Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia
Abstract
1. Introduction
2. Basic Properties and Substrate Specificity of Grimelysin and Protealysin
3. Specific Actinase Activity of Grimelysin (ECP 32) and Protealysin
4. Specific Properties of Protease-Cleaved Actin
5. Actin-Like Proteins of Bacteria
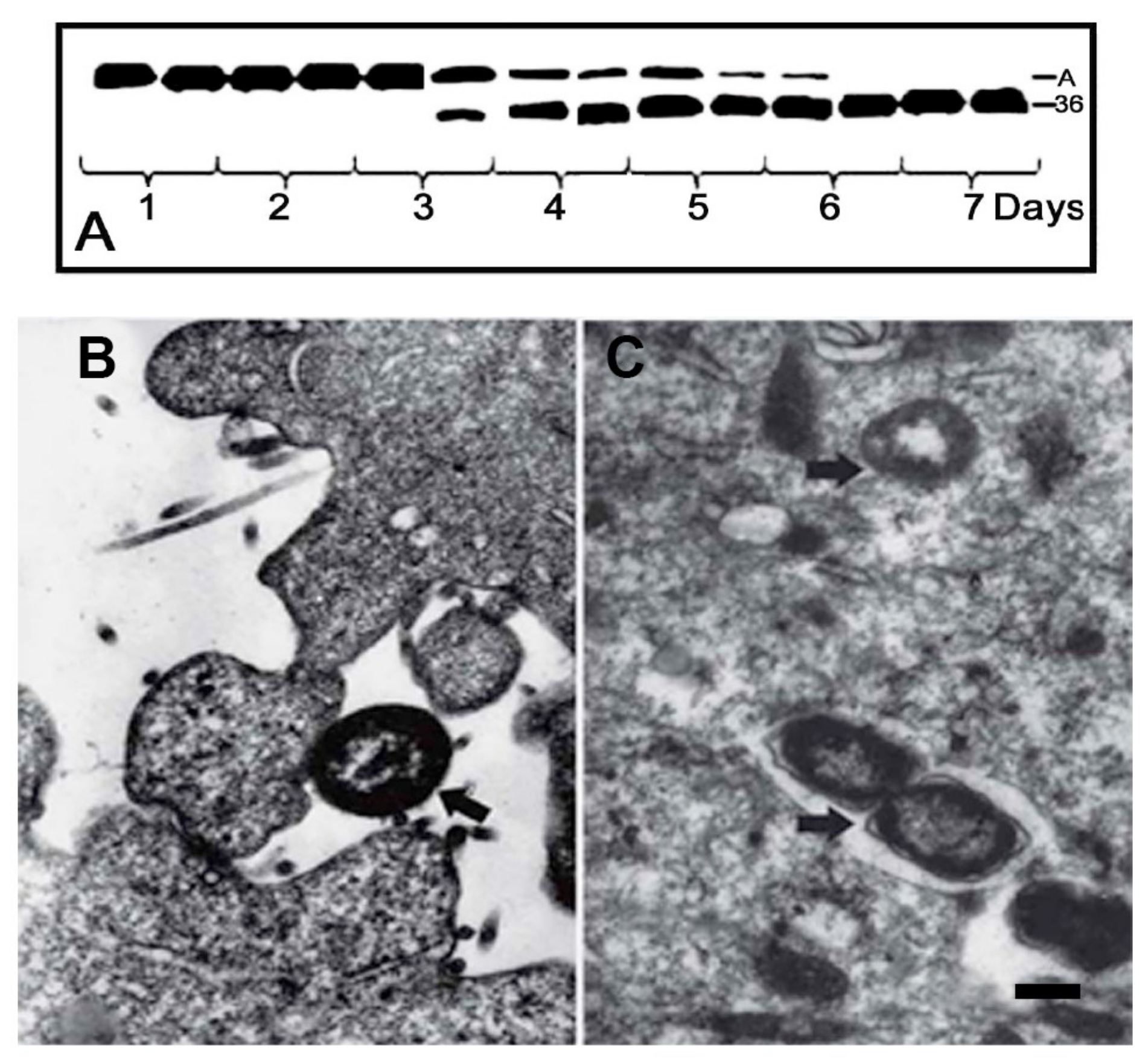
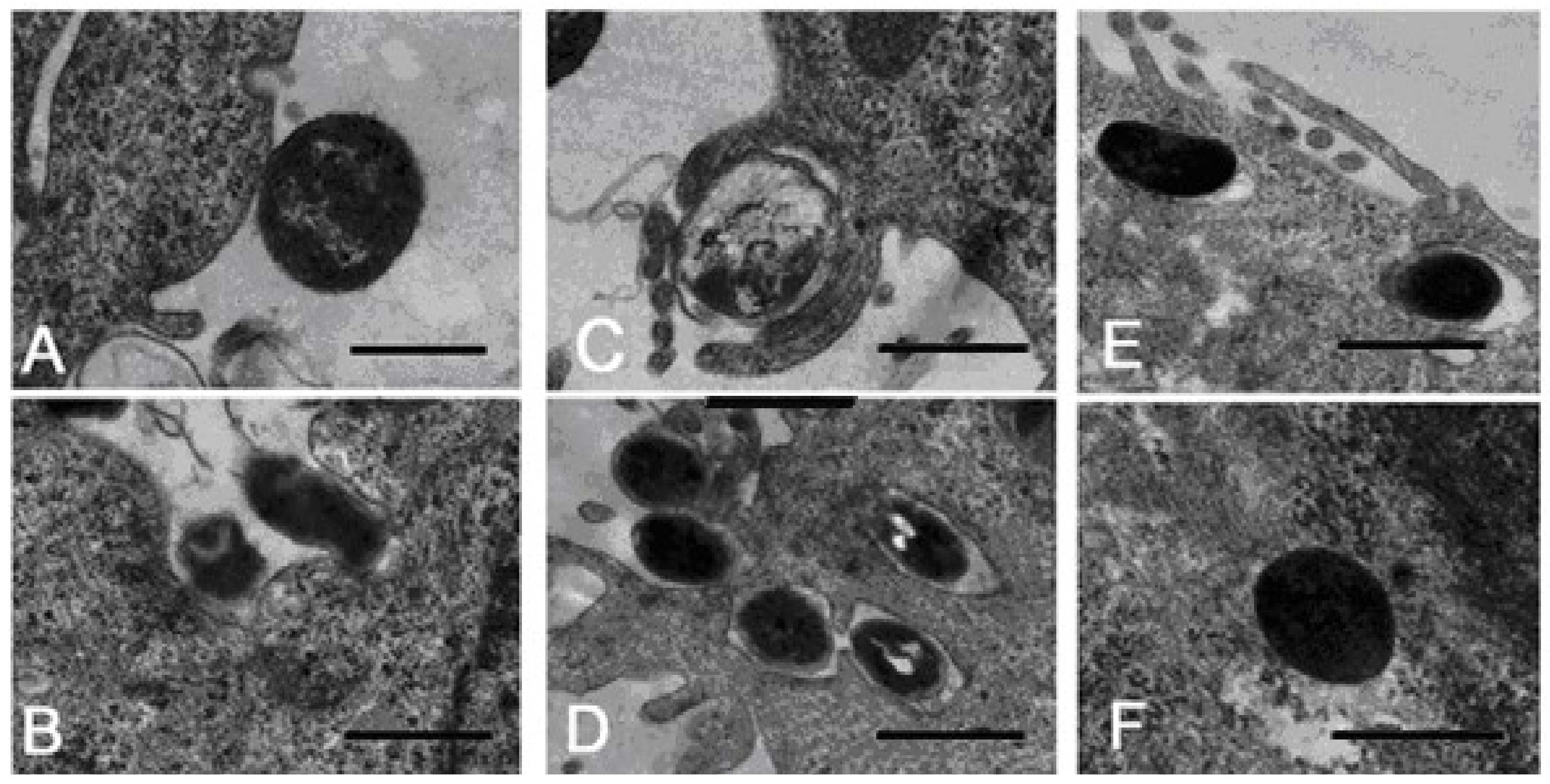
6. Invasive Activity of Bacteria Serratia grimesii and Serratia proteamaculans
7. Bacterial Virulence Factors Involved in Serratia Invasion
8. Cellular Factors Involved in Serratia Invasion
9. Conclusions
Funding
Conflicts of Interest
References
- Bhavsar, A.P.; Guttman, J.A.; Finlay, B.B. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007, 449, 827–834. [Google Scholar] [CrossRef]
- Schnupf, P.; Sansonetti, P.J. Shigella Pathogenesis: New Insights through Advanced Methodologies. Microbiol. Spectr. 2019, 7, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sutton, S.E.; Wallenfang, A.J.; Orchard, R.C.; Wu, X.; Feng, Y.; Chai, J.; Alto, N.M. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat. Struct. Mol. Biol. 2009, 16, 853–860. [Google Scholar] [CrossRef]
- Pinaud, L.; Sansonetti, P.J.; Phalipon, A. Host Cell Targeting by Enteropathogenic Bacteria T3SS Effectors. Trends Microbiol. 2018, 26, 266–283. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, M.; Hayer, A.; Theriot, J. A endothelial cells use a formin-dependent phagocytosis-like process to internalize the bacterium Listeria monocytogenes. PLoS Pathog. 2016, 12, e1005603. [Google Scholar] [CrossRef] [PubMed]
- Mantulenko, V.B.; Khaitlina, S.Y..; Sheludko, N.S. High molecular weight proteolysis-resistant actin fragment. Biochemistry 1983, 48, 69–74. [Google Scholar]
- Usmanova, A.M.; Khaitlina, S.Y. A specific actin-digesting protease from the bacterial strain E.coli A2. Biochemistry 1989, 54, 1074–1079. [Google Scholar]
- Matveyev, V.V.; Usmanova, A.M.; Morozova, A.B.; Khaitlina, S.Y. Purification and characterization of the proteinase ECP-32 from Escherichia coli A2 strain. Biochim. Biophys. Acta 1996, 1296, 55–62. [Google Scholar] [CrossRef]
- Khaitlina, S.Y..; Smirnova, T.D.; Usmanova, A.M. Limited proteolysis of actin by a specific bacterial protease. FEBS Lett. 1988, 28, 72–74. [Google Scholar] [CrossRef]
- Khaitlina, S.Y..; Collins, J.H.; Kusnetsova, I.M.; Pershina, V.P.; Synakevich, I.G.; Turoverov, K.K.; Usmanova, A.M. Physico-chemical properties of actin cleaved with bacterial protease from E. coli A2 strain. FEBS Lett. 1991, 279, 49–51. [Google Scholar] [CrossRef]
