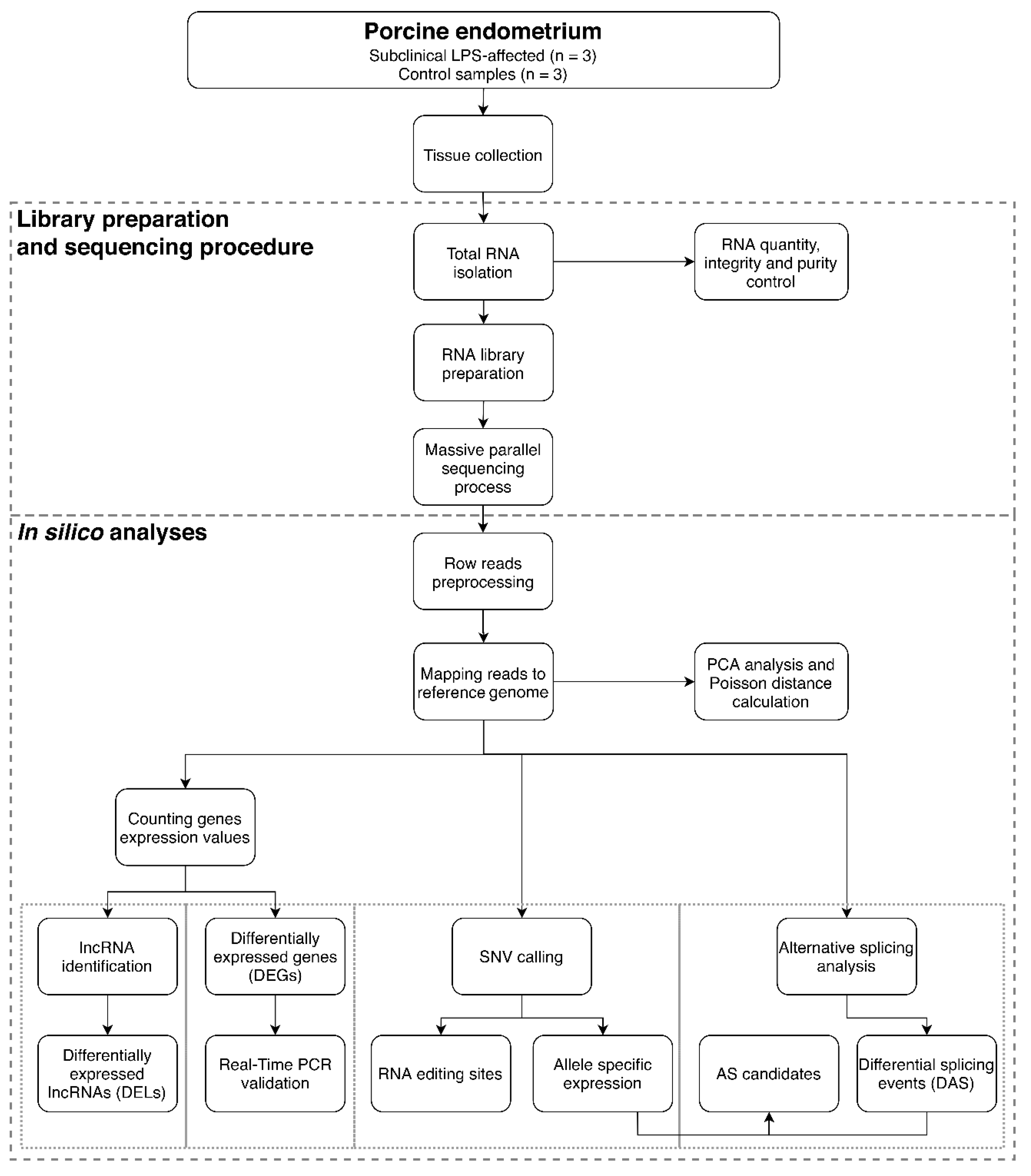
2.1. Mapping and Clustering of RNA-Seq Libraries
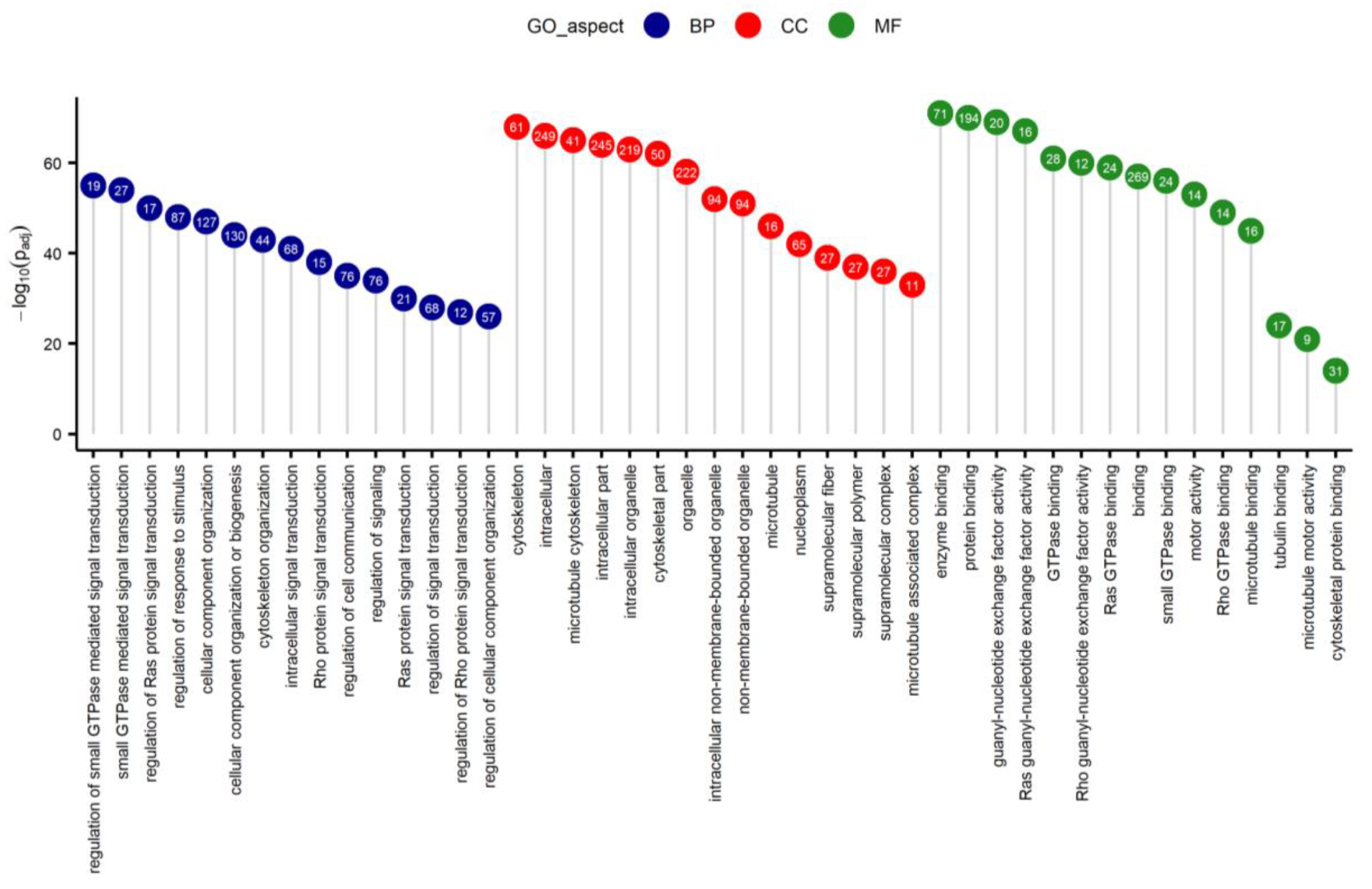
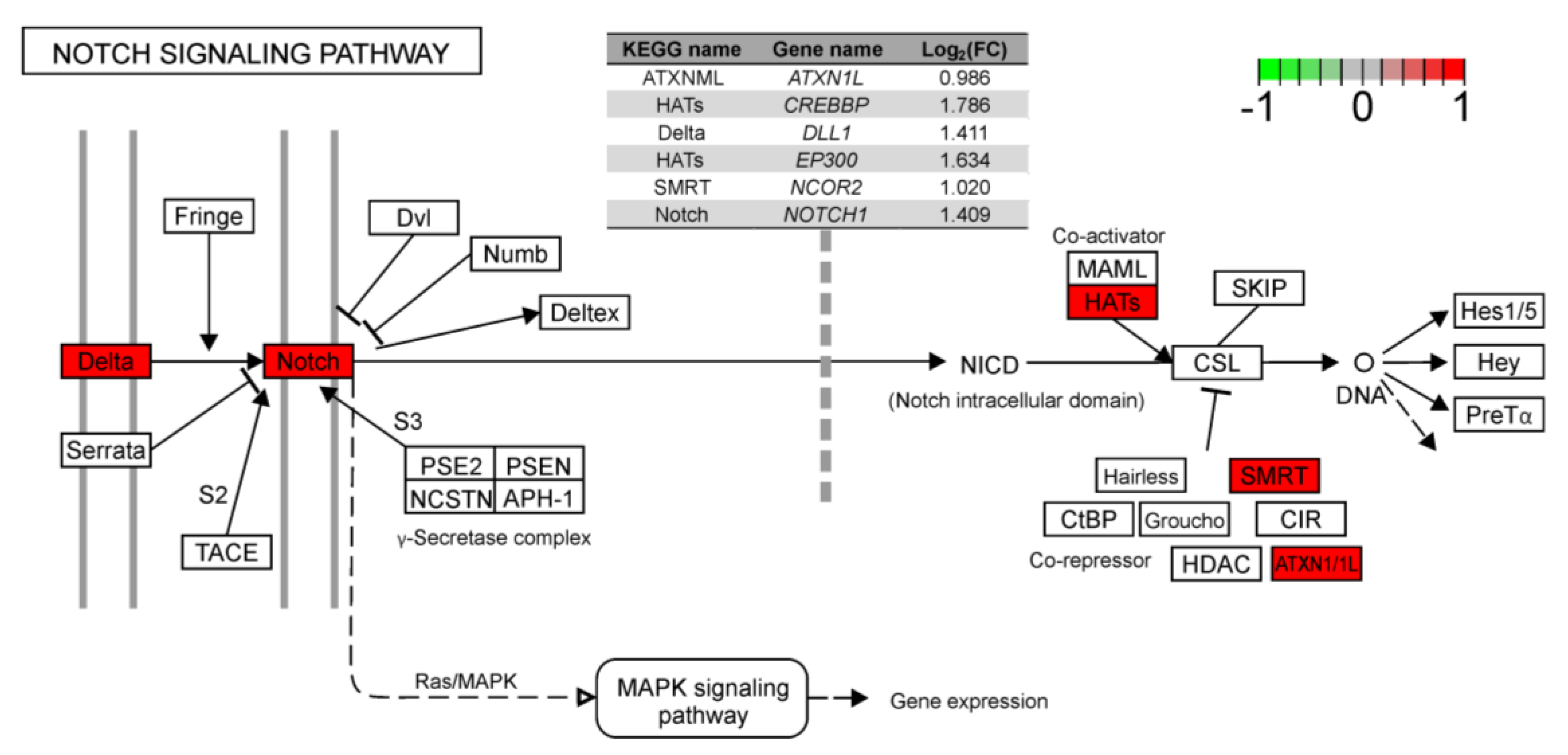
2.2. Differentially Expressed Genes (DEGs) and Functional Annotations
2.3. Long noncoding RNA (lncRNA) Identification and Cis/Trans-Connection with Protein-Coding Genes
2.4. Differentially Alternative Splicing (AS) Events
2.5. Allele-Specific Expression (ASE)
2.6. RNA Editing Prediction
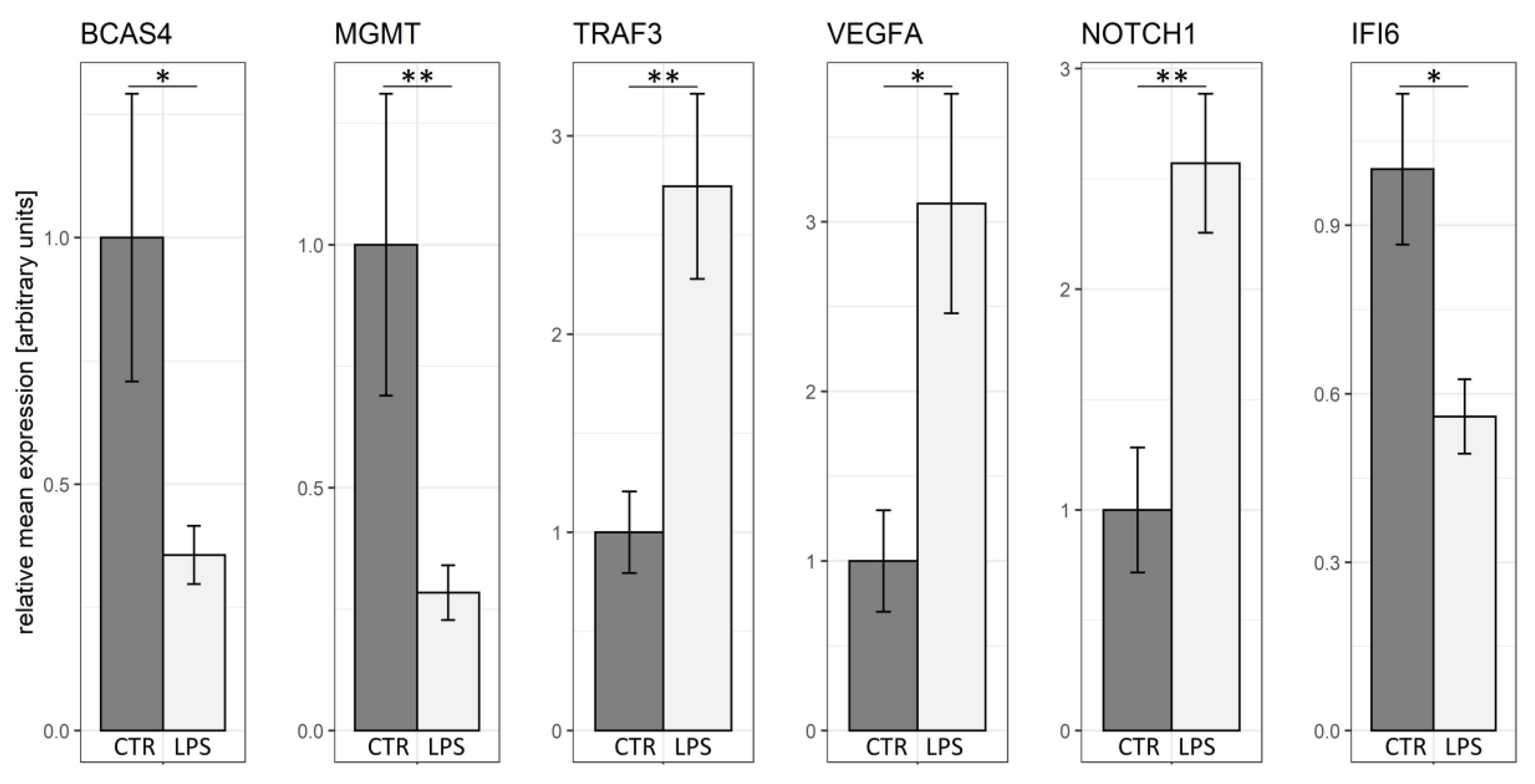
2.7. Real-Time PCR Validation
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Collection of Samples
4.2. Library Preparation and RNA-Seq Sequencing
4.3. Transcriptome Expression Profiling
4.4. Identification and Expression Profiling of Long Noncoding RNA (lncRNAs)
4.5. Alternative Splicing Events
4.6. Allele-Specific Expression (ASE) Variants
4.7. RNA Editing Sites Prediction
4.8. Quantitative Reverse Transcription PCR (qRT-PCR) of DEGs
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Supporting Data
Abbreviations
| lncRNAs | long non-coding RNAs |
| LPS | lipopolysaccharide |
| RNA-seq | RNA-sequencing |
| bp | base pair |
| NGS | next-generation sequencing |
| CPAT | Coding-Potential Assessment Tool |
| CPC | Coding Potential Calculator |
| FEELnc | Flexible Extraction of LncRNAs |
| Pfam | protein family database |
| DELs | differentially expressed lncRNAs |
| GO | Gene Ontology database |
| TAR | transcriptionally active region |
| DEGs | differentially expressed genes |
| SNV | single nucleotide variant |
| SNP | single nucleotide polymorphism |
| PGE2 | prostaglandin E2 |
| TNF-α | tumor necrosis factor alpha |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| IFNs | interferons |
| PCA | principal component analysis |
| FPKM | fragments per kilobase of transcript per million mapped reads |
| FDR | false discovery rate |
| AS | alternative splicing |
| rMATS | replicate multivariate analysis of transcript splicing |
| DAS | differentially alternative splicing |
| A5SS | alternative 5′splice site |
| A3SS | alternative 3′splice site |
| MXE | mutually exclusive exons |
| RI | retention intron |
| SE | skipping exon |
| AF | alternative first |
| AL | last exon |
| PSI | percent splicing inclusion |
| SSR | single sequence repeat |
| ASE | allele-specific expression |
| AAF | alternative allele fraction |
| TLR | Toll-like receptor |
| I 5 | intron 5 |
| dsRNA | double stranded RNA |
| SINE | short interspersed nuclear element |
| A-to-I | adenosine to inosine RNA editing |
| T4 | thyroxine |
| T3 | triiodothyronine |
| MXC-I | major histocompatibility complex I |
| RhoGTPases | Rho family of GTPase |
| MAPK | mitogen-activated protein kinas |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| JNK | c-Jun N-terminal kinases |
| RIG-I signaling pathway | Retinoic acid-Inducible Gene I signaling pathway |
| heteroref | heterozygous variants in which reference allele was predominant |
| heteroalt | heterozygous variants in which alternate allele was predominant |
| true heterozygotes | heterozygous sites with no predominant variant |
| GATK | The Genome Analysis Toolkit |
| ΔPSI | difference in PSI values |
| BP | biological process |
| MF | molecular function |
| CC | cellular components |
| ΔΔCT | comparative cycle threshold method |
| rs ID | SNP annotation in database |
| C-to-U | cytidine to uridine RNA editing |
| VEP | Variant Effect Predictor |
| VCF | variant calling format |
| r | Pearson’s correlation coefficient |
| KEGG | Kyoto Encyclopaedia of Genes and Genomes database |
| GO | Gene Ontology |
| ENA | European Nucleotide Archive |
References
- Hohmann, E.L. Nontyphoidal Salmonellosis. Clin. Infect. Dis. 2001, 32, 263–269. [Google Scholar] [CrossRef]
