Abstract
Production of wheat-alien disomic addition lines is of great value to the exploitation and utilization of elite genes originated from related species to wheat. In this study, a novel wheat-Aegilops biuncialis 5Mb disomic addition line WA317 was characterized by in situ hybridization (ISH) and specific-locus amplified fragment sequencing (SLAF-seq) markers. Compared to its parent Chinese Spring (CS), the glumes of WA317 had black color and were difficult to remove after harvesting, suggesting chromosome 5Mb carried gene(s) related to glume development and Triticeae domestication process. A total of 242 Ae. biuncialis SLAF-based markers (298 amplified patterns) were developed and further divided into four categories by Ae. biuncialis Y17, Ae. umbellulata Y139 and Ae. comosa Y258, including 172 markers amplifying the same bands of U and M genome, six and 102 markers amplifying U-specific and M-specific bands, respectively and eighteen markers amplifying specific bands in Y17. Among them, 45 markers had the specific amplifications in WA317 and were 5Mb specific markers. Taken together, line WA317 with tenacious and black glumes should serve as the foundation for understanding of the Triticeae domestication process and further exploitation of primitive alleles for wheat improvement. Ae. biuncialis SLAF-based markers can be used for studying syntenic relationships between U and M genomes as well as rapid tracking of U and M chromosomal segments in wheat background.
1. Introduction
Transferring desirable genes from wild relatives into common wheat is considered an efficient strategy for wheat genetic improvement. Wheat alien disomic addition lines are usually used as intermediate materials to further produce alien translocation and introgression lines for wheat breeding. On the other hand, alien disomic addition lines are commonly used for sorting alien chromosomes [1], homology comparison between wheat and alien relatives [2], chromosomal localization of alien genes [3] and development of molecular markers [4]. To date, a large number of wheat–alien chromosome addition lines and amphiploids/introgression lines have been obtained from crossing with wild species, including Secale cereale [5], Haynaldia villosa [6], Thinopyrum ponticum [7], Aegilops speltoides [8] and Agropyron cristatum [9].
Cytogenetic techniques have always been an important methodology of detecting wild relative introgressions [10,11]. New experimental protocols [12], oligonucleotide probes [13] and other innovations improved the procedures of the preparation of root tip cells and detecting probes, as well as the process of in situ hybridization. Despite this, molecular markers are considerably more popular in identifying alien chromosomes because of their high efficiency and throughput. Simple sequence repeats (SSR) [14,15], expressed sequence tag (EST) [16], sequence-tagged sites (STS) [17], cleaved amplification polymorphism sequence (CAPS) [18] and competitive allele-specific PCR (KASP) [19] markers have been successfully used to identify alien derivative lines in large numbers. Specific-locus amplified fragment sequencing (SLAF)-seq technology has several obvious advantages, including high throughput, high accuracy and low cost and provides an important tool for developing specific markers of wheat wild relatives, such as Thinopyrum elongatum 7E [20], Agropyron cristatum [21], Thinopyrum ponticum 4Ag [22], rye 4R Ku [23] and 6R Ku [24] chromosomes. These SLAF-based markers could be used for tracking specific alien chromosomal segments and alien genes in wheat background. To date, no Ae. biuncialis specific SLAF-based markers have been reported.
Modern common wheat varieties differ from their wild ancestors due to the acquisition of domestication traits. Discovering genes governing these traits will provide more insight into the domestication process and lead to further exploitation of primitive alleles for wheat improvement [25,26]. Threshability is mainly controlled by two genes—the major domestication gene Q and the homoeologous tenacious glume (Tg) genes. The Q gene on chromosome arm 5AL, a member of the APETALA2 (AP2) family of transcription factors, affects multiple agronomic traits, such as threshability, rachis fragility, plant height, spike length and glume morphology. Sharma et al. [27] mapped these major quantitative trait loci (QTL) loci through a population of recombinant inbred lines derived from a cross between durum and cultivated emmer. Tg gene was located on the homoeologous group 2, such as 2A in spelt [28], 2B in wild emmer [29], 2D in Ae. tauschii [30,31,32] and 2E in Lophopyrum elongatum [33]. Zhang et al. [34] demonstrated that Q governs threshability through extensive modification of wheat glumes including their structure, cell wall thickness and chemical composition. The glume color is an important taxonomic discriminator in wheat [35,36] and is associated with the crop’s adaptability [37,38]. The genes controlling the glume color are mainly located on the homoeologous group 1, including Rg-A1, Rg-B1 and Rg-D1 in common wheat and its related species [39]. Four alleles of Rg-A1 determine the red color (Rg-A1b), the black color (Rg-A1c and Rg-A1d) and the absence of color (Rg-A1a). Rg-B1b and Rg-D1b control the red color of glumes, while Rg-B1a and Rg-D1a do not impart color. The Rg gene expression in glumes is involved in the biosynthesis of flavonoid pigments phlobaphenes and/or 3-desoxyanthocyanidines [40]. In addition to the red and black glumes, smoky-grey glumes [41] and purple glumes [42] have been also mapped on chromosome 1D of Triticum aestivum and chromosome 2A of Triticum durum, respectively. A deeper comprehension of the genetic basis of glume traits (threshability and color) will be beneficial to understanding spike developmental mechanism in Triticeae species.
Aegilops genus comprises 11 diploid, 10 tetraploid and two hexaploid species [43] with various genomic compositions of the C, D, N, M, S and U genomes [44]. Most Aegilops species have been successfully introgressed into common wheat. Ae. biuncialis (2n = 4x = 28, UUMM) displays a series of agronomically useful traits including special high molecular weight glutenin subunits, disease resistance and drought and salt tolerance [45,46,47]. Molnár-Láng team reported seven wheat–Ae. biuncialis disomic addition lines (2Ub, 2Mb, 3Ub, 3Mb, 5Ub, 6Mb and 7Ub) in the background of winter wheat line Mv9kr1 [48,49]. During their research, Schneider et al. [50] screened two markers (GWM44 and GDM61) from 108 wheat SSR markers, which amplified specific PCR products from wheat-Ae. biuncialis 2Mb and 3Mb addition lines and Molnár et al. [51] mapped a series of gene-based conserved orthologous set (COS) markers to U and M genomes by wheat-Aegilops chromosome introgression lines. Zhou et al. [47] obtained five Ae. biuncialis 1Ub specific markers from 48 polymerase chain reaction (PCR)-based landmark unique gene (PLUG) markers and created a wheat–Ae.biuncialis 1Ub disomic addition line using T. aestivum cv. Chuannong 19 as receptor parent. To date, most Ae. biuncialis specific markers have originated from wheat markers and acquired low efficiency. New Ae. biuncialis markers need to be urgently developed to accelerate the process of theoretical research and elite gene utilization in wheat breeding. In this study, a new 5Mb addition line derived from the cross between T. aestivum cv. Chinese Spring and Ae. biuncialis was identified by in situ hybridization (ISH) and SLAF-based markers. Its agronomic performance was also evaluated.