- Kabsch, W.; Mannherz, H.G.; Suck, D.; Pai, E.F.; Holmes, K.C. Atomic structure of the actin:DNase I complex. Nature 1990, 347, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.C.; Popp, D.; Gebhard, W.; Kabsch, W. Atomic model of the actin filament. Nature 1990, 347, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Khaitlina, S.Y..; Moraczewska, J.; Strzelecka-Golaszewska, H. The actin-actin interactions involving the N-terminal portion of the DNase I-binding loop are crucial for stabilization of the actin filament. Eur. J. Biochem. 1993, 218, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Khaitlina, S.; Hinssen, H. Conformational changes in actin Induced by Its Interaction with gelsolin. Biophys. J. 1997, 73, 929–937. [Google Scholar] [CrossRef]
- Khaitlina, S.Y.; Strzelecka-Golaszewska, H. Role of the DNase-I-binding loop in dynamic properties of actin filament. Biophys. J. 2002, 82, 321–334. [Google Scholar] [CrossRef]
- Moraczewska, J.; Gruszczynska-Biegala, J.; Redowicz, M.J.; Khaitlina, S.; Strzelecka-Golaszewska, H. The DNase-I binding loop of actin may play a role in the regulation of actin-myosin interaction by tropomyosin/troponin. J. Biol. Chem. 2004, 279, 31197–31204. [Google Scholar] [CrossRef]
- Khaitlina, S.; Tsaplina, O.; Hinssen, H. Cooperative effects of tropomyosin on the dynamics of the actin filament. Febs Lett. 2017, 591, 1884–1891. [Google Scholar] [CrossRef]
- Bozhokina, E.; Khaitlina, S.; Adam, T. Grimelysin, a novel metalloprotease from Serratia grimesii, is similar to ECP 32. Biochem Biophys Res Commun. 2006, 367, 888–892. [Google Scholar] [CrossRef]
- Demidyuk, I.V.; Kalashnikov, A.E.; Gromova, T.Y.; Gasanov, E.V.; Safina, D.R.; Zabolotskaya, M.V.; Rudenskaya, G.N.; Kostrov, S.V. Cloning, sequencing, expression, and characterization of protealysin, a novel neutral proteinase from Serratia proteamaculans representing a new group of thermolysin-like proteases with short N-terminal region of precursor. Protein Expr. Purif. 2006, 47, 551–561. [Google Scholar] [CrossRef]
- Efremova, T.; Ender, N.; Brudnaja, M.; Komissarchik, Y.; Khaitlina, S. Specific invasion of transformed cells by Escherichia coli A2 strain. Cell Biol. Intern. 2001, 25, 557–561. [Google Scholar] [CrossRef]
- Bozhokina, E.S.; Tsaplina, O.A.; Efremova, T.N.; Kever, L.V.; Demidyuk, I.V.; Kostrov, S.V.; Adam, T.; Komissarchik, Y.Y.; Khaitlina, S.Y. Bacterial invasion of eukaryotic cells can be mediated by actin-hydrolysing metalloproteases grimelysin and protealysin. Cell Biol Int. 2011, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, O.A.; Efremova, T.N.; Kever, L.V.; Komissarchik, Y.Y.; Demidyuk, I.V.; Kostrov, S.V.; Khaitlina, S.Y. Probing for actinase activity of protealysin. Biochemistry 2009, 74, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kazanina, G.A.; Mirgorodskaia, E.P.; Mirgorodskaia, O.A.; Khaitlina, S.Y.. ECP 32 proteinase: Characteristics of the enzyme, study of specificity. Bioorg. Khim. 1995, 21, 761–766. [Google Scholar] [PubMed]