- Thompson Bastin, M.L.; Neville, N.R.; Parsons, R.E.; Flannery, A.H.; Tennant, S.J.; Johnson, C.A. An unusual case of Salmonella Enteritidis causing pneumonia, septic shock and multiple organ failure in an immunocompetent patient. IDCases 2016, 6, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zenk, S.F.; Jantsch, J.; Hensel, M. Role of Salmonella enterica Lipopolysaccharide in Activation of Dendritic Cell Functions and Bacterial Containment. J. Immunol. 2009, 183, 2697–2707. [Google Scholar] [CrossRef]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. Lipopolysaccharide Appears to Activate Human Endometrial Endothelial Cells Through TLR-4-Dependent and TLR-4-Independent Mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef]
- Deb, K.; Chaturvedi, M.M.; Jaiswal, Y.K. A “minimum dose” of lipopolysaccharide required for implantation failure: Assessment of its effect on the maternal reproductive organs and interleukin-1α expression in the mouse. Reproduction 2004, 128, 87–97. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Złotkowska, D. Neuroimmunological Implications of Subclinical Lipopolysaccharide from Salmonella Enteritidis. Int. J. Mol. Sci. 2018, 19, 3274. [Google Scholar] [CrossRef]
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.J.; Crump, J.A. Global burden of invasive nontyphoidal salmonella disease, 2010. Emerg. Infect. Dis. 2015, 21, 941–949. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Jones, B.D.; Ghori, N.; Falkow, S. Salmonella typhlrnurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the peyer’s patches. J. Exp. Med. 1994, 180, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Hine, B.C.; Wallace, O.A.M.; Callaghan, M.; Bibiloni, R. Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Cunha, F.; Vieira-Neto, A.; Bicalho, R.C.; Lima, S.; Bicalho, M.L.; Galvão, K.N. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.; Utekar, T.; Ray, R. Preterm delivery and neonatal meningitis due to transplacental acquisition of non-typhoidal Salmonella serovar montevideo. Case Rep. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Singla, N. Salmonella Typhi Isolation in a Pregnant Woman: Determining the Importance. J. Clin. Diagn. Res. 2013, 7, 2100–2101. [Google Scholar] [CrossRef] [PubMed]
- Rupasri, A.; Shivakumar, K.R.; Sreenath, B.R.; Seshagiri, P.B. Assessment of developmental retardation and abnormality of in vivo produced preimplantation embryos in rat. Indian J. Exp. Biol. 1995, 33, 911–916. [Google Scholar] [PubMed]
- Wira, C.R.; Grant-Tschudy, K.S.; Crane-Godreau, M.A. Epithelial Cells in the Female Reproductive Tract: A Central Role as Sentinels of Immune Protection. Am. J. Reprod. Immunol. 2005, 53, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction 2014, 148. [Google Scholar] [CrossRef]
- Cronin, J.G.; Turner, M.L.; Goetze, L.; Bryant, C.E.; Sheldon, I.M. Toll-Like Receptor 4 and MYD88-Dependent Signaling Mechanisms of the Innate Immune System Are Essential for the Response to Lipopolysaccharide by Epithelial and Stromal Cells of the Bovine Endometrium1. Biol. Reprod. 2012, 86. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Rycroft, A.N.; Dogan, B.; Craven, M.; Bromfield, J.J.; Chandler, A.; Roberts, M.H.; Price, S.B.; Gilbert, R.O.; Simpson, K.W. Specific Strains of Escherichia coli Are Pathogenic for the Endometrium of Cattle and Cause Pelvic Inflammatory Disease in Cattle and Mice. PLoS ONE 2010, 5, e9192. [Google Scholar] [CrossRef]
- Jursza, E.; Szóstek, A.Z.; Kowalewski, M.P.; Boos, A.; Okuda, K.; Siemieniuch, M.J. LPS-challenged TNF α production, prostaglandin secretion, and TNF α /TNFRs expression in the endometrium of domestic cats in estrus or diestrus, and in cats with pyometra or receiving medroxyprogesterone acetate. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef]
- Jana, B.; Czarzasta, J. Effect of lipopolysaccharide and cytokines on synthesis and secretion of leukotrienes from endometrial epithelial cells of pigs. Anim. Reprod. Sci. 2016, 168, 116–125. [Google Scholar] [CrossRef]
