Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets
Abstract
1. Introduction
2. Results and Discussion
2.1. Computational Analysis
2.1.1. Nuclei Segmentation and Image Correction
2.1.2. Feature Extraction
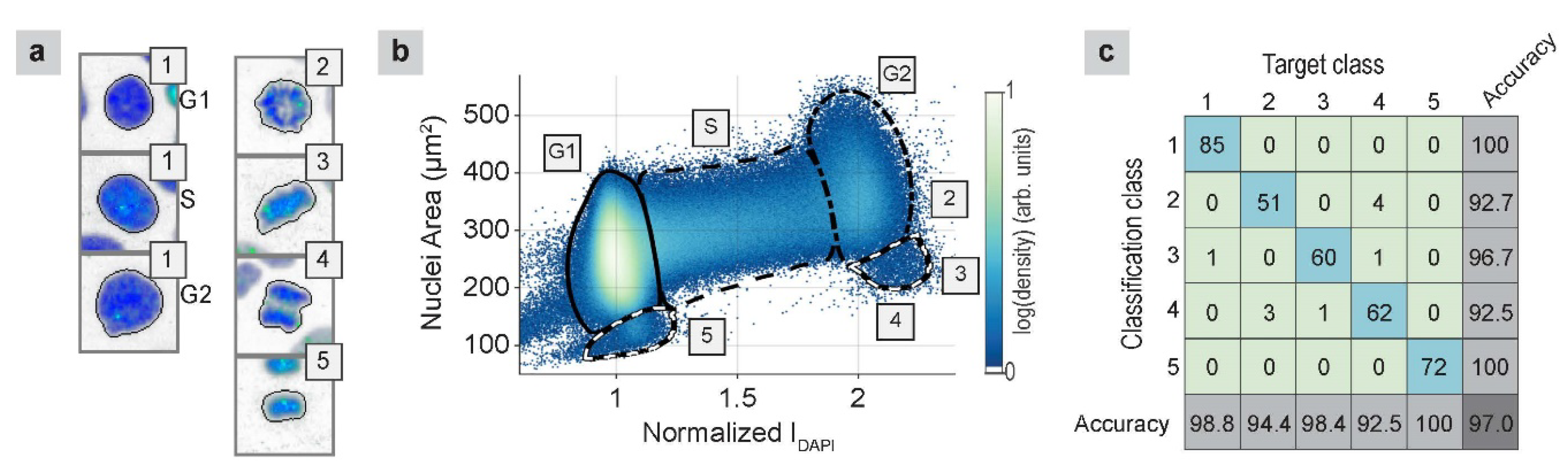
2.1.3. Cell-Cycle Classification
2.1.4. Damage Quantification
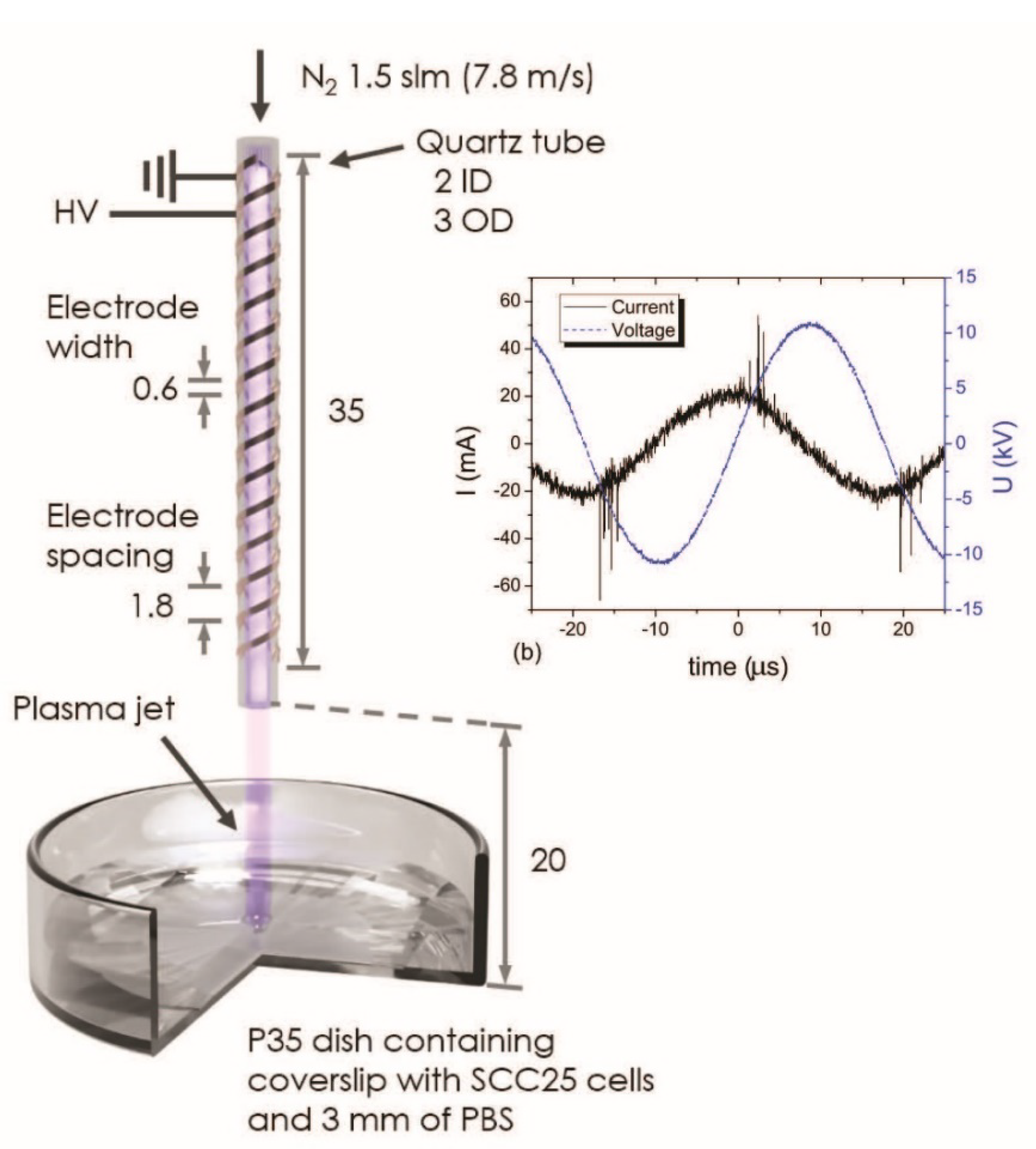
2.2. APPJ Irradiation
2.2.1. APPJ-Irradiated Malignant Cells
2.2.2. APPJ-Irradiated Nonmalignant Cells
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Extracted Features

| Feature Group | Feature Name |
|---|---|
| Shape | Area Major axis length Minor axis length Eccentricity solidity form factor Fourier descriptors (20) |
| Intensity | Integrated mean deviation Kurtosis |
| Radial intensity (N3 W4) | Mean Standard deviation |