- Mirgorodskaya, O.; Kazanina, G.; Mirgorodskaya, E.; Matveyev, V.; Thiede, B.; Khaitlina, S.Y.. Proteolytic cleavage of mellitin with the actin-digesting protease. Protein Pept. Lett. 1996, 3, 81–88. [Google Scholar]
- Morozova, A.V.; Khaitlina, S.Y.; Malinin, A.Y. Heat Shock Protein DnaK—Substrate of actin-specific bacterial protease ECP 32. Biochemistry 2011, 76, 455–461. [Google Scholar] [CrossRef]
- Khaitlina, S.; Lindberg, U. Dissociation of profilactin as a two-step process. J. Muscle Res. Cell Motil. 1995, 16, 188–189. [Google Scholar]
- Rawlings, N.D.; Burrett, A.J. Evolutionary families of metallopeptidases. Methods Enzym. 1995, 248, 183–228. [Google Scholar] [PubMed]
- Shinde, U.; Inouye, M. Intramolecular chaperones: Polypeptide extensions that modulate protein folding. Semin. Cell Dev. Biol. 2000, 11, 35–44. [Google Scholar] [CrossRef]
- Gromova, T.Y.; Demidyuk, I.V.; Kozlovskiy, V.I.; Kuranova, I.P.; Kostrov, S.V. Processing of protealysin precursor. Biochimie 2009, 91, 639–645. [Google Scholar] [CrossRef]
- Demidyuk, I.V.; Gasanov, E.V.; Safina, D.R.; Kostrov, S.V. Structural organization of precursors of thermolysin-like proteinases. Protein J. 2008, 27, 343–354. [Google Scholar] [CrossRef]
- Demidyuk, I.V.; Gromova, T.Y.; Polyakov, K.M.; Melik-Adamyan, W.R.; Kuranova, I.P.; Kostrov, S.V. Crystal structure of the protealysin precursor: Insights into propeptide function. J. Biol.Chem. 2010, 285, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Demidyuk, I.V.; Shubin, A.V.; Gasanov, E.V.; Kostrov, S.V. Propeptides as modulators of functional activity of proteases. Biomol. Concepts 2010, 1, 305–322. [Google Scholar] [CrossRef]
- Tsaplina, O.; Efremova, T.; Demidyuk, I.; Khaitlina, S. Filamentous actin is a substrate for protealysin, a metalloprotease of invasive Serratia proteamaculans. Febs J. 2012, 279, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, M.; Collins, J.H.; Kuehl, W.M.; Adelstein, R.S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc. Natl. Acad. Sci. USA 1973, 70, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Klenchin, V.A.; Khaitlina, S.Y.; Rayment, I. Crystal structure of polymerization-competent actin. J. Mol. Biol. 2006, 362, 140–150. [Google Scholar] [CrossRef]