- Aisemberg, J.; Vercelli, C.A.; Bariani, M.V.; Billi, S.C.; Wolfson, M.L.; Franchi, A.M. Progesterone Is Essential for Protecting against LPS-Induced Pregnancy Loss. LIF as a Potential Mediator of the Anti-inflammatory Effect of Progesterone. PLoS ONE 2013, 8, e56161. [Google Scholar] [CrossRef] [PubMed]
- Paukszto, L.; Mikolajczyk, A.; Szeszko, K.; Smolinska, N.; Jastrzebski, J.P.; Kaminski, T. Transcription analysis of the response of the porcine adrenal cortex to a single subclinical dose of lipopolysaccharide from Salmonella Enteritidis. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Mikolajczyk, A.; Calka, J.; Gonkowski, S. Neurochemical characterization of nerve fibers in the porcine gallbladder wall under physiological conditions and after the administration of Salmonella enteritidis lipopolysaccharides (LPS). Toxicol. Res. Camb 2018, 7, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, A.; Kozłowska, A.; Gonkowski, S. Distribution and Neurochemistry of the Porcine Ileocaecal Valve Projecting Sensory Neurons in the Dorsal Root Ganglia and the Influence of Lipopolysaccharide from Different Serotypes of Salmonella spp. on the Chemical Coding of DRG Neurons in the Cell Cul. Int. J. Mol. Sci. 2018, 19, 2551. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Złotkowska, D. Subclinical Lipopolysaccharide from Salmonella Enteritidis Induces Dysregulation of Bioactive Substances from Selected Brain Sections and Glands of Neuroendocrine Axes. Toxins Basel. 2019, 11, 91. [Google Scholar] [CrossRef]
- Xu, D.-X.; Wang, H.; Zhao, L.; Ning, H.; Chen, Y.-H.; Zhang, C. Effects of low-dose lipopolysaccharide (LPS) pretreatment on LPS-induced intra-uterine fetal death and preterm labor. Toxicology 2007, 234, 167–175. [Google Scholar] [CrossRef]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef]
- Chae, B.S. Pretreatment of low-dose and super-low-dose LPS on the production of in vitro LPS-induced inflammatory mediators. Toxicol. Res. 2018. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Correa, W.; Brandenburg, K.; Zähringer, U.; Ravuri, K.; Khan, T.; von Wintzingerode, F. Biophysical Analysis of Lipopolysaccharide Formulations for an Understanding of the Low Endotoxin Recovery (LER) Phenomenon. Int. J. Mol. Sci. 2017, 18, 2737. [Google Scholar] [CrossRef]
- Nordgreen, J.; Munsterhjelm, C.; Aae, F.; Popova, A.; Boysen, P.; Ranheim, B.; Heinonen, M.; Raszplewicz, J.; Piepponen, P.; Lervik, A.; et al. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol. Behav. 2018, 195, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.W.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Lilly, S.T.; Fischer, D.P.; Williams, E.J.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Bacterial lipopolysaccharide induces an endocrine switch from prostaglandin F2alpha to prostaglandin E2 in bovine endometrium. Endocrinology 2009, 150, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Nakamura, T.; Hodgson, D.M. Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress 2010, 13, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.Q.; Mitchell, M.D.; Almughlliq, F.B.; Vaswani, K.; Peiris, H.N. Regulation of inflammatory mediator expression in bovine endometrial cells: Effects of lipopolysaccharide, interleukin 1 beta, and tumor necrosis factor alpha. Physiol. Rep. 2018, 6, e13676. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Mansouri-Attia, N.; Mansourri-Attia, N.; Fahey, A.G.; Browne, J.; Forde, N.; Roche, J.F.; Lonergan, P.; Fair, T. Characterization of the Th profile of the bovine endometrium during the oestrous cycle and early pregnancy. PLoS ONE 2013, 8, e75571. [Google Scholar] [CrossRef]
- Ibrahim, S.; Szóstek-Mioduchowska, A.; Skarzynski, D. Expression profiling of selected miRNAs in equine endometrium in response to LPS challenge in vitro: A new understanding of the inflammatory immune response. Vet. Immunol. Immunopathol. 2019, 209, 37–44. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Luo, N.; Li, C.; Chen, R.; Qu, X.; Liu, M.; Kang, L.; Cheng, Z. LPS/TLR4-mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci. Rep. 2016, 6, 21416. [Google Scholar] [CrossRef]