| Haralick texture (3, 7) | Contrast Correlation Difference entropy Difference variance Energy Entropy Information measures correlation 1 Information measures correlation 2 Inverse difference moment Sum average Sum entropy Sum of squares variance Sum variance |
| Granularity | ---- |
Appendix B. Cell Culture Medium Recipes
Appendix B.1. SCC25 Cell Culture Medium: ~500 mL
- Dulbecco’s modified Eagle medium (DMEM): 250 mL;
- Ham’s nutrient mixture F-12 (F-12): 250 mL;
- Fetal bovine serum (FBS): 50 mL;
- Penicillin streptomycin (Pen Strep): 5 mL;
- Amphotericin B: 0.5 mL.
Appendix B.2. OKF6/T Cell Culture Medium: ~500 mL
- Keratinocyte-serum free medium (SFM): 500 mL;
- Epidermal growth factor 1-52 (EGF 1-53, 0.03 μg/μL): 3.3 μL;
- Bovine pituitary extract (BPE, 10 mg/mL): 1.25 mL;
- Calcium chloride (CaCl2, 2M): 77.5 μL;
- Penicillin streptomycin (Pen Strep): 5 mL.
Appendix C. Cell Culture Procedure
Appendix C.1. Primary Culture
- Remove the cell culture medium in the p100 dish and wash the cells with PBS (1X) twice.