- Tsaplina, O.A.; Khaitlina, S.Y. Sodium fluoride as a nucleating factor for Mg-actin polymerization. Biochem. Biophys. Res. Commun. 2016, 479, 1746–1774. [Google Scholar] [CrossRef]
- Wawro, B.; Khaitlina, S.Y.; Galinska-Rakoczy, A.; Strzelecka-Goaszewska, H. Role of DNase I-binding loop in myosin subfragment 1-induced actin polymerization. Implications to the polymerization mechanism. Biophys. J. 2005, 88, 2883–2896. [Google Scholar] [CrossRef]
- Morozova, A.V.; Skovorodkin, I.N.; Khaitlina, S.Y.; Malinin, A.Y. Bacterial protease ECP 32 specifically hydrolyzing actin and its effect on cytoskeleton in vivo. Biochemistry 2001, 66, 83–90. [Google Scholar] [CrossRef]
- Eun, Y.J.; Kapoor, M.; Hussain, S.; Garner, E.C. Bacterial filament systems: Towards understanding their emergent behavior and cellular functions. J. Biol. Chem. 2015, 290, 17181–17189. [Google Scholar] [CrossRef]
- Gayathry, P. Bacterial actins and their interaction. Curr. Top. Microbiol. Immunol. 2017, 399, 22–1242. [Google Scholar]
- Van den Ent, F.; Amos, L.; Löwe, J. Bacterial ancestry of actin and tubulin. Curr Opin Microbiol. 2001, 4, 634–638. [Google Scholar] [CrossRef]
- Van den Ent, F.; Amos, L.A.; Löwe, J. Prokaryotic origin of the actin cytoskeleton. Nature 2001, 413, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Efremova, T.N.; Ender, N.A.; Brudnaia, M.S.; Komissarchik, I.I.; Khaĭtlina, S.I. Invasion of Escherichia coli A2 induces reorganization of actin microfilaments in Hep-2 cells. Tsitologia 1998, 40, 524–528. [Google Scholar]
- Fedorova, Z.F.; Khaitlina, S.Y. Detection of actin-specific protease in the revertants of Shigella flexnery L form. Bull. Exp. Biol Med. 1990, 7, 46–48. [Google Scholar]
- Efremova, T.N.; Gruzdeva, I.G.; Matveev, I.V.; Bozhokina, E.S.; Komissarchuk, Y.Y.; Fedorova, Z.F.; Khaitlina, S.Y. Invasive characteristics of apathogenic Shigella flexneri 5a2c mutant obtained under the effect of furazolidone. Bull. Exp. Biol. Med. 2004, 137, 479–482. [Google Scholar] [CrossRef]
- Ivlev, A.P.; Efremova, T.N.; Khaitlina, S.Y..; Bozhokina, E.S. Difference in susceptibility of 3T3 and 3T3-SV40 cells to invasion by opportunistic pathogens Serratia grimesii. Cell Tissue Biol. 2018, 12, 33–40. [Google Scholar] [CrossRef]
- Velge, P.; Bottreau, E.; Kaeffer, B.; Pardon, P. Cell immortalization enhances Listeria monocytogenes invasion. Med. Microbiol. Immunol. 1994, 183, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Velge, P.; Kaeffer, B.; Bottreau, E.; Van Langendonck, N. The loss of contact inhibition and anchorage-dependent growth are key steps in the acquisition of Listeria monocytogenes susceptibility phenotype by non-phagocytic cells. Biol. Cell. 1995, 85, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, A.K.S.; Pan, W.-A.; Trejo, J.A. GPCRs in cancer: Protease-activated receptors, endocytic adaptors and Signaling. Int. J. Mol. Sci. 2018, 19, 1886–1910. [Google Scholar] [CrossRef]