- Häcker, H.; Redecke, V.; Blagoev, B.; Kratchmarova, I.; Hsu, L.-C.; Wang, G.G.; Kamps, M.P.; Raz, E.; Wagner, H.; Häcker, G.; et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 2006, 439, 204–207. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Bedford, C.; Jacca, S.; Rosamilia, A.; de Lima, V.F.; Donofrio, G.; Sheldon, I.M.; Cronin, J.G. Coordinated Role of Toll-Like Receptor-3 and Retinoic Acid-Inducible Gene-I in the Innate Response of Bovine Endometrial Cells to Virus. Front. Immunol. 2017, 8, 996. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Chen, B. SIGIRR participates in negative regulation of LPS response and tolerance in human bladder epithelial cells. BMC Immunol. 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Chuang, H.C.; Tan, T.H. Regulation of dual-specificity phosphatase (Dusp) ubiquitination and protein stability. Int. J. Mol. Sci. 2019, 20, 2668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Van Putten, V.; Zarinetchi, F.; Nicks, M.E.; Thaler, S.; Heasley, L.E.; Nemenoff, R.A. Suppression of smooth-muscle α-actin expression by platelet-derived growth factor in vascular smooth-muscle cells involves Ras and cytosolic phospholipase A2. Biochem. J. 1997, 327, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, V.; Refaat, Z.; Dessev, C.; Blaine, S.; Wick, M.; Butterfield, L.; Han, S.Y.; Heasley, L.E.; Nemenoff, R.A. Induction of cytosolic phospholipase A2 by oncogenic Ras is mediated through the JNK and ERK pathways in rat epithelial cells. J. Biol. Chem. 2001, 276, 1226–1232. [Google Scholar] [CrossRef]
- Grewal, S.; Carver, J.; Ridley, A.J.; Mardon, H.J. Human Endometrial Stromal Cell Rho GTPases Have Opposing Roles in Regulating Focal Adhesion Turnover and Embryo Invasion In Vitro. Biol. Reprod. 2010, 83, 75. [Google Scholar] [CrossRef]
- Maldonado, M.D.M.; Dharmawardhane, S. Targeting Rac and Cdc42 GTPases in Cancer. Cancer Res. 2018, 78, 3101–3111. [Google Scholar] [CrossRef]
- Rashidi, B.; Malekzadeh, M. Evaluation of Endometrial Angiogenesis in Mice Uterus Before Implantation in Natural Cycles Followed by Use of Human Menopausal Gonadotropin—Human Chorionic Gonadotropin Drugs and Epigallocatechin Gallate. Adv. Biomed. Res. 2017, 6, 138. [Google Scholar] [CrossRef]
- Tabernero, J. The role of VEGF and EGFR inhibition: Implications for combining anti-VEGF and anti-EGFR agents. Mol. Cancer Res. 2007, 5, 203–220. [Google Scholar] [CrossRef]
- Cobellis, L.; Caprio, F.; Trabucco, E.; Mastrogiacomo, A.; Coppola, G.; Manente, L.; Colacurci, N.; De Falco, M.; De Luca, A. The pattern of expression of Notch protein members in normal and pathological endometrium. J. Anat. 2008, 213, 464–472. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Tsao, P.-N.; Wei, S.-C.; Huang, M.-T.; Lee, M.-C.; Chou, H.-C.; Chen, C.-Y.; Hsieh, W.-S. Lipopolysaccharide-induced Notch signaling activation through JNK-dependent pathway regulates inflammatory response. J. Biomed. Sci. 2011, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Degaki, K.Y.; Chen, Z.; Yamada, A.T.; Croy, B.A. Delta-like ligand (DLL)1 expression in early mouse decidua and its localization to uterine natural killer cells. PLoS ONE 2012, 7, e52037. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Brüne, B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Tucker, D.; Burchett, S.; Leslie, C. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 2006, 45, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Földi, J.; Kulcsár, M.; Pécsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.C.M.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Ibrahim, S.; Gebremedhn, S.; Tesfaye, D.; Heppelmann, M.; Bollwein, H.; Pfarrer, C.; Tholen, E.; Neuhoff, C.; Schellander, K.; et al. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genom. 2016, 17, 218. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoo, J.-Y.; Choi, K.-C.; Shin, J.-H.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.-G.; Jeong, J.-W. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci. Transl. Med. 2019, 11, eaaf7533. [Google Scholar] [CrossRef]