2. Results
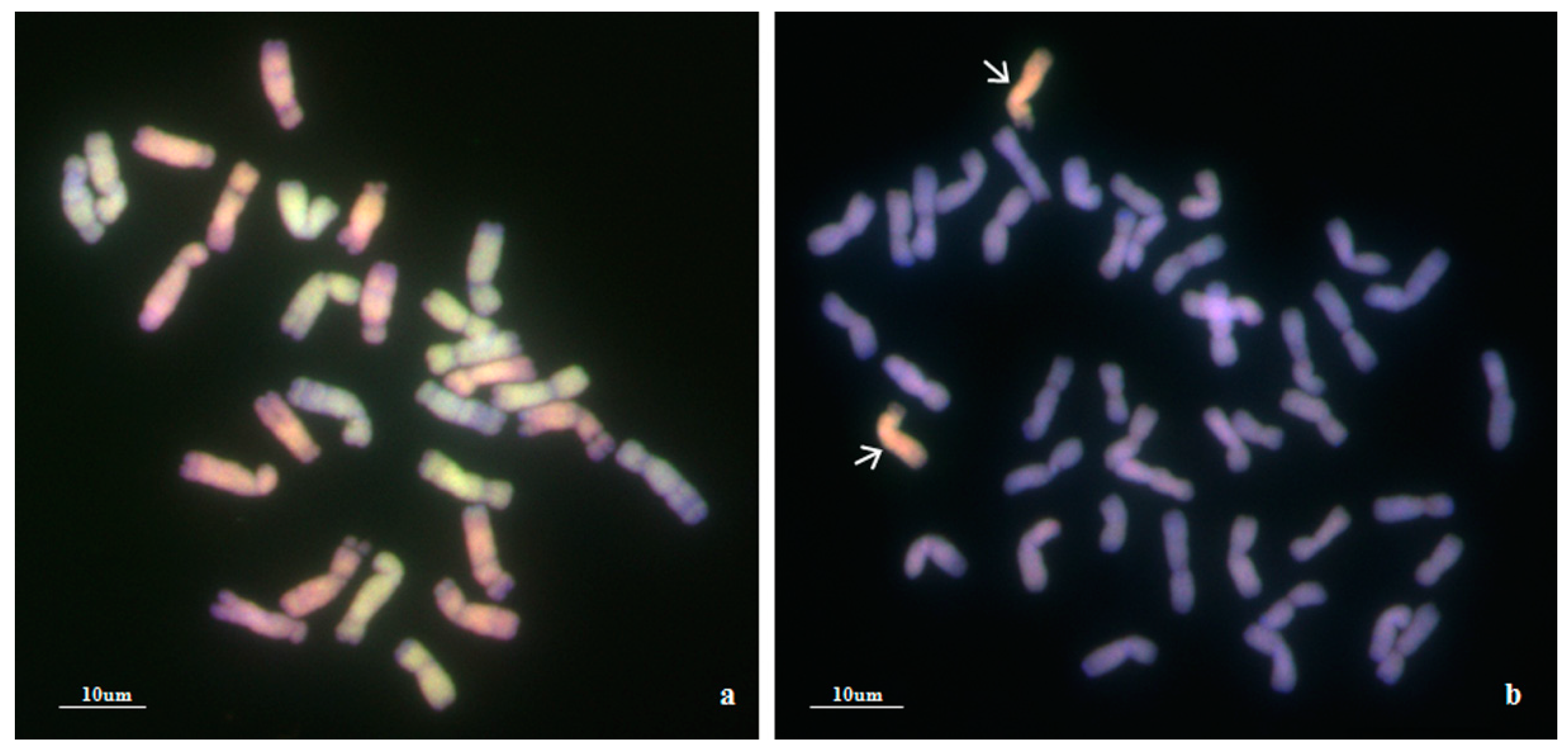
2.1. Genomic In Situ Hybridization (GISH) Analysis
Using Ae. biuncialis genome DNA as a probe, GISH detection was performed to verify the presence of Ae. biuncialis chromatin in WA317 (Figure 1). The result showed that WA317 had 44 chromosomes, including 42 wheat chromosomes stained blue by DAPI and a pair of intact chromosomes with red hybridization signal, which suggested that the alien chromosomes had successfully been introgressed into Chinese Spring (CS) background (Figure 1a,c). GISH analysis of the offspring plants from five consecutive generations of selfing WA317 further confirmed that it had high cytological stability. Therefore, WA317 was a genetically stable disomic addition line.
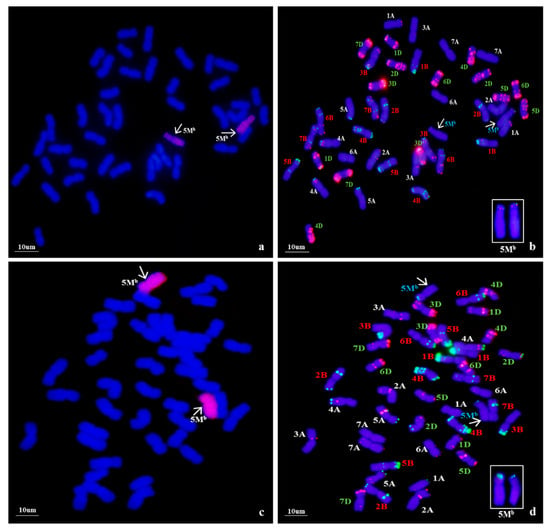
Figure 1.
Genomic in situ hybridization (GISH) and non-denaturing fluorescence in situ hybridization (ND-FISH) detection of mitotic chromosomes in the root tips of wheat-Ae. biuncialis 5Mb addition line WA317. The arrows indicate the 5Mb additional chromosome. (a,c) GISH detection of WA317 using Ae. biuncialis genome DNA as probe; (b) ND-FISH pattern of the corresponding slide using probes Oligo-pAs1.0 (red) and OligopSc119.2 (green); (d) ND-FISH pattern of the corresponding slide using probes Oligo-pTa535 (red) and OligopSc119.2 (green).
2.2. Non-Denaturing Fluorescence In Situ Hybridization (ND-FISH) Analysis
Following GISH analysis, ND-FISH analysis with three probes, Oligo-pSc119.2 and Oligo-pAs1.0 (or Oligo-pTa535), was used to characterize the Ae. biuncialis and wheat chromosomes (Figure 1). With Oligo-pAs1.0 and Oligo-pSc119.2 as probes, the additional chromosomes had both signals in the terminal end of the short arm (Figure 1b). The same hybridization pattern of Oligo-pSc119.2 was also seen on additional Ae. biuncialis chromosomes when labeled with Oligo-pSc119.2 and Oligo-pTa535 (Figure 1d). Meanwhile, the two chromosomes did not bear any Oligo-pTa535 hybridization signals. In addition, the arm ratio (L/S) was also a clue to identify the alien chromosome. In WA317, the arm ratio of Ae. biuncialis chromosomes was calculated to be 2.26.