- Bozhokina, E.S.; Kever, L.V.; Komissarchik, Y.Y.; Khaitlina, S.Y.; Efremova, T.N. Entry of facultative pathogen Serratia grimesii into Hela cells. Electron microscopic analysis. Cell Tissue Biol. 2016, 10, 60–68. [Google Scholar] [CrossRef]
- Tiney, L.G.; Portnoy, D.A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 1989, 1091, 597–608. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Kühbacher, A.; Cossart, P. Entry of Listeria monocytogenes in mammalian epithelial cells: An updated view. Cold Spring Harb. Perspect. Med. 2012, 2, a010009. [Google Scholar] [CrossRef]
- Pizarro-Cerdá, J.; Cossart, P. Listeria monocytogenes: Cell biology of invasion and intracellular growth. Microbiol Spectr. 2018, 6, GPP3-00132018. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens. The paradigm of Shigella. Folia Microbiol. 1998, 43, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Cossart, P.; Sansonetti, P.J. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science 2004, 304, 242–248. [Google Scholar] [CrossRef]
- Carayol, N.; Tran Van Nhieu, G. Tips and tricks about Shigella invasion of epithelial cells. Curr Opin Microbiol. 2013, 16, 32–37. [Google Scholar] [CrossRef]
- Valencia-Gallardo, C.M.; Carayol, N.; Tran Van Nhieu, G. Cytoskeletal mechanics during Shigella invasion and dissemination in epithelial cells. Cell Microbiol. 2015, 17, 174–182. [Google Scholar] [CrossRef]
- Rosselin, M.; Virlogeux-Payant, I.; Roy, C.; Bottreau, E.; Sizaret, P.Y.; Mijouin, L.; Germon, P.; Caron, E.; Velge, P.; Wiedemann, A. Rck of Salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res. 2010, 20, 647–664. [Google Scholar] [CrossRef]
- Velge, P.; Wiedemann, A.; Rosselin, M.; Abed, N.; Boumart, Z.; Chausse, A.M.; Grepinet, O.; Namdari, F.; Roche, S.M.; Rossignol, A.; et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiol. Open 2012, 1, 243–258. [Google Scholar] [CrossRef]
- Boumart, Z.; Velge, P.; Wiedemann, A. Multiple invasion mechanisms and different intracellular behaviors: A new vision of Salmonella–host cell interaction. Fems Microbiol Lett. 2014, 361, 1–7. [Google Scholar] [CrossRef]
- Mambu, J.; Virlogeux-Payant, I.; Holbert, S.; Grépinet, O.; Velge, P.; Wiedemann, A. An Updated view on the Rck Invasin of Salmonella: Still much to discover. Front. Cell Infect. Microbiol. 2017, 7, 500. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, O. Cleavage of Ompx with protealysin can regulate Serratia proteamaculans invasion. In Abstract Book of the 3rd International Conference SmartBio; Vytautas Magnus University: Kaunas, Lithuania, 2019; p. 113. [Google Scholar]