- Choe, C.; Park, J.-W.; Kim, E.-S.; Lee, S.-G.; Park, S.-Y.; Lee, J.-S.; Cho, M.-J.; Kang, K.R.; Han, J.; Kang, D. Proteomic analysis of differentially expressed proteins in bovine endometrium with endometritis. Korean J. Physiol. Pharmacol. 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, Y.S.; Yoon, T.K.; Lee, W.S. Chronic endometritis and infertility. Clin. Exp. Reprod. Med. 2016, 43, 185–192. [Google Scholar] [CrossRef]
- Doǧan-Ekici, A.I.; Usubütün, A.; Küçükali, T.; Ayhan, A. Xanthogranulomatous endometritis: A challenging imitator of endometrial carcinoma. Infect. Dis. Obstet. Gynecol. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Himes, K.P.; Handley, D.; Chu, T.; Burke, B.; Bunce, K.; Simhan, H.N.; Peters, D.G. Comprehensive analysis of the transcriptional response of human decidual cells to lipopolysaccharide stimulation. J. Reprod. Immunol. 2012, 93, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Momtazi, A.A.; Banach, M.; Sahebkar, A. PCSK9 inhibitors in sepsis: A new potential indication? Expert Opin. Investig. Drugs 2017, 26, 137–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paciullo, F.; Fallarino, F.; Bianconi, V.; Mannarino, M.R.; Sahebkar, A.; Pirro, M. PCSK9 at the crossroad of cholesterol metabolism and immune function during infections. J. Cell. Physiol. 2017, 232, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.G.; Zasłona, Z.; Galván-Peña, S.; Koppe, E.L.; Sévin, D.C.; Angiari, S.; Triantafilou, M.; Triantafilou, K.; Modis, L.K.; O’Neill, L.A. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 2018, 293, 5509–5521. [Google Scholar] [CrossRef]
- Shiozawa, T.; Shih, H.-C.; Miyamoto, T.; Feng, Y.-Z.; Uchikawa, J.; Itoh, K.; Konishi, I. Cyclic Changes in the Expression of Steroid Receptor Coactivators and Corepressors in the Normal Human Endometrium. J. Clin. Endocrinol. Metab. 2003, 88, 871–878. [Google Scholar] [CrossRef][Green Version]
- Hall, A. Chapter 19 The role of ras in signal transduction. In Principles of Medical Biology; Elsevier Inc.: Amsterdam, The Netherlands, 1997; Volume 7, pp. 451–468. [Google Scholar]
- Lu, J.; Teh, B.K.; Wang, L.; Wang, Y.; Tan, Y.S.; Lai, M.C.; Reid, K.B.M. The classical and regulatory functions of C1q in immunity and autoimmunity. Cell. Mol. Immunol. 2008, 5, 9–21. [Google Scholar] [CrossRef]
- Yanai, H.; Ban, T.; Taniguchi, T. Essential role of high-mobility group box proteins in nucleic acid-mediated innate immune responses. J. Intern. Med. 2011, 270, 301–308. [Google Scholar] [CrossRef]
- Yehia, L.; Jindal, S.; Komar, A.A.; Eng, C. Non-canonical role of cancer-associated mutant SEC23B in the ribosome biogenesis pathway. Hum. Mol. Genet. 2018, 27, 3154–3164. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, T.; Wu, L.; Liu, X.; Xue, S.; Lei, M. Identification of non-coding and coding RNAs in porcine endometrium. Genomics 2017, 109, 43–50. [Google Scholar] [CrossRef]
- Noh, K.T.; Jung, I.D.; Cha, G.S.; Han, M.-K.; Park, Y.-M. Platelet-activating Factor Mediates Endotoxin Tolerance by Regulating Indoleamine 2,3-Dioxygenase-dependent Expression of the Suppressor of Cytokine Signaling 3. J. Biol. Chem. 2017, 292, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Broeke, S.W.T.; Klift, H.M.V.; Tops, C.M.J.; Aretz, S.; Bernstein, I.; Buchanan, D.D.; de la Chapelle, A.; Capella, G.; Clendenning, M.; Engel, C.; et al. Cancer Risks for PMS2-associated lynch syndrom. J. Clin. Oncol. 2018, 36, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Broaddus, R.; Sun, L.; Xue, F.; Zhang, W. Exon 3 mutations of CTNNB1 drive tumorigenesis: A review. Oncotarget 2018, 9, 5492–5508. [Google Scholar] [CrossRef] [PubMed]