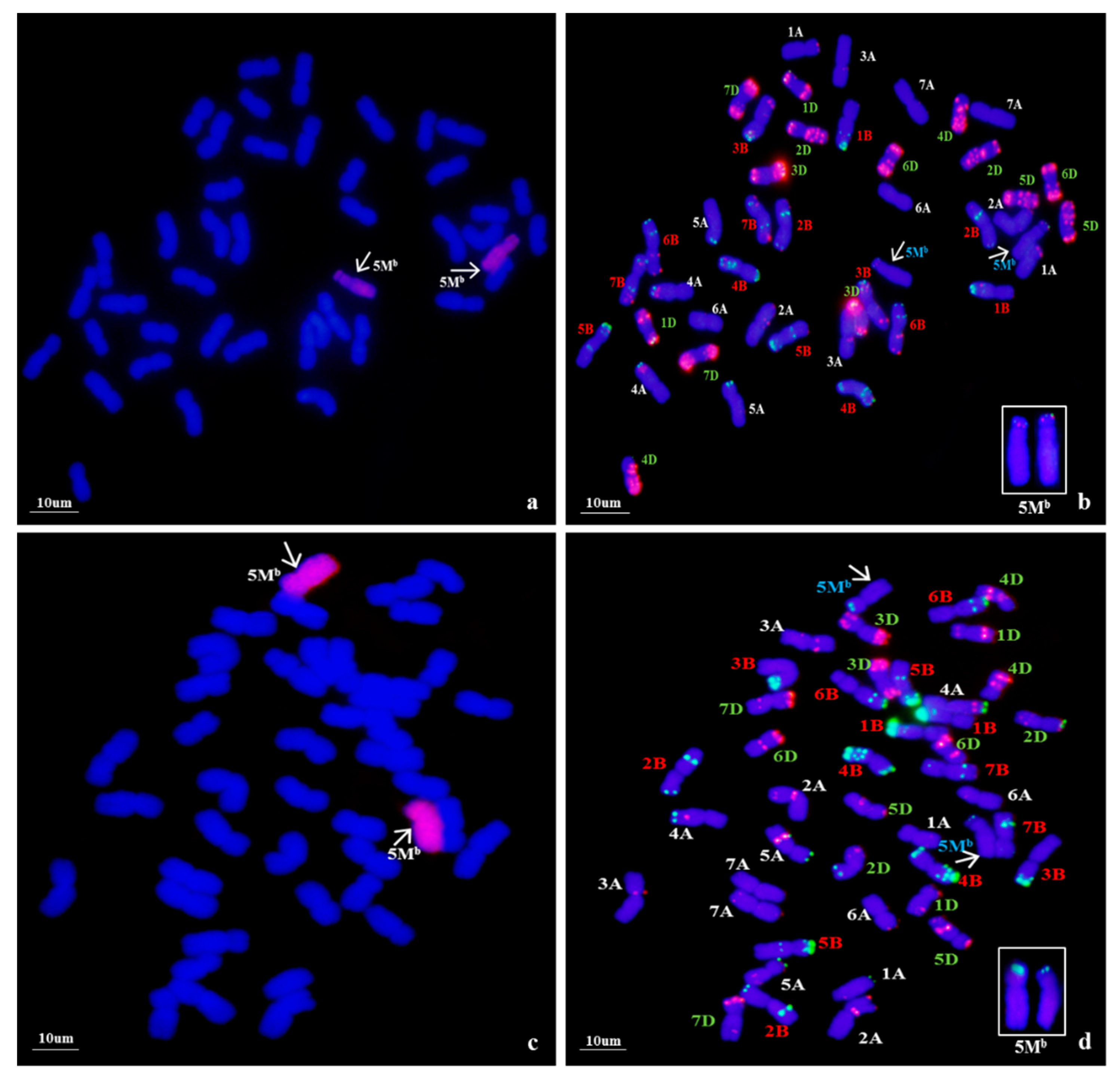
2.3. Multicolor GISH (mcGISH) Analysis
To confirm the genomic origin of the alien chromosomes of WA317, mcGISH was performed using Ae. umbellulata and Ae. comosa genomic DNA as probes (Figure 2). In Figure 2a, mcGISH could clearly distinguish the Ub (green) and Mb (red) genomes of Ae. biuncialis. In line WA317 (Figure 2b), the hybridization signal of alien chromosomes was consistent with that of Mb genome, indicating that a pair of Mb chromosomes were transferred into the wheat background. Among seven Mb chromosomes, the FISH pattern and arm ratio were closer to the characters of Ae. biuncialis 5Mb chromosome described by Wang et al. [52]. Therefore, WA317 was a wheat-Ae. biuncialis 5Mb addition line.
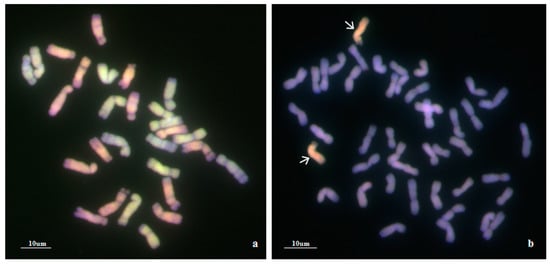
Figure 2.
mcGISH detection of mitotic chromosomes in the root tips of Ae. biuncialis and wheat–Ae. biuncialis 5Mb addition line WA317 using Ae. umbellulata and Ae. comosa genomic DNA as probes. (a) mcGISH of Ae. biuncialis, U and M chromosomes are green and red, respectively; (b) mcGISH of WA317, the additional chromosomes are red and wheat chromosomes are blue. Arrows indicate the alien chromosomes from Ae. biuncialis.
2.4. Development of Ae. Biuncialis Specific Markers and Molecular Detection of Line WA317
High-throughput sequencing identified a total of 643,071 effective SLAFs for Ae. biuncialis Y17. Sequence comparison showed that 9342 sequences had less than 50% homology with CS, which were designed to develop Ae. biuncialis specific markers. To date, a total of 600 primer pairs have been designed and amplified from Ae. biuncialis and CS. Among them, 242 markers showed Ae. biuncialis specific bands with a success rate up to 40.33% (Table S1).
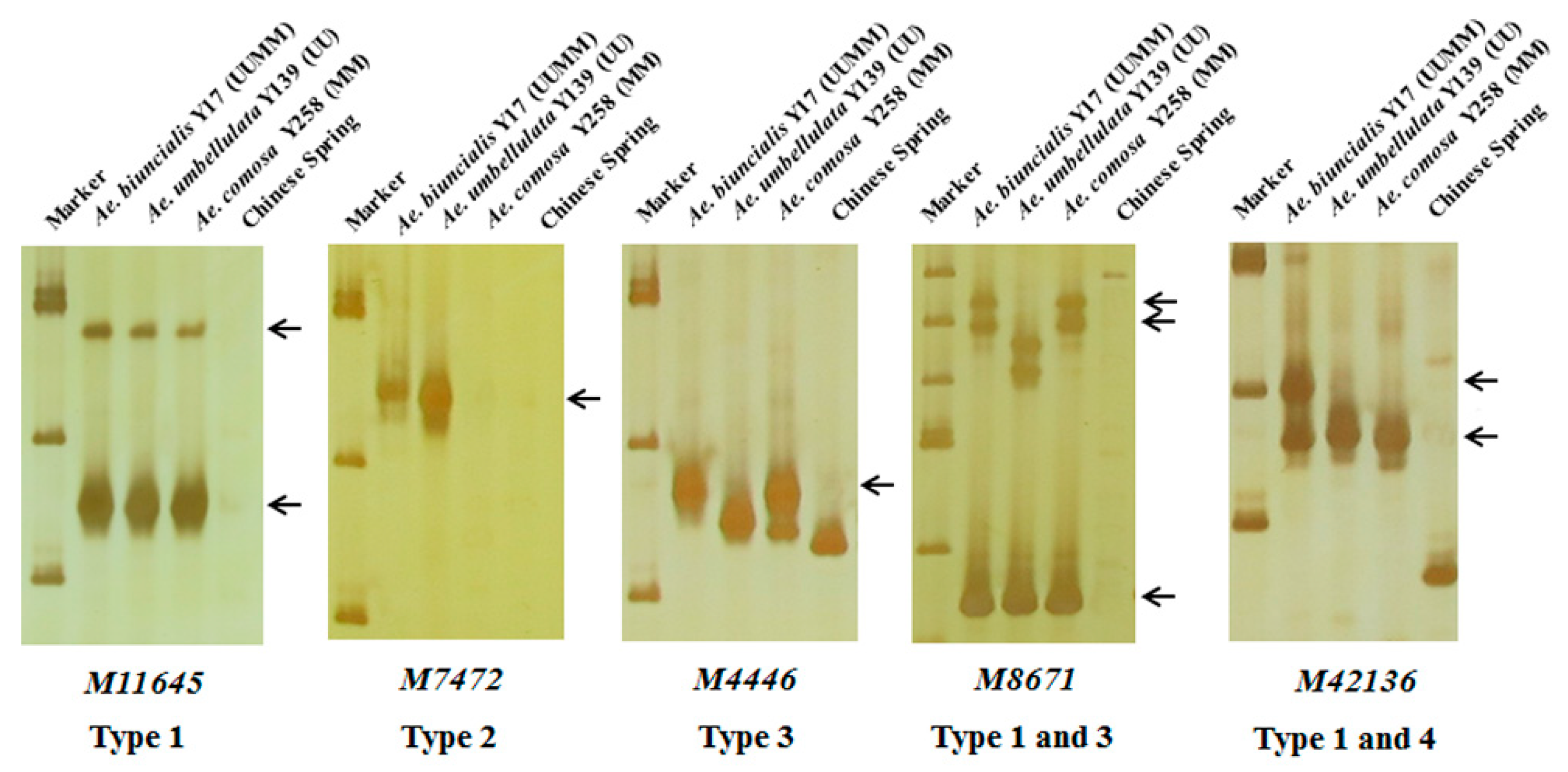
In order to further map these specific markers into U and/or M genomes, 242 markers were used to amplify DNAs of Ae. biuncialis Y17 (UUMM genome), Ae. umbellulata Y139 (UU genome), Ae. comosa Y258 (MM genome) and the negative control CS. The results showed that the 242 markers could amplify 298 specific bands and be divided into four categories (Table 1, Table S1, Figure 3). A total of 172 markers had the same amplified bands in Y17, Y139 and Y258, suggesting U and M genomes shared the specific bands (Type 1); six markers had the U-specific amplified bands in Y17 and Y139 but had no corresponding fragments in Y258 (Type 2); 102 markers amplified M-specific diagnostic bands in Y17 and Y258 but did not amplify the corresponding fragments in Y139 (Type 3); eighteen markers amplified the specific bands in Y17 (Type 4).