- Tsaplina, O.; Bozhokina, E.; Mardanova, A.; Khaitlina, S. Virulence factors contributing to invasive activities of Serratia grimesii and Serratia proteamaculans. Arch. Microbiol. 2015, 197, 481–488. [Google Scholar] [CrossRef]
- Grimont, P.A.D.; Grimont, F.; Irino, K. Biochemical characterization of Serratia liquefaciens sensu stricto, Serratia proteamaculans, and Serratia grimesii sp. Curr. Microbiol. 1982, 7, 69–74. [Google Scholar] [CrossRef]
- Grimont, F.; Grimont, P.A.D. The genus Serratia. Prokaryotes 2006, 6, 219–244. [Google Scholar] [CrossRef]
- Mahlen, S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef] [PubMed]
- Hertle, R.; Schwarz, H. Serratia marcescens internalization and replication in human bladder epithelial cells. BMC Infect. Dis. 2004, 4, 16. [Google Scholar] [CrossRef]
- Hertle, R. The family of Serratia type pore forming toxins. Curr. Protein Pept. Sci. 2005, 6, 313–325. [Google Scholar] [CrossRef]
- Marty, K.B.; Williams, C.L.; Guynn, L.J.; Benedik, M.J.; Blanke, S.R. Characterization of a cytotoxic factor in culture filtrates of Serratia marcescens. Infect. Immun. 2002, 70, 1121–1128. [Google Scholar] [CrossRef]
- Kolodziejek, A.M.; Sinclair, D.J.; Seo, K.S.; Schnider, D.R.; Deobald, C.F.; Rohde, H.N.; Viall, A.K.; Minnich, S.S.; Hovde, C.J.; Minnich, S.A.; et al. Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM. Microbiology 2007, 153, 2941–2951. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.P.; Choi, J.; Lim, J.A.; Lee, J.; Hwang, S.; Ryu, S. Outer membrane proteins A (OmpA) and X (OmpX) are essential for basolateral invasion of Cronobacter sakazakii. Appl. Environ. Microbiol. 2010, 76, 5188–5198. [Google Scholar] [CrossRef]
- Meng, X.; Liu, X.; Zhang, L.; Hou, B.; Li, B.; Tan, C.; Li, Z.; Zhou, R.; Li, S. Virulence characteristics of extraintestinal pathogenic Escherichia coli deletion of gene encoding the outer membrane protein X. J. Vet. Med. Sci. 2016, 78, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, O.A. Participation of Serratia proteamaculans outer membrane protein (OmpX) in bacterial adhesion of eukaryotic cells. Tsitologia (Rus). 2018, 50, 817–820. [Google Scholar] [CrossRef]