- Add 12 mL fresh medium in the dish.
- Return the dish to the incubator.
Appendix C.2. Subculture
- Remove cell culture medium in the p100 dish and wash the cells with PBS (1X) twice.
- Add trypsin 2 mL (2 mL is enough for covering the surface of a p100 dish) and transfer the cell dish into an incubator for 3 min.
- Observe the cells under a microscope to check if the cells are detached from the dish surface. If not, prolong the incubation time or tab the side of the dish gently to expedite cell detachment.
- Add 4 mL fresh medium to the dish (make sure this amount is at least twice the volume of the added trypsin). Disperse the medium by pipetting the cell suspension over the cell layer surface several times.
- Harvest all the cell suspension into a 15 mL centrifuge tube.
- Centrifuge the tube at 1200 rpm for 2 min.
- Remove the supernatant above the cell pellet. Add 4 mL fresh medium, resuspend the cell pellet by pipetting and mixing.
- Add 11.5 mL fresh medium in a new p100 dish.
- Distribute 0.5 mL cells suspension into the new dish. As a result, the cells are seeded with 1:8 ratio in 12 mL medium in the p100 dish. The seeding ratio can be varied according to experimental needs. With 1:8 ratio dilution, SCC25 cells usually become confluent in approximately 4 days, and OKF6/T in approximately 6 days.
- Move the dish in four directions (forward, backward, left, and right) horizontally several times before transferring it to the incubator. This procedure will facilitate the cells to be seeded uniformly throughout the whole dish area.
Appendix D. Freezing and Thawing Cell Procedures
Appendix D.1. Freezing
- Conduct the first 6 steps in Appendix C.2.
- Remove the supernatant above the cell pellet. Add 1.2 mL prewarmed freezing medium, resuspend the cell pellet by pipetting and mixing.
- Dispense the cell suspension in two cryogenic storage vials with 0.5 mL each.
- Place the vials in the isopropanol chamber and store it at −80 °C overnight.
- Transfer the vials to a storage unit in a liquid nitrogen tank.
Appendix D.2. Thawing
- Prepare a p100 dish with 10 mL fresh prewarmed cell culture medium in a sterilized laminar flow hood.
- Thaw the frozen cells rapidly (<1 min) by gently swirling the vial in a 37 °C water bath until the ice remains a little bit in the vial. At this step, a face mask or goggles are required in addition to gloves for PPE for the hazard of vial explosion.
- Sterilize the vial surface with 70% ethanol before transferring to the hood.
- Transfer the cells into the prepared dish filled with fresh medium with micropipette. Add 10 mL more fresh medium to the dish and gently mix with the cells.
- Move the dish in four directions horizontally several times before transferring it to the incubator.
Appendix E. Immunofluorescence Staining Procedures
Appendix E.1. Single Antibody (AB) Staining
- (1)
- Wash cells with PBS (1X) twice.
- (2)
- Fix the cells by adding 1 mL PFA (4%) to the dish for 20 min.
- (3)
- Remove PFA to a biowaste container. Since PFA is toxic, it should not be discarded with other conventional biowaste solutions.
- (4)
- Rinse the cells with PBS (1X) twice.
- (5)
- Permeabilize the cells with Triton X-100 (0.3%) for 5 min.
- (6)
- Remove the Triton X-100 and wash the cells with PBS (1X) three times.
- (7)
- Apply blocking solution BSA (3%) to the cells for 1 h.
- (8)
- Prepare primary AB solution. For example, anti-phospho-histone H2AX needs to be diluted with a ratio of 1:250 in 3% BSA. The amount of antibody needed can be calculated as follows:
- (9)
- Prepare a flat parafilm sheet as a platform for AB incubation. The film can be placed at the bottom inside a square plastic container (e.g., 10 × 10 × 15 mm square petri dish with lid, SKS Science Products). The inside of the lid is covered with a wet Kimwipe tissue, which will facilitate maintaining the moisture during AB incubation process.
- (10)
- Carefully add 75 μL primary AB each at a set of dispersed locations (the number of locations equals to Nsample) on the parafilm avoiding bubbles while pipetting.
- (11)
- Take out the coverslips immersed in BSA from the dishes.