Table 1.
Detection of Ae. biuncialis accessions and line WA317 using Ae. biuncialis specific-locus amplified fragment sequencing (SLAF) markers.
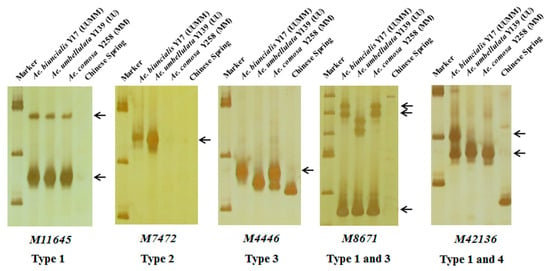
Figure 3.
Amplification patterns of Aegilops accessions using Ae. biuncialis SLAF markers M11645, M7472, M4446, M8671 and M42136. Type 1: the same amplified bands in Ae. biuncialis Y17 (UUMM), Ae. umbellulata Y139 (UU) and Ae. comosa Y258 (MM); Type 2: the U-specific amplified bands in Y17 and Y139; Type 3: the M-specific amplified bands in Y17 and Y258; Type 4: the specific amplified bands in Y17. Arrows indicate the specific bands of Aegilops accessions.
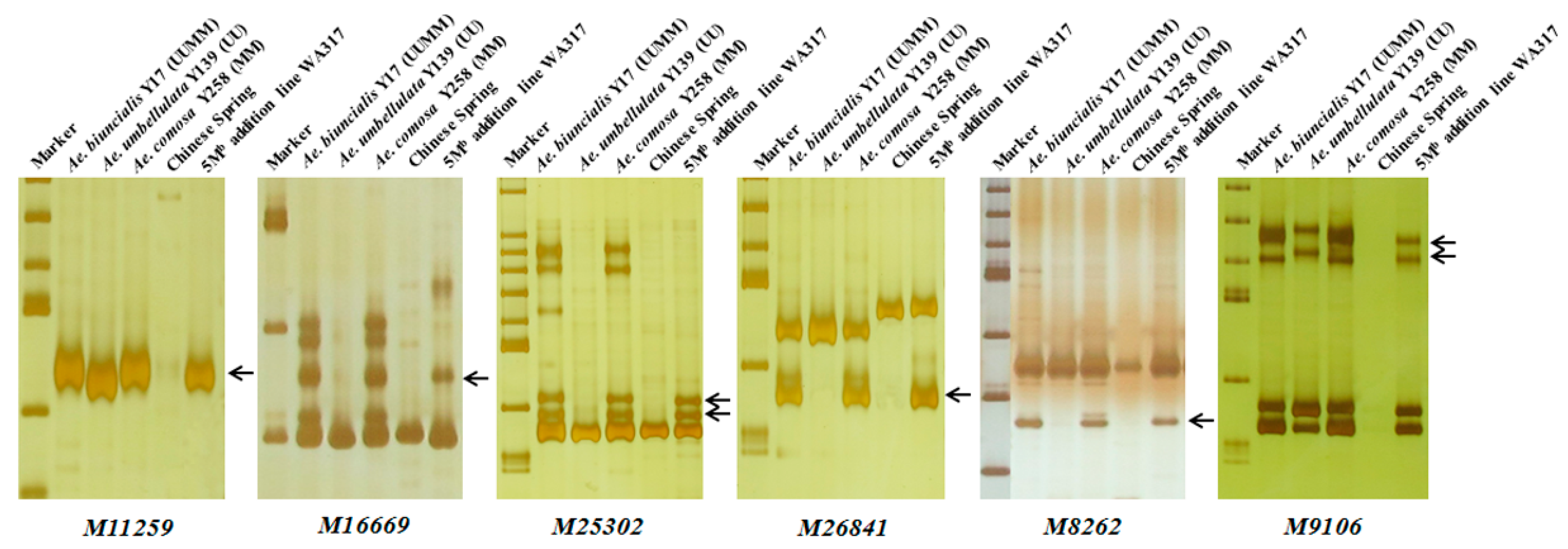
Using the 242 Ae. biuncialis specific markers to identify WA317, it was found that 45 markers showed specific bands in WA317 (Table 1, Table S1), of which, 37 markers amplified the same patterns in Y17, Y139, Y258 and WA317 (Type 1); two markers (M6201 and M90496) had the same amplified bands in Y17 and WA317, without the corresponding bands in Y139 and Y258 (Type 4). Six markers (M11259, M16669, M25302, M26841, M8262 and M9106) amplified the same bands in Y17, Y258 and WA317, excluding Y139 (Type 3) (Figure 4). These results further confirmed that a pair of Mb genome chromosomes were added to the CS background. These 45 markers could be used to identify the additional chromosome in WA317.
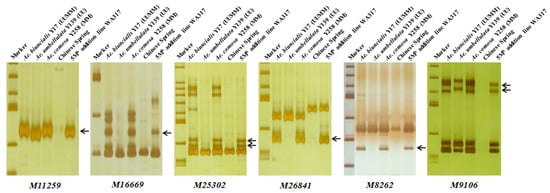
Figure 4.
Polymerase chain reaction (PCR) amplification of six markers M11259, M16669, M25302, M26841, M8262 and M9106 for detection of 5Mb in WA317. These markers had the same Mb-specific amplified bands in Y17, Y258 and WA317, which further confirmed that the alien chromosome in WA317 belonged to the Mb genome. Arrows indicate the M-specific bands in WA317.