- Da Silva, C.V.; Cruz, L.; Araújo, N.S.; Angeloni, M.B.; Fonseca, B.B.; Gomes, A.O.; dos Carvalho, F.R.; Gonçalves, A.L.; Barbosa, B.F. A glance at Listeria and Salmonella cell invasion: Different strategies to promote host actin polymerization. Int. J. Med. Microbiol. 2012, 302, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Cossart, P. Listeria monocytogenes: Towards a complete picture of its physiology and pathogenesi. Nat. Rev. Microbiol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Parasassi, T.; Brunelli, R.; Costa, G.; De Spirito, M.; Krasnowska, E.; Lundeberg, T.; Pittaluga, E.; Ursini, F. Thiol redox transitions in cell signaling: A lesson from N-acetylcysteine. Sci. World J. 2010, 10, 1192–1202. [Google Scholar] [CrossRef]
- Parasassi, T.; Brunelli, R.; Bracci-Laudiero, L.; Greco, G.; Gustafsson, A.C.; Krasnowska, E.K.; Lundeberg, J.; Lundeberg, T.; Pittaluga, E.; Romano, M.C.; et al. Differentiation of normal and cancer cells induced by sulfhydryl reduction: Biochemical and molecular mechanisms. Cell Death Differ. 2005, 12, 51128–51296. [Google Scholar] [CrossRef]
- Gamalei, I.A.; Efremova, T.N.; Kirpichnikova, K.M.; Komissarchik, Y.Y.; Kever, L.V.; Polozov, Y.V.; Khaitlina, S.Y. Decreased sensitivity of transformed 3T3-SV40 cells treated with N-acetylcysteine to bacterial invasion. Bull. Exp. Biol. Med. 2006, 142, 90–93. [Google Scholar] [CrossRef]
- Bozhokina, E.; Vakhromova, E.; Gamaley, I.; Khaitlina, S. N-Acetylcysteine increases susceptibility of HeLa cells to bacterial Invasion. J. Cell. Biochem. 2013, 114, 1568–1574. [Google Scholar] [CrossRef]
- Bonazzi, M.; Veiga, E.; Pizarro-Cerda, J.; Cossart, P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol 2008, 10, 2208–2222. [Google Scholar] [CrossRef]
- Bonazzi, M.; Lecuit, M.; Cossart, P. Listeria monocytogenes internalin and E-cadherin: From structure to pathogenesis. Cell Microbiol. 2009, 11, 693–702. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015, 17, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bozhokina, E.S.; Tsaplina, O.A.; Khaitlina, S.Y. The opposite effects of ROCK and Src kinase inhibitors on susceptibility of eukaryotic cells to invasion by bacteria Serratia grimesii. Biochemistry 2019, 84, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Tsaplina, A. Redistribution of the EGF receptor and α5-, β1-integins in response to infection of epithelial cells by Serratia proteamaculans. Tsitologiya 2020, 62, 349–355. [Google Scholar] [CrossRef]
- Sasakawa, C. A new paradigm of bacteria-gut interplay brought through the study of Shigella. Proc. Jpn. Acad. Ser. B. 2010, 86, 229–243. [Google Scholar] [CrossRef]