- (12)
- Flip the coverslips enabling the face of the coverslip that having cells facing down towards the parafilm. Rest one side edge of the coverslip on the film, then carefully lower down the other side of the coverslip, till the cells on the entire coverslip are in contact with the solution. Make sure there are no bubbles in between the coverslip and the parafilm.
- (13)
- After finishing the previous step for all the samples, put on the wet-tissue-covered lid. Transfer the container to a 4 °C room overnight.
- (14)
- Fill coverglass staining jars with 1X PBS. Each jar can contain four coverslips vertically.
- (15)
- Transfer the coverslips from the container to the staining jars. Make sure to record the orientation of the coverslip faces that have cells.
- (16)
- Remove the PBS inside the jars. Fill the jars with fresh PBS and put the jars on a shaker (Orbit shaker, Barnstead Lab-Line, 600 rpm) for 5 min. Repeat this step one more time.
- (17)
- Prepare secondary AB solution. For example, Alexa Fluor 488 goat anti-mouse needs to be diluted with a ratio of 1:400 in 3% BSA. The amount of the antibody needed can be calculated in the same method as Equation (E1) with a dilution factor of 400. During preparation, light exposure to the secondary AB must to be kept as short as possible.
- (18)
- Repeat step (9), (10) with secondary AB, (11) with taking out coverslips immersed in PBS from the jars, and (12).
- (19)
- After finishing the previous step for all samples, close the container with the wet-tissue-covered lid. Keep the container in dark for 20 min.
- (20)
- Repeat step (15). Similar as the step (16), wash the coverslips in PBS on a shaker four times with 5 min each. Repeat washing the slides once with reverse osmosis (RO) water on a shaker for 5 min. Keep the entire washing process in dark.
- (21)
- Take out the coverslips from the jars. Place the coverslips on a Kimwipe tissue with the cells facing up. Air dry the coverslips in dark.
- (22)
- Prepare glass microscope slides with 20 μL DAPI droplets on top. One DAPI drop per slide is preferred. Be careful to protect DAPI from light.
- (23)
- Repeat step (12) for staining the cells with DAPI solution. Press the coverslips on the slides vertically to remove extra DAPI with Kimwipe tissues.
- (24)
- If the DAPI solution used is a soft-mounting medium, apply clear nail polish on four edges of the coverslips to seal the slides. If the DAPI used is a hard-mounting medium, no further sealing process is needed.
- (25)
- Store the slides in a slide storage box in a 4 °C room in dark.
Appendix E.2. Double Antibody Staining
- (26)
- Prepare AB solution with two primary ABs. For example, anti-phospho-histone H2AX and cleaved caspase-3 antibody need to be diluted in 3% BSA with a ratio of 1:250 and 1:400, respectively. The amount of antibody can be calculated as follows:
- (27)
- Prepare AB solution with two secondary ABs. For example, both Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit need to be diluted in 3% BSA with the ratio of 1:400. The amount of each antibody can be calculated in the same method as Equations (4) and (5) with a dilution factor of 400.
Appendix E.3. S Phase Cell Staining with Click-iT EdU Alexa Fluor 647 Imaging Kit and Single AB
- (28)
- Incubate cells with fresh medium containing 10 μM EdU for 2.5 h.
- (29)
- Wash cells with PBS (1X) twice.
- (30)
- Fix the cells by adding 1 mL PFA (4%) for 15 min.
- (31)
- Remove PFA to a biowaste container. Since PFA is toxic, it should not be discarded with other conventional biowaste solutions.
- (32)
- Wash cells with 1 mL 3% BSA twice.
- (33)
- Permeabilize the cells with Triton X-100 (0.3%) for 20 min.
- (34)
- Prepare the reaction buffer additive.
- (35)
- Prepare the reaction cocktail according to the manufacture protocol [48] by adding the ingredients in the listed order. This cocktail solution should be used within 15 min of preparation.
- (36)
- Wash cells with 1 mL 3% BSA twice.
- (37)
- Add 500 μL reaction cocktail to each cell dish, incubate for 30 min in dark.
- (38)
- Wash cells with 1 mL 3% BSA once.
- (39)
- Follow single cell staining steps (8)−(25). If no AB staining needed (only EdU staining), proceed with steps (21)−(25).