2.5. The Agronomic Traits of Line WA317
The agronomic traits of WA317 were investigated in both 2017–2018 and 2018–2019 seasons. WA317 glumes grew gradually black and became tenacious during the grain-filling stage, compared to CS (Figure 5). As a result, the glumes of WA317 were difficult to remove after harvesting. In its parents, the glumes of Ae. biuncialis were tenacious and difficult to remove (Figure S1), while it was much easier to remove the glumes of CS. These results suggested that the 5Mb chromosome carried gene(s) related to glume development.

Figure 5.
Morphological traits of wheat–Ae. biuncialis 5Mb addition line WA317 and its parent CS. (a) Plants of Chinese Spring (CS) and WA317 (left and right), (b) spikes of CS and WA317 (left and right), (c) Spikelets of CS (c1) and WA317 (c2) after threshing.
The agronomic traits, including plant height, grain number per spike and thousand grain weight of WA317, were significantly different from its receptor parent CS, due to the alien addition of a pair of intact chromosomes (Table 2).

Table 2.
Agronomic traits of wheat-Ae. biuncialis 5Mb addition line WA317 and CS.
3. Discussion
The production of wheat-Ae. biuncialis disomic addition lines enables the study of the genetic effects of individual alien chromosomes in common wheat. In previous studies, eight wheat-Ae. biuncialis disomic addition lines (1Ub, 2Ub, 2Mb, 3Ub, 3Mb, 5Ub, 6Mb, 6Ub and 7Ub) were created in different wheat backgrounds [47,48,49,53]. These addition lines showed distinct agronomic traits, such as seed storage protein profile [47], disease resistance [54] and drought tolerance [53], which were of great importance to discovering genes carried by each Ae. biuncialis chromosome.
In this study, a new wheat-Ae. biuncialis 5Mb addition line was identified in the background of Chinese Spring based on in situ hybridization and SLAF markers. mcGISH enables the parental genomes to be discriminated in allopolyploid plants using different total genomic DNA as probes [55]. Molnár and Molnár-láng [56] clearly discriminated the Ub and Mb chromosomes in Ae. biuncialis and wheat-Ae. biuncialis amphiploids using the total genomic DNA of Ae. umbellulata and Ae. comosa as U and M genomic probes. In this paper, mcGISH showed that a pair of Mb chromosomes were introgressed into the CS background. This result was also confirmed by SLAF markers. Six markers (M11259, M16669, M25302, M26841, M8262 and M9106) could amplify M specific bands in Y17, Y258 and WA317 (Figure 4). The karyotypes of U and M genomes from Ae. biuncialis accessions could discriminate different chromosomes based on FISH probes [52]. The arm ratio was also a clue for identifying alien additional chromosomes. The additional chromosome in WA317 had both signals of pAs1.0 and pSc119.2 in the short arm. From 1Mb to 7Mb chromosomes, only the 5Mb short arm had both signals of pAs1.0 and pSc119.2 [52]. In addition, the L/S arm ratio of the alien chromosome was 2.26, which was near the result of 5Mb ratio described by Wang et al. [52]. Based on the above results, we concluded that the additional chromosome was 5Mb. The alien chromosome in WA317 had no obvious signal on the long arm, while the 5Mb long arm had the pAs1.0 signal in Wang et al. [52]. This difference might be the result of the genetic diversity of Ae. biuncialis accessions or other unknown events.
This addition line WA317 had obvious tenacious glumes, suggesting that Ae. biuncialis 5Mb chromosome carries gene(s) functioning like gene Q which is located on wheat chromosome 5A. Interestingly, the glume of wheat-Ae. biuncialis 5Mb addition line was significantly black compared to the receptor parent CS, implying that chromosome 5Mb would carry glume color related gene(s). In Triticeae species, most glume color genes were reported to locate on homoeologous group 1 [38]. Chromosome 1Mg of Ae. geniculata had a black glume color gene, wheat-Ae. geniculata 1Mg (1B) substitution line exhibited a darker color than 1Mg (1A) and 1Mg (1D) substitution line due to conditional epistasis [57]. We do not know whether the glume color change accompanied with the 5Mb addition line was related to the structural rearrangement of M genome during allotetraploid formation; the Ae. biuncialis chromosome 5Mb addition line could be useful for better understanding of the domestication process of Triticeae species.
Aegilops contribute to the evolution of cultivated wheat and are important sources of genes for wheat improvement. There exists wide genetic variation among Aegilops accessions. DNA clones (pSc119.2 and pAs1) display different hybridization signals in four Ae. biuncialis accessions and its two diploid progenitor species [48]. Molnár et al. [51,58] compared the syntenic relationships between U and M genomes among diploid (Ae. umbellulata and Ae. comosa) and allotetraploid (Ae. biuncialis and Ae. geniculata) Aegilops by COS markers and revealed more significant chromosome rearrangements in U genome than M genome, suggesting that Aegilops 4, 6 and 7 chromosomes have undergone clear structural rearrangements relative to wheat. Therefore, development of specific markers helps in investigation of the genetic constitutions of alien chromosomes, homoeologous relationships with wheat and allopolyploidization-related molecular processes, such as the P genome of Ae. cristatum chromosome [59] and E genome of Thinopyrum elongatum [60].
In this study, 242 Ae. biuncialis SLAF-based markers (298 amplified patterns) were developed and could be potentially used for tracking the U and M chromosomal segments in the wheat background. Among them, 172 markers (71.07%) amplified the same bands in Y17, Y139 and Y258, suggesting a high syntenic relationship between U and M genomes. Eighteen markers amplified the specific bands in Ae. biuncialis Y17 compared to Ae. umbellulata Y139 and Ae. comosa Y258, implying that the chromosome region in which the 18 corresponding markers were located might undergo chromosome rearrangement events during allopolyploidization. Few U and M specific markers were reported before [47,50,51], so these markers developed in this study can enrich U and M genome marker availability, facilitate the detection of U and M genomes in the wheat background and accelerate elite gene utilization in wheat breeding. It should be noted that there was only an Ae. biuncialis (Y17), an Ae. biuncialis (Y139) and an Ae. comosa accession (Y258) involved in this study. If more Aegilops accessions containing the U and/or M genome were used to identify by these markers, the results might be different to some extent, due to the genetic diversity and evolutionary chromosome rearrangements. This means that these Ae. biuncialis specific markers can serve as molecular tools to analyze the syntenic relationships of Aegilops species, which would eventually help deep understanding of U and M genomes. Moreover, a total of 45 markers has the specific amplifications in wheat-Ae. biuncialis 5Mb addition line, which could be used for tracking 5Mb chromosomal segments in the wheat background.
4. Materials and Methods
4.1. Plant Materials
The plant materials in this study included T. aestivum cv. ‘Chinese Spring’ (CS) (AABBDD), Ae. biuncialis accession Y17 (2n = 4X = 28, UbUbMbMb), Ae. umbellulata accession Y139 (2n = 18, UU), Ae. comosa accession Y258 (2n = 14, MM) and wheat–Ae. biuncialis 5Mb disomic addition line WA317 (2n = 44). The 5Mb addition line WA317 was a BC2F8 line derived from the cross between CS and Ae. biuncialis with the former as the recurrent parent. The creation procedure was as follows—Three F0 hybrid seeds were produced by distant hybridization of common wheat CS × Ae. biuncialis, two F1 hybrid plants survived and one F1 plant was successively backcrossed twice with CS as the male parent and then selfed. During this process, the plants were bagged to prevent any cross pollination. Aegilops accessions Y17, Y139, Y258 were kept at the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, China.