- Bazak, L.; Haviv, A.; Barak, M.; Jacob-Hirsch, J.; Deng, P.; Zhang, R.; Isaacs, F.J.; Rechavi, G.; Li, J.B.; Eisenberg, E.; et al. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res. 2014, 24, 365–376. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Peng, Z.; Zhang, R.; Yang, X.-Z.; Tan, B.C.-M.; Fang, H.; Liu, C.-J.; Shi, M.; Ye, Z.-Q.; Zhang, Y.E.; et al. RNA Editome in Rhesus Macaque Shaped by Purifying Selection. PLoS Genet. 2014, 10, e1004274. [Google Scholar] [CrossRef]
- Funkhouser, S.A.; Steibel, J.P.; Bates, R.O.; Raney, N.E.; Schenk, D.; Ernst, C.W. Evidence for transcriptome-wide RNA editing among Sus scrofa PRE-1 SINE elements. BMC Genom. 2017, 18, 360. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, M.; Fan, X.; Yao, Y.; Yan, J.; Tang, Y.; Liu, S.; Li, K.; Tang, Z. Developmental atlas of the RNA editome in Sus scrofa skeletal muscle. DNA Res. 2019, 26, 261–272. [Google Scholar] [CrossRef]
- Maekawa, R.; Taketani, T.; Mihara, Y.; Sato, S.; Okada, M.; Tamura, I.; Jozaki, K.; Kajimura, T.; Asada, H.; Tamura, H.; et al. Thin endometrium transcriptome analysis reveals a potential mechanism of implantation failure. Reprod. Med. Biol. 2017, 16, 206–227. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Choy, Y.S.; Talib, A.; Norsiah, M.D.; van den Heuvel, L.P.; Rodenburg, R.J. Infantile Progressive Hepatoencephalomyopathy with Combined OXPHOS Deficiency due to Mutations in the Mitochondrial Translation Elongation Factor Gene GFM1. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 113–122. [Google Scholar]
- Mikołajczyk, A.; Gonkowski, S.; Złotkowska, D. Modulation of the main porcine enteric neuropeptides by a single low-dose of lipopolysaccharide (LPS) Salmonella Enteritidis. Gut Pathog. 2017, 9, 73. [Google Scholar] [CrossRef]
- Mikołajczyk, A.; Złotkowska, D. Subclinical lipopolysaccharide from Salmonella Enteritidis induces neuropeptide dysregulation in the spinal cord and the dorsal root ganglia. BMC Neurosci. 2019, 20, 18. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinforma. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 January 2020).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/ (accessed on 1 February 2020).
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Mccarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma. Appl. NOTE 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. g:Profiler—A web server for functional interpretation of gene lists. Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.-P.; Li, W. CPAT: Coding-Potential Assessment Tool using an alignment-free logistic regression model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, D.C.; Kong, L.; Hou, M.; Meng, Y.Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef] [PubMed]
- Wucher, V.; Legeai, F.; Hédan, B.; Rizk, G.; Lagoutte, L.; Leeb, T.; Jagannathan, V.; Cadieu, E.; David, A.; Lohi, H.; et al. FEELnc: A tool for long non-coding RNA annotation and its application to the dog transcriptome. Nucleic Acids Res. 2017, gkw1306. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam protein families database. Nucleic Acids Res. 2010, 38, D211–D222. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. Rfam: An RNA family database. Nucleic Acids Res. 2003, 31, 439–441. [Google Scholar] [CrossRef]
- Trincado, J.L.; Entizne, J.C.; Hysenaj, G.; Singh, B.; Skalic, M.; Elliott, D.J.; Eyras, E. SUPPA2: Fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 2018, 19, 40. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.-X.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593–E5601. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Veiga, D. Maser: Mapping Alternative Splicing Events to pRoteins. R Package Version 1.0.0. Available online: https://github.com/DiogoVeiga/maser (accessed on 1 February 2020).