References
- Hou, J.; Ma, J.; Yu, K.N.; Li, W.; Cheng, C.; Bao, L.; Han, W. Non-thermal plasma treatment altered gene expression profiling in non-small-cell lung cancer A549 cells. BMC Genom. 2015, 16, 435. [Google Scholar] [CrossRef]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’Connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- Gumbel, D.; Bekeschus, S.; Gelbrich, N.; Napp, M.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold Atmospheric Plasma in the Treatment of Osteosarcoma. Int. J. Mol. Sci. 2017, 18, 2004. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Klas, M.; Liu, Y.; Stack, M.S.; Ptasinska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 233703. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef]
- Akter, M.; Jangra, A.; Choi, A.S.; Choi, E.H.; Han, I. Non-Thermal Atmospheric Pressure Bio-Compatible Plasma Stimulates Apoptosis via p38/MAPK Mechanism in U87 Malignant Glioblastoma. Cancers 2020, 12, 245. [Google Scholar] [CrossRef]
- Liu, J.-R.; Wu, Y.-M.; Xu, G.-M.; Gao, L.-G.; Ma, Y.; Shi, X.-M.; Zhang, G.-J. Low-temperature plasma induced melanoma apoptosis by triggering a p53/PIGs/caspase-dependent pathway in vivo and in vitro. J. Phys. D Appl. Phys. 2019, 52, 315204. [Google Scholar] [CrossRef]
- Chernets, N.; Kurpad, D.S.; Alexeev, V.; Rodrigues, D.B.; Freeman, T.A. Reaction Chemistry Generated by Nanosecond Pulsed Dielectric Barrier Discharge Treatment is Responsible for the Tumor Eradication in the B16 Melanoma Mouse Model. Plasma Process. Polym. 2015, 12, 1400–1409. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of Indirect Nonequilibrium Atmospheric Pressure Plasma on Anti-Proliferative Activity against Chronic Chemo-Resistant Ovarian Cancer Cells in Vitro and in Vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Piroozmand, S.; Soleimani, N.; Jalali Faharani, N.; Ghomi, H.; Fotovat Eskandari, H.; Sharifi, A.M.; Mirpour, S.; Eftekhari, M.; Nikkhah, M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016, 6, 29048. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.R.W.; Ostrikov, K.K. The Emerging Role of Gas Plasma in Oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Craniomaxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Pranda, M.A.; Murugesan, B.J.; Knoll, A.J.; Oehrlein, G.S.; Stroka, K.M. Sensitivity of tumor versus normal cell migration and morphology to cold atmospheric plasma-treated media in varying culture conditions. Plasma Process. Polym. 2020, 17, 1900103. [Google Scholar] [CrossRef]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—a review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauere, G.; Metelmanng, P.; Seebauer, C. Side Effects in Cold Plasma Treatment of Advanced Oral Cancer—Clinical Data and Biological Interpretation. Clin. Plasma Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Jablonowski, L.; Kocher, T.; Schindler, A.; Muller, K.; Dombrowski, F.; von Woedtke, T.; Arnold, T.; Lehmann, A.; Rupf, S.; Evert, M.; et al. Side Effects by Oral Application of Atmospheric Pressure Plasma on the Mucosa in Mice. PLoS ONE 2019, 14, e0215099. [Google Scholar] [CrossRef]
- Itooka, K.; Takahashi, K.; Izawa, S. Fluorescence microscopic analysis of antifungal effects of cold atmospheric pressure plasma in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 9295–9304. [Google Scholar] [CrossRef]
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarle, S.; Vergani, C.E. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef]
- Smolkova, B.; Lunova, M.; Lynnyk, A.; Uzhytchak, M.; Churpita, O.; Jirsa, M.; Kubinova, S.; Lunov, O.; Dejneka, A. Non-Thermal Plasma, as a New Physicochemical Source, to Induce Redox Imbalance and Subsequent Cell Death in Liver Cancer Cell Line. Cell Physiol. Biochem. 2019, 52, 119–140. [Google Scholar] [CrossRef] [PubMed]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence microscopy. Cold Spring Harb. Protoc. 2014, 10. [Google Scholar] [CrossRef] [PubMed]