4.2. GISH Analysis
The mitotic chromosome spreads from root tip cells were prepared and observed as described by Liu et al. [61]. GISH was performed for detection of Ae. biuncialis chromatin in line WA317 as described by Liu et al. [61]. The Ae. biuncialis Y17 and CS genomic DNAs were utilized as probe and block, at a 1:250 ratio, respectively. The Digoxigenin-Nick Translation Mix, anti-digoxigenin-rhodamine (red) were purchased from Roche, Mannheim, Germany. All the images were observed under a Nikon Eclipse E600 (Japan) fluorescence microscope and captured with a CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA).
4.3. Non-Denaturing FISH Analysis
The synthetic oligonucleotides Oligo-pAs1.0 (or Oligo-pTa535) and Oligo-pSc119.2 can be used to distinguish wheat chromosomes [13]. Wang et al. [52] identified an Ae. biuncialis accession karyotype formula and FISH pattern using pAs1.0 and pSc119.2 as probe. The arm ratios (L/S) of 5Mb chromosome was near 2.23 and the short arm had the hybridization signals of pAs1.0 and pSc119.2. Therefore, these oligonucleotide probes could be used to characterize wheat chromosomes and Ae. biuncialis 5Mb chromosome. The oligonucleotides were synthesized by Shanghai Invitrogen Biotechnology Co. Ltd. (Shanghai, China), as described by Tang et al. [13] and ND-FISH analysis was described by Fu et al. [62].
In order to estimate the arm ratio (L/S), we compared the relative length of the long arm and short arm of the additional chromosomes. We photographed 5–10 root-cells at mitotic metaphase from WA317 and measured the length of both arms using the software Image J. According to the ND-FISH signals and arm ratio, we determined alien chromosome constitution as described in a previous report [52].
4.4. Multicolor GISH (mcGISH) Analysis
The total genomic DNA of Ae. umbellulata Y139 (UU) was labeled with fluorescein-12-dUTP and total genomic DNA of Ae. comosa Y258 (MM) was labeled with Texas-red-5-dUTP, while total genomic DNA of durum wheat (Triticum turgidum, 2n = 4X = 28, AABB) was used for blocking with a ratio of 1:30 [56]. The hybridization mixture (8 μL per slide) included 50 ng of each U and M genome probe and 1.5 μg competitor DNA in 2× SSC and 1× TE buffer. The denaturation and hybridization conditions were previously described by Han et al. [63].
4.5. Development of Ae. Biuncialis Specific Markers and Identification of The Addition Line WA317
SLAF-seq of Ae. biuncialis Y17 was performed with some modification by the Beijing Biomarker Technologies Corporation [22,64]. In order to increase marker specificity and efficiency, the SLAFs were compared with CS sequences [22] The SLAFs with less than 50% homology were used to design molecular markers. All these primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China).
These Ae. biuncialis specific markers were verified when their PCR bands were present in Ae. biuncialis Y17 but absent in common wheat CS. Those verified markers were further divided into U-specific and M-specific markers by amplifying Ae. umbellulata Y139 and Ae. comosa Y258. In addition, Ae. biuncialis specific markers (Table S2) were used to characterize the 5Mb addition line. Here, Ae. biuncialis, Ae. umbellulata and Ae. comosa were used as positive controls, while CS as negative control.
PCR amplification was performed as described previously by Luan et al. [65]. The amplified products were separated by polyacrylamide gel electrophoresis (PAGE) with an acrylamide concentration of 8% and displayed by silver staining. The annealing temperatures of SLAF-based markers were 59 °C.
4.6. Agronomic Trait Determination of WA317
The wheat-Ae. biuncialis 5Mb addition line WA317 and its receptor parent CS were planted in a randomized complete block design with three replicates in Shijiazhuang, Hebei province of China. Thirty seeds of each line were evenly planted in six 2.25 m long rows, spaced 0.25 m apart. The agronomic traits were evaluated across 2017–2018 to 2018–2019 seasons.
At the physiology maturity stage, WA317 and CS were manually harvested. Measurement and counting were done on spike length, spikelet number per spike, grain number per spike and thousand-grain weight for 10 representative plants randomly selected in each plot. Statistical analyses were conducted using the Statistical Analysis System version 9.2 (SAS Institute Inc., Cary, NC, USA) and the t-test was used to test the difference of the agronomic traits between the addition line WA317and the receptor parent CS.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/11/4053/s1.
Author Contributions
L.S., L.L. and J.L. designed the research project. L.S., Z.Z. and S.Z. carried out the experiments. H.Z., L.S., S.Z., J.L. (Jiajia Liu), W.Z., N.Z. and J.J. collected the phenotypic data. L.S. wrote the manuscript. J.L. (Junming Li) revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful to An Diaoguo for her kind help in mcGISH analysis. This research was jointly supported by the National Natural Science Foundation of China (No. 31701422), the National Key Research and Development Program of China (2017YFD0100600), the Natural Science Foundation of Hebei Province (C2019503066) and China Agriculture Research System (CARS-03-01B).
Acknowledgments
In this section you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CS | Chinese Spring |
| DAPI | 4,6-Diamidino-2-phenylindole |
| GISH | Genomic in situ hybridization |
| ISH | in situ hybridization |
| ND-FISH | Non-denaturing fluorescence in situ hybridization |
| SLAF | Specific-locus amplified fragment sequencing |
| PCR | Polymerase chain reaction |
References
- Doležel, J.; Kubaláková, M.; Bartoš, J.; Macas, J. Flow cytogenetics and plant genome mapping. Chromosome Res. 2004, 12, 77–91. [Google Scholar] [CrossRef]
- Han, H.M.; Bai, L.; Su, J.J.; Zhang, J.P.; Song, L.Q.; Gao, A.N.; Yang, X.M.; Li, X.Q.; Liu, W.H.; Li, L.H. Genetic rearrangements of six wheat-Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS ONE 2014, 9, e91066. [Google Scholar] [CrossRef] [PubMed]
- Triebe, B.; Mukai, Y.; Dhaliwal, H.S.; Martin, T.J.; Gill, B.S. Identification of alien chromatin specifying resistance to wheat streak mosaic and greenbug in wheat germ plasm by C-banding and in situ hybridization. Theor. Appl. Genet. 1991, 81, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, P.P.; Peterson, T.S.; Xu, S.S. Cytogenetic and molecular characterization of a durum alien disomic addition line with enhanced tolerance to Fusarium head blight. Genome 2009, 52, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Chapman, V.; Riley, R. Disomic addition of rye chromosome II to wheat. Nature 1955, 175, 1091–1092. [Google Scholar] [CrossRef]
- Ying, J.; Chen, P.C. Studies of development of disomic addition lines of Triticum aestivum-Haynaldia villosa via AABBDDDD octaploid. Yi Chuan Xue Bao 2000, 27, 506–510. [Google Scholar] [PubMed]