- Xie, Z.J.; Tseng, Y.-T.; Xing, Y. rmats2sashimiplot. Available online: https://github.com/Xinglab/rmats2sashimiplot (accessed on 1 February 2020).
- Wang, J.; Pan, Y.; Shen, S.; Lin, L.; Xing, Y. rMATS-DVR: rMATS discovery of differential variants in RNA. Bioinformatics 2017, 33, 2216–2217. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010. [Google Scholar] [CrossRef]
- Wang, X.; Lu, P.; Luo, Z. GMATo: A novel tool for the identification and analysis of microsatellites in large genomes. Bioinformation 2013, 9, 541–544. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly Austin 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- DREP: Database of RNA Editing in Pig. Available online: http://www.rnanet.org/editing/home.html (accessed on 4 May 2020).
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



| SAMPLE | CTR1 | CTR2 | CTR3 | LPS1 | LPS2 | LPS3 |
|---|---|---|---|---|---|---|
| Raw reads | 107,429,702 | 89,680,918 | 84,472,314 | 97,318,676 | 90,921,644 | 95,504,908 |
| Trimmed reads | 99,350,630 | 82,942,056 | 78,186,984 | 89,746,640 | 83,948,534 | 87,774,992 |
| Mapped reads | 97,654,932 | 81,309,628 | 76,784,982 | 88,226,984 | 82,560,754 | 85,830,024 |
| Uniquely mapped reads | 89,527,396 | 60,499,072 | 58,793,660 | 80,185,286 | 70,781,032 | 73,217,060 |
| Multi-mapped reads | 8,066,184 | 20,785,528 | 17,964,626 | 7,990,104 | 11,741,434 | 12,567,688 |
| Reads mapped with too many loci | 61,352 | 25,028 | 26,696 | 51,594 | 38,288 | 45,276 |
| Gene_ID | Log2(FC) | P-Adj | Chr/Contig | Start | End | Strand | ENSEMBL ID |
|---|---|---|---|---|---|---|---|
| MSTRG.3558 | 1.42 | 2.44E-03 | 12 | 47,554,682 | 47,562,366 | − | NA |
| MSTRG.9270 | −0.95 | 1.41E-02 | 2 | 11,923,739 | 11,924,922 | + | NA |
| MSTRG.15739 | −1.38 | 4.80E-03 | 6 | 88,799,040 | 88,800,470 | − | NA |
| MSTRG.19846 | 1.86 | 1.64E-06 | AEMK02000682.1 | 1,430,615 | 1,441,540 | + | ENSSSCG00000035563 |
| Gene Name | Chrom.: Site | Alt. Allele Freq. in CTR | Alt. Allele Freq. in LPS | Variant Annotation |
|---|---|---|---|---|
| FAM214A | 1:119024113 * | 0.611, 0.474, 0.222 | 0.571, 0.333, 0.167 | intron_variant |
| FAM214A | 1:119024125 * | 0.471, 0.389, 0.125 | 0.333, 0.154, 0.214 | intron_variant |
| SPPL2A | 1:121133488 | 0.0, 0.071, 0.0 | 0.273, 0.333, 0.167 | 3_prime_UTR_variant |
| SPPL2A | 1:121133557 | 0.304, 0.367, 0.333 | 0.5, 0.419, 0.263 | 3_prime_UTR_variant |
| SPPL2A | 1:121133571 | 0.36, 0.447, 0.147 | 0.2, 0.289, 0.261 | 3_prime_UTR_variant |
| ENSSSCG00000004985 | 1:169701294 | 0.143, 0.154, 0.0 | 0.375, 0.083, 0.0 | 3_prime_UTR_variant |
| DHRS7 | 1:189153076 * | 0.125, 0.111, 0.25 | 0.182, 0.2, 0.071 | 3_prime_UTR_variant |
| DHRS7 | 1:189153088 * | 0.059, 0.125, 0.286 | 0.1, 0.25, 0.0 | 3_prime_UTR_variant |
| DENND4C | 1:203449165 | 0.333, 0.1, 0.2 | 0.0, 0.0, 0.0 | downstream_gene_variant |
| DENND4C | 1:203449178 | 0.333, 0.222, 0.2 | 0.111, 0.0, 0.0 | downstream_gene_variant |
| HDHD3 | 1:254011510 | 0.25, 0.167, 0.5 | 0.5, 0.167, 0.667 | upstream_gene_variant |
| RC3H2 | 1:263824986 | 0.444, 0.4, 0.333 | 0.444, 0.25, 0.0 | upstream_gene_variant |
| ENSSSCG00000038757 | 1:66466635 | 0.167, 0.25, 0.4 | 0.333, 0.5, 0.429 | intron_variant |
| ENSSSCG00000038757 | 1:66466642 | 0.429, 0.333, 0.2 | 0.5, 0.571, 0.167 | intron_variant |
| ENSSSCG00000038757 | 1:66466658 | 0.25, 0.5, 0.0 | 0.333, 0.25, 0.0 | intron_variant |
| TRAF3IP2 | 1:77485788 | 0.167, 0.182, 0.571 | 0.143, 0.0, 0.143 | downstream_gene_variant |
| ARL14EP | 2:30295149 | 0.167, 0.167, 0.143 | 0.444, 0.0, 0.375 | 3_prime_UTR_variant |
| ARL14EP | 2:30295163 | 0.1, 0.0, 0.167 | 0.111, 0.2, 0.0 | 3_prime_UTR_variant |
| TMEM161A | 2:58744433 | 0.3, 0.3, 0.286 | 0.125, 0.0, 0.0 | 3_prime_UTR_variant |
| GNG3 | 2:9050458 | 0.333, 0.1, 0.143 | 0.077, 0.0, 0.0 | downstream_gene_variant |
| RPS15A | 3:26783904 | 0.0, 0.375, 0.043 | 0.188, 0.0, 0.143 | 3_prime_UTR_variant |
| ZNF484 | 3:41895871 * | 0.375, 0.071, 0.429 | 0.3, 0.0, 0.333 | 3_prime_UTR_variant |
| CRIPT | 3:93916337 * | 0.438, 0.4, 0.333 | 0.4, 0.333, 0.25 | splice_region_variant&intron_variant |
| ENSSSCG00000021180 | 4:36847416 * | 0.563, 0.571, 0.333 | 0.375, 0.435, 0.333 | intron_variant |
| RDH10 | 4:62570908 | 0.0, 0.571, 0.375 | 0.577, 0.543, 0.391 | 3_prime_UTR_variant |
| SFT2D2 | 4:82714164 | 0.143, 0.214, 0.333 | 0.25, 0.222, 0.0 | intron_variant |
| SFT2D2 | 4:82714165 | 0.5, 0.462, 0.667 | 0.5, 0.667, 0.0 | intron_variant |