- Blasi, T.; Hennig, H.; Summers, H.D.; Theis, F.J.; Cerveira, J.; Patterson, J.O.; Davies, D.; Filby, A.; Carpenter, A.E.; Rees, P. Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Kapaldo, J.; Han, X.; Mery, D. Seed-Point Detection of Clumped Convex Objects by Short-Range Attractive Long-Range Repulsive Particle Clustering. arXiv 2018, arXiv:1804.04071. [Google Scholar]
- Kapaldo, J. Seed-Point Based Geometric Partitioning of Nuclei Clumps. arXiv 2018, arXiv:1804.04549. [Google Scholar]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 6, 610–621. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Zahn, C.T.; Roskies, R.Z. Fourier Descriptors for Plane Closed Curves. IEEE Trans. Comput. 1972, 100, 269–281. [Google Scholar] [CrossRef]
- Connolly, A.J.; Genovese, C.; Moore, A.W.; Nichol, R.C.; Schneider, J.; Wasserman, L. Fast Algorithms and Efficient Statistics: Density Estimation in Large Astronomical Datasets. arXiv 2000, arXiv:astro-ph/0008187. [Google Scholar]
- Graves, D.B. Oxy-nitroso shielding burst model of cold atmospheric plasma therapeutics. Clin. Plasma Med. 2014, 2, 38–49. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Stack, M.S.; Ptasinska, S. 3D Mapping of plasma effective areas via detection of cancer cell damage induced by atmospheric pressure plasma jets. J. Phys. Conf. Ser. 2014, 565, 012011. [Google Scholar] [CrossRef]
- Klas, M.; Ptasinska, S. Characteristics of N2 and N2/O2 atmospheric pressure glow discharges. Plasma Sources Sci. Technol. 2013, 22, 025013. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA-a review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Obrusnik, A.; Jones, B.T.; Zajickova, L.; Ptasinska, S. Effect of Additive Oxygen on the Reactive Species Profile and Microbicidal Property of a Helium Atmospheric Pressure Plasma Jet. Plasma Process. Polym. 2016, 13, 1089–1105. [Google Scholar] [CrossRef]
- Kapaldo, J.; Han, X.; Ptasinska, S. Shielding-gas-controlled atmospheric pressure plasma jets: Optical emission, reactive oxygen species, and the effect on cancer cells. Plasma Process. Polym. 2019, 16, 1800169. [Google Scholar] [CrossRef]
- Gweon, B.; Kim, M.; Bee Kim, D.; Kim, D.; Kim, H.; Jung, H.; Shin, J.H.; Choe, W. Differential responses of human liver cancer and normal cells to atmospheric pressure plasma. Appl. Phys. Lett. 2011, 99, 063701. [Google Scholar] [CrossRef]
- Panngom, K.; Baik, K.Y.; Nam, M.K.; Han, J.H.; Rhim, H.; Choi, E.H. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013, 4, e642. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; Garland Science, Taylor & Francis Group, LLC.: New York, NY, USA, 2014. [Google Scholar]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Life Technologies Corporation. Click-iT EdU Imaging Kits (Mannual); Life Technologies Corp.: Carlsbad, CA, USA, 2011. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Kapaldo, J.; Liu, Y.; Stack, M.S.; Alizadeh, E.; Ptasinska, S. Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. Int. J. Mol. Sci. 2020, 21, 4127. https://doi.org/10.3390/ijms21114127
Han X, Kapaldo J, Liu Y, Stack MS, Alizadeh E, Ptasinska S. Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. International Journal of Molecular Sciences. 2020; 21(11):4127. https://doi.org/10.3390/ijms21114127
Chicago/Turabian StyleHan, Xu, James Kapaldo, Yueying Liu, M. Sharon Stack, Elahe Alizadeh, and Sylwia Ptasinska. 2020. "Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets" International Journal of Molecular Sciences 21, no. 11: 4127. https://doi.org/10.3390/ijms21114127
APA StyleHan, X., Kapaldo, J., Liu, Y., Stack, M. S., Alizadeh, E., & Ptasinska, S. (2020). Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. International Journal of Molecular Sciences, 21(11), 4127. https://doi.org/10.3390/ijms21114127