- Zheng, Q.; Lv, Z.L.; Niu, Z.X.; Li, B.; Li, H.W.; Xu, S.S.; Han, F.P.; Li, Z.S. Molecular cytogenetic characterization and stem rust resistance of five wheat-Thinopyrum ponticum partial amphiploids. J. Genet. Genom. 2014, 41, 591–599. [Google Scholar] [CrossRef]
- Friebe, B.; Qi, L.L.; Nasuda, S.; Zhang, P.; Tuleen, N.A.; Gill, B.S. Development of a complete set of Triticum aestivum-Aegilops speltoides chromosome addition lines. Theor. Appl. Genet. 2000, 101, 51–58. [Google Scholar] [CrossRef]
- Wu, J.; Yang, X.M.; Wang, H.; Li, H.J.; Li, L.H.; Li, X.Q.; Liu, W.H. The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor. Appl. Genet. 2006, 114, 13–20. [Google Scholar] [CrossRef]
- Gill, B.S.; Friebe, B.; Endo, T.R. Standard karyotype and nomenclature system for description of chromosome bands and structural aberrations in wheat (Triticum aestivum). Genome 1991, 34, 830–839. [Google Scholar] [CrossRef]
- Cuadrado, A.; Vitellozzi, F.; Jouve, N.; Ceoloni, C. Fluorescence in situ hybridization with multiple repeated DNA probes applied to the analysis of wheat-rye chromosome pairing. Theor. Appl. Genet. 1997, 94, 347–355. [Google Scholar] [CrossRef]
- Han, F.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Adonina, I.G.; Salina, E.A.; Efremova, T.T.; Pshenichnikova, T.A. The study of introgressive lines of Triticum aestivum × Aegilops speltoides by in situ and SSR analyses. Plant Breed 2004, 123, 220–224. [Google Scholar] [CrossRef]
- Wang, R.R.C.; Larson, S.R.; Jensen, K.B. Analyses of Thinopyrum bessarabicum, T. elongatum, and T. junceum chromosomes using EST-SSR markers. Genome 2010, 53, 1083–1089. [Google Scholar] [CrossRef]
- Hagras, A.A.-A.; Kishii, M.; Sato, K.; Tanaka, H.; Tsujimoto, H. Extended application of barley EST markers for the analysis of alien chromosomes added to wheat genetic background. Breed. Sci. 2005, 55, 335–341. [Google Scholar] [CrossRef]
- Zhang, J.P.; Liu, W.H.; Lu, Y.Q.; Liu, Q.X.; Yang, X.M.; Li, X.Q.; Li, L.H. A resource of large-scale molecular markers for monitoring Agropyron cristatum chromatin introgression in wheat background based on transcriptome sequences. Sci. Rep. 2017, 7, 11942. [Google Scholar] [CrossRef]
- Shen, X.; Kong, L.; Ohm, H. Fusarium head blight resistance in hexaploid wheat (Triticum aestivum)-Lophopyrum genetic lines and tagging of the alien chromatin by PCR markers. Theor. Appl. Genet. 2003, 108, 808–813. [Google Scholar] [CrossRef]
- Grewal, S.; Hubbart-Edwards, S.; Yang, C.Y.; Devi, U.; Baker, L.; Heath, J.; Ashling, S.; Scholefield, D.; Howells, C.; Yarde, J.; et al. Rapid identification of homozygosity and site of wild relative introgressions in wheat through chromosome-specific KASP genotyping assays. Plant Biotechnol. J. 2020, 18, 743–755. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Z.; Dai, Y.; Qin, S.; Gao, Y.; Zhang, L.; Gao, Y.; Chen, J. The development of 7E chromosome-specific molecular markers for Thinopyrum elongatum based on SLAF-seq technology. PLoS ONE 2013, 8, e65122. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.P.; Huang, L.; Gao, A.N.; Zhang, J.; Yang, X.M.; Liu, W.H.; Li, X.Q.; Li, L.H. A high-density genetic map for P genome of Agropyron Gaertn. based on specific-locus amplified fragment sequencing (SLAF-seq). Planta 2015, 242, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luo, Q.; Teng, W.; Li, B.; Li, H.; Li, Y.; Li, Z.; Zheng, Q. Development of Thinopyrum ponticum-specific molecular markers and FISH probes based on SLAF-seq technology. Planta 2018, 247, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Wang, Y.Y.; Qiu, L.; Ren, T.H.; Li, Z.; Fu, S.L.; Tang, Z.X. Physical location of new PCR-based Markers and powdery mildew resistance gene(s) on rye (Secale cereale L.) chromosome 4 using 4R dissection lines. Front. Plant Sci. 2017, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Tang, Z.; Duan, Q.; Tang, S.; Fu, S. Using the 6RL(Ku) minichromosome of rye (Secale cereale L.) to create wheat-rye 6D/6RL(Ku) small segment translocation lines with powdery mildew resistance. Int. J. Mol. Sci. 2018, 19, 3933. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.J.; Fellers, J.P.; Trick, H.N.; Zhang, Z.; Tai, Y.S.; Gill, B.S.; Faris, J.D. Molecular characterization of the major wheat domestication gene Q. Genetics 2006, 172, 547–555. [Google Scholar] [CrossRef]
- Sharma, J.S.; Running, K.L.D.; Xu, S.S.; Zhang, Q.; Peters Haugrud, A.R.; Sharma, S.; McClean, P.E.; Faris, J.D. Genetic analysis of threshability and other spike traits in the evolution of cultivated emmer to fully domesticated durum wheat. Mol. Genet. Genom. 2019, 294, 757–771. [Google Scholar] [CrossRef]
- Dvorak, J.; Deal, K.R.; Luo, M.C.; You, F.M.; von Borstel, K.; Dehghani, H. The origin of spelt and free-threshing hexaploid wheat. J. Hered. 2012, 103, 426–441. [Google Scholar] [CrossRef]
- Faris, J.D.; Zhang, Z.; Chao, S. Map-based analysis of the tenacious glume gene Tg-B1 of wild emmer and its role in wheat domestication. Gene 2014, 542, 198–208. [Google Scholar] [CrossRef]
- Kerber, E.R.; Rowland, G.G. Origin of the free threshing character in hexaploid wheat. Can. J. Genet. Cytol. 1974, 16, 145–154. [Google Scholar] [CrossRef]
- Nalam, V.J.; Vales, M.I.; Watson, C.J.W.; Kianian, S.F.; Riera-Lizarazu, O. Map-based analysis of genes affecting the brittle rachis character in tetraploid wheat (Triticum turgidum L.). Theor. Appl. Genet. 2005, 112, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Kuraparthy, V.; Bai, G.; Gill, B.S. The major threshability genes soft glume (sog) and tenacious glume (Tg), of diploid and polyploid wheat, trace their origin to independent mutations at non-orthologous loci. Theor. Appl. Genet. 2009, 119, 341–351. [Google Scholar] [CrossRef]
- Dvořák, J.; Chen, K.C. Phylogenetic relationships between chromosomes of wheat and chromosome 2E of Elytrigia elongata. Can. J. Genet. Cytol. 1984, 26, 128–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, A.; Song, G.; Geng, S.; Gill, B.S.; Faris, J.D.; Mao, L. Comprehensive analysis of Q gene near isogenic lines reveals key molecular pathways for wheat domestication and improvement. Plant J. 2020, 102, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Mansfeld, R. Das morphologische System des Saatweizens, Triticum aestivum L.s.l. Der Züchter 1951, 21, 41–60. [Google Scholar] [CrossRef]
- Dorofeev, V.F.; Filatenko, A.A.; Migushova, E.F.; Udachin, R.A.; Jacubziner, M.M. Flora of Cultivated Plants; Dorofeev, V.F., Korovina, O.N., Eds.; Pshenitsa (Wheat), Leningrad: Kolos, Russia, 1979. [Google Scholar]