| SFT2D2 | 4:82714213 * | 0.545, 0.609, 0.6 | 0.214, 0.5, 0.308 | intron_variant |
| SFT2D2 | 4:82714216 * | 0.667, 0.227, 0.182 | 0.267, 0.25, 0.308 | intron_variant |
| SFT2D2 | 4:82714221 * | 0.538, 0.364, 0.167 | 0.176, 0.304, 0.2 | intron_variant |
| SFT2D2 | 4:82714222 * | 0.154, 0.091, 0.0 | 0.235, 0.167, 0.067 | intron_variant |
| ATF6 | 4:88593317 * | 0.4, 0.667, 0.2 | 0.364, 0.25, 0.333 | 3_prime_UTR_variant |
| ENSSSCG00000040782 | 4:98777743 | 0.125, 0.182, 0.4 | 0.0, 0.636, 0.111 | downstream_gene_variant |
| ENSSSCG00000040782 | 4:98777751 | 0.385, 0.5, 0.667 | 0.333, 0.375, 0.444 | downstream_gene_variant |
| KIAA1551 | 5:42263049 | 0.2, 0.0, 0.143 | 0.167, 0.364, 0.0 | downstream_gene_variant |
| KIAA1551 | 5:42263098 | 0.333, 0.222, 0.143 | 0.182, 0.0, 0.0 | downstream_gene_variant |
| ENSSSCG00000027085 | 6:39367588 * | 0.286, 0.259, 0.158 | 0.261, 0.4, 0.222 | 3_prime_UTR_variant |
| CATSPERG | 6:47279285 | 0.091, 0.25, 0.0 | 0.429, 0.111, 0.105 | intron_variant |
| ENSSSCG00000033351 | 6:62013919 * | 0.25, 0.25, 0.286 | 0.111, 0.0, 0.071 | 3_prime_UTR_variant |
| PLOD1 | 6:72021487 * | 0.286, 0.25, 0.25 | 0.455, 0.375, 0.25 | downstream_gene_variant |
| PLA2G7 | 7:41487728 | 0.059, 0.2, 0.059 | 0.286, 0.0, 0.111 | 3_prime_UTR_variant |
| FCF1 | 7:97961485 | 0.2, 0.0, 0.375 | 0.5, 0.429, 0.333 | downstream_gene_variant |
| NDUFC1 | 8:87702830 | 0.182, 0.0, 0.176 | 0.333, 0.125, 0.333 | 3_prime_UTR_variant |
| PPP1R15B | 9:65058149 | 0.346, 0.393, 0.344 | 0.373, 0.221, 0.342 | 3_prime_UTR_variant |
| PPP1R15B | 9:65058152 * | 0.156, 0.226, 0.121 | 0.25, 0.107, 0.158 | 3_prime_UTR_variant |
| AAED1 | 10:25443226 * | 0.217, 0.194, 0.333 | 0.167, 0.139, 0.077 | 3_prime_UTR_variant |
| AAED1 | 10:25443245 * | 0.36, 0.385, 0.522 | 0.458, 0.395, 0.263 | 3_prime_UTR_variant |
| RSAD1 | 12:26775820 | 0.0, 0.111, 0.0 | 0.333, 0.143, 0.222 | downstream_gene_variant |
| ENSSSCG00000011234 | 13:18421001 * | 0.111, 0.2, 0.143 | 0.167, 0.077, 0.222 | intron_variant |
| GFM1 | 13:98408126 | 0.25, 0.0, 0.429 | 0.333, 0.222, 0.25 | upstream_gene_variant |
| GFM1 | 13:98408170 | 0.0, 0.077, 0.0 | 0.111, 0.273, 0.143 | upstream_gene_variant |
| GFM1 | 13:98408190 | 0.375, 0.292, 0.333 | 0.111, 0.214, 0.143 | upstream_gene_variant |
| ENSSSCG00000031589 | 14:60792171 | 0.167, 0.333, 0.333 | 0.182, 0.0, 0.0 | downstream_gene_variant |
| ZNF277 | 18:33313035 | 0.143, 0.333, 0.429 | 0.2, 0.286, 0.182 | intron_variant |
| ZNF277 | 18:33313036 * | 0.286, 0.0, 0.429 | 0.0, 0.286, 0.231 | intron_variant |
| ENSSSCG00000023820 | 18:50580239 * | 0.571, 0.0, 0.0 | 0.273, 0.313, 0.179 | downstream_gene_variant |
| ENSSSCG00000023820 | 18:50580258 * | 0.571, 0.364, 0.6 | 0.625, 0.688, 0.318 | downstream_gene_variant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paukszto, L.; Mikolajczyk, A.; Jastrzebski, J.P.; Majewska, M.; Dobrzyn, K.; Kiezun, M.; Smolinska, N.; Kaminski, T. Transcriptome, Spliceosome and Editome Expression Patterns of the Porcine Endometrium in Response to a Single Subclinical Dose of Salmonella Enteritidis Lipopolysaccharide. Int. J. Mol. Sci. 2020, 21, 4217. https://doi.org/10.3390/ijms21124217
Paukszto L, Mikolajczyk A, Jastrzebski JP, Majewska M, Dobrzyn K, Kiezun M, Smolinska N, Kaminski T. Transcriptome, Spliceosome and Editome Expression Patterns of the Porcine Endometrium in Response to a Single Subclinical Dose of Salmonella Enteritidis Lipopolysaccharide. International Journal of Molecular Sciences. 2020; 21(12):4217. https://doi.org/10.3390/ijms21124217
Chicago/Turabian StylePaukszto, Lukasz, Anita Mikolajczyk, Jan P. Jastrzebski, Marta Majewska, Kamil Dobrzyn, Marta Kiezun, Nina Smolinska, and Tadeusz Kaminski. 2020. "Transcriptome, Spliceosome and Editome Expression Patterns of the Porcine Endometrium in Response to a Single Subclinical Dose of Salmonella Enteritidis Lipopolysaccharide" International Journal of Molecular Sciences 21, no. 12: 4217. https://doi.org/10.3390/ijms21124217
APA StylePaukszto, L., Mikolajczyk, A., Jastrzebski, J. P., Majewska, M., Dobrzyn, K., Kiezun, M., Smolinska, N., & Kaminski, T. (2020). Transcriptome, Spliceosome and Editome Expression Patterns of the Porcine Endometrium in Response to a Single Subclinical Dose of Salmonella Enteritidis Lipopolysaccharide. International Journal of Molecular Sciences, 21(12), 4217. https://doi.org/10.3390/ijms21124217