- Bahia, D.; Satoskar, A.R.; Dussurget, O. Editorial: Cell signaling in host–pathogen interactions: The host point of view. Front. Immunol. 2018, 9, 221. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, S.; Kim, E.; Hwang, S.Y.; Yoon, H. Secretion of Salmonella pathogenicity island 1-encoded type III secretion system effectors by outer membrane vesicles in Salmonella enterica serovar Typhimurium. Front. Microbiol. 2018, 23, 2810. [Google Scholar] [CrossRef]
- Chatterjee, D.; Chaudhuri, K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Gayathri, P.; Gunasekaran., P.; Lagannadham, M.V.; Rajendhran, J. Comprehensive proteomic analysis and pathogenic role of membrane vesicles of Listeria monocytogenes serotype 4b reveals proteins associated with virulence and their possible interaction with host. Int. J. Med. Microbiol. 2019, 309, 199–212. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.B.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef]
- Vanhove, A.S.; Duperthuy, M.; Charrie‘re, G.M.; Le Roux, F.; Goudene‘ge, D.; Gourbal, B. Outer membrane vesicles are vehicles for the delivery of Vibrio tasmaniensis virulence factors to oyster immune cells. Environ. Microbiol. 2015, 17, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Bozhokina, E.; Kever, L.; Komissarchik, Y. Secretion and internalization of outer membrane vesicles (OMV) from Serratia grimesii during bacterial invasion. In Abstracts of the 2nd International Conference Smart Bio; Vytautas Magnus University: Kaunas, Lithuania, 2018; p. 237. [Google Scholar]
- Bozhokina, E. Outer membrane vesicles (OMV) of gram-negative bacteria Serratia grimesii: A role in bacterial invasion. In Abstracts of the 3rd International Conference Smart Bio; Vytautas Magnus University: Kaunas, Lithuania, 2019; p. 71. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaitlina, S.; Bozhokina, E.; Tsaplina, O.; Efremova, T. Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia. Int. J. Mol. Sci. 2020, 21, 4025. https://doi.org/10.3390/ijms21114025
Khaitlina S, Bozhokina E, Tsaplina O, Efremova T. Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia. International Journal of Molecular Sciences. 2020; 21(11):4025. https://doi.org/10.3390/ijms21114025
Chicago/Turabian StyleKhaitlina, Sofia, Ekaterina Bozhokina, Olga Tsaplina, and Tatiana Efremova. 2020. "Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia" International Journal of Molecular Sciences 21, no. 11: 4025. https://doi.org/10.3390/ijms21114025
APA StyleKhaitlina, S., Bozhokina, E., Tsaplina, O., & Efremova, T. (2020). Bacterial Actin-Specific Endoproteases Grimelysin and Protealysin as Virulence Factors Contributing to the Invasive Activities of Serratia. International Journal of Molecular Sciences, 21(11), 4025. https://doi.org/10.3390/ijms21114025