- Börner, A.; Schäfer, M.; Schmidt, A.; Grau, M.; Vorwald, J. Associations between geographical origin and morphological characters in bread wheat. Plant Genet. Resour. 2005, 3, 360–372. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Pshenichnikova, T.A.; Röder, M.S.; Salina, E.A.; Arbuzova, V.S.; Börner, A. Comparative mapping of genes for glume colouration and pubescence in hexaploid wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 801–807. [Google Scholar] [CrossRef]
- Kozub, N.A.; Sozinov, I.A.; Niniyeva, A.K.; Tverdokhleb, Y.V.; Blume, Y.B.; Boguslavskii, R.L. Genetic marking of glume color in Triticum spelta L. var. caeruleum using gliadins. Cytol Genet Cytol. Genet. 2016, 50, 168–172. [Google Scholar] [CrossRef]
- Sobko, T.A.; Sozinov, A.A. Mapping of loci that control morphological spike traits and reserve grain proteins in 1A chromosome of winter common wheat. Tsitol. Genet. 1997, 31, 18–26. [Google Scholar]
- Pshenichnikova, T.A.; Bokarev, I.E.; Shchukina, L.V. Hybrid and monosomic analyses of smoky coloration of the ear in common wheat. Russ. J. Genet. 2005, 41, 1147–1149. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Röder, M.S.; Börner, A. Mapping genes controlling anthocyanin pigmentation on the glume and pericarp in tetraploid wheat (Triticum durum L.). Euphytica 2010, 171, 65–69. [Google Scholar] [CrossRef]
- Van Slageren, M.W. A Monograph of Aegilops L. and Amblyopyrum (Jaub and Spach) Eig (Poaceae). In Wageningen Agricultural University Papers 94-7; Wageningen Agricultural University: Wageningen, The Netherlands, 1994; p. 514. [Google Scholar]
- Mirzaghaderi, G.; Mason, A.S. Broadening the bread wheat D genome. Theor. Appl. Genet. 2019, 132, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Gáspár, L.; Sárvári, É.; Dulai, S.; Hoffmann, B.; Molnár-láng, M.; Galiba, G. Physiological and morphological responses to water stress in Aegilops biuncialis and Triticum aestivum genotypes with differing tolerance to drought. Funct. Plant Boil. 2004, 31, 1149–1159. [Google Scholar] [CrossRef]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef]
- Zhou, J.P.; Yao, C.H.; Yang, E.N.; Yin, M.Q.; Liu, C.; Ren, Z.L. Characterization of a new wheat Aegilops biuncialis addition line conferring quality-associated HMW glutenin subunits. Genet. Mol. Res. 2014, 13, 660–669. [Google Scholar] [CrossRef]
- Schneider, A.; Linc, G.; Molnár, I.; Molnár-Láng, M. Molecular cytogenetic characterization of Aegilops biuncialis and its use for the identification of 5 derived wheat-Aegilops biuncialis disomic addition lines. Genome 2005, 48, 1070–1082. [Google Scholar] [CrossRef]
- Schneider, A.; Molnár-láng, M. Detection of various U and M chromosomesin wheat-Aegilops biuncialis hybrids and derivatives using fluorescence in situ hybridisation and molecular markers. Czech J. Genet. Plant Breed. 2012, 48, 169–177. [Google Scholar] [CrossRef]
- Schneider, A.; Molnár, I.; Molnár-Láng, M. Selection of U and M genome-specific wheat SSR markers using wheat-Aegilops biuncialis and wheat-Ae. geniculata addition lines. Euphytica 2010, 175, 357–364. [Google Scholar] [CrossRef]
- Molnár, I.; Šimková, H.; Leverington-Waite, M.; Goram, R.; Cseh, A.; Vrána, J.; Farkas, A.; Doležel, J.; Molnár-Láng, M.; Griffiths, S. Syntenic relationships between the U and M genomes of Aegilops, wheat and the model species Brachypodium and rice as revealed by COS markers. PLoS ONE 2013, 8, e70844. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zhao, H.; Li, F.R.; Wang, Z.G.; Ji, J.; Zhang, X.Q.; Wang, D.W.; Li, J.M. Molecular cytogenetic characterization of the Aegilops biuncialis karyotype. Genet. Mol. Res. 2013, 12, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, W.; Wang, J.Y.; Li, F.R.; Cui, F.; Ji, J.; Wang, D.W.; Li, J.M. Comparative study on drought tolerance of wheat and wheat-Aegilops biuncialis 6Ub addition lines. J. Food Agric. Environ. 2013, 11, 1046–1052. [Google Scholar]
- Li, H.; Dong, Z.; Ma, C.; Tian, X.; Xiang, Z.; Xia, Q.; Ma, P.; Liu, W. Discovery of powdery mildew resistance gene candidates from Aegilops biuncialis chromosome 2Mb based on transcriptome sequencing. PLoS ONE 2019, 14, e0220089. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Nakahara, Y.; Yamamoto, M. Simultaneous discrimination of the 3 genomes in hexaploid wheat by multicolour fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 1993, 36, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Molnár-láng, M. Visualization of U and M genome chromosomes by multicolour genomic in situ hybridization in Aegilops biuncialis and Triticum aestivum-Ae. biumcialis amphiploids. Acta Agron. Hung. 2010, 58, 195–202. [Google Scholar] [CrossRef]
- Garg, M.; Tsujimoto, H.; Gupta, R.K.; Kumar, A.; Kaur, N.; Kumar, R.; Chunduri, V.; Sharma, N.K.; Chawla, M.; Sharma, S.; et al. Chromosome specific substitution lines of Aegilops geniculata alter parameters of bread making quality of wheat. PLoS ONE 2016, 11, e0162350. [Google Scholar] [CrossRef] [PubMed]
- Molnár, I.; Vrána, J.; Burešová, V.; Cápal, P.; Farkas, A.; Darkó, É.; Cseh, A.; Kubaláková, M.; Molnár-Láng, M.; Doležel, J. Dissecting the U, M, S and C genomes of wild relatives of bread wheat (Aegilops spp.) into chromosomes and exploring their synteny with wheat. Plant J. 2016, 88, 452–467. [Google Scholar] [CrossRef]
- Zhou, S.H.; Zhang, J.P.; Che, Y.H.; Liu, W.H.; Lu, Y.Q.; Yang, X.M.; Li, X.Q.; Jia, J.Z.; Liu, X.; Li, L.H. Construction of Agropyron Gaertn. genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2017, 16, 818–827. [Google Scholar] [CrossRef]
- Gaál, E.; Valárik, M.; Molnár, I.; Farkas, A.; Linc, G. Identification of COS markers specific for Thinopyrum elongatum chromosomes preliminary revealed high level of macrosyntenic relationship between the wheat and Th. elongatum genomes. PLoS ONE 2018, 13, e0208840. [Google Scholar] [CrossRef]
- Liu, W.H.; Luan, Y.; Wang, J.C.; Wang, X.G.; Su, J.J.; Zhang, J.P.; Yang, X.M.; Gao, A.N.; Li, L.H. Production and identification of wheat-Agropyron cristatum (1·4P) alien translocation lines. Genome 2010, 53, 472–481. [Google Scholar] [CrossRef]
- Fu, S.L.; Chen, L.; Wang, Y.Y.; Li, M.; Yang, Z.J.; Qiu, L.; Yan, B.J.; Ren, Z.L.; Tang, Z.X. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015, 5, 10552. [Google Scholar] [CrossRef]
- Han, F.P.; Gao, Z.; Birchler, J.A. Centromere inactivation and reactivation reveals both epigenetic and genetic components for centromere specification. Plant Cell 2009, 21, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.W.; Liu, D.Y.; Zhang, X.F.; Li, W.B.; Liu, H.; Hong, W.G.; Jiang, C.B.; Guan, N.; Ma, C.X.; Zeng, H.P.; et al. SLAF-seq: An efficient method of large-scale de novo SNP discovery and genotyping using high-throughput sequencing. PLoS ONE 2013, 8, e58700. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Wang, X.G.; Liu, W.H.; Li, C.Y.; Zhang, J.P.; Gao, A.N.; Wang, Y.D.; Yang, X.M.; Li, L.H. Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 2010, 232, 501–510. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).