Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer
Abstract
1. Introduction
2. Results
2.1. Characteristics of BCa Patients and Control Subjects
2.2. Correlations between the Analyzed miRNAs
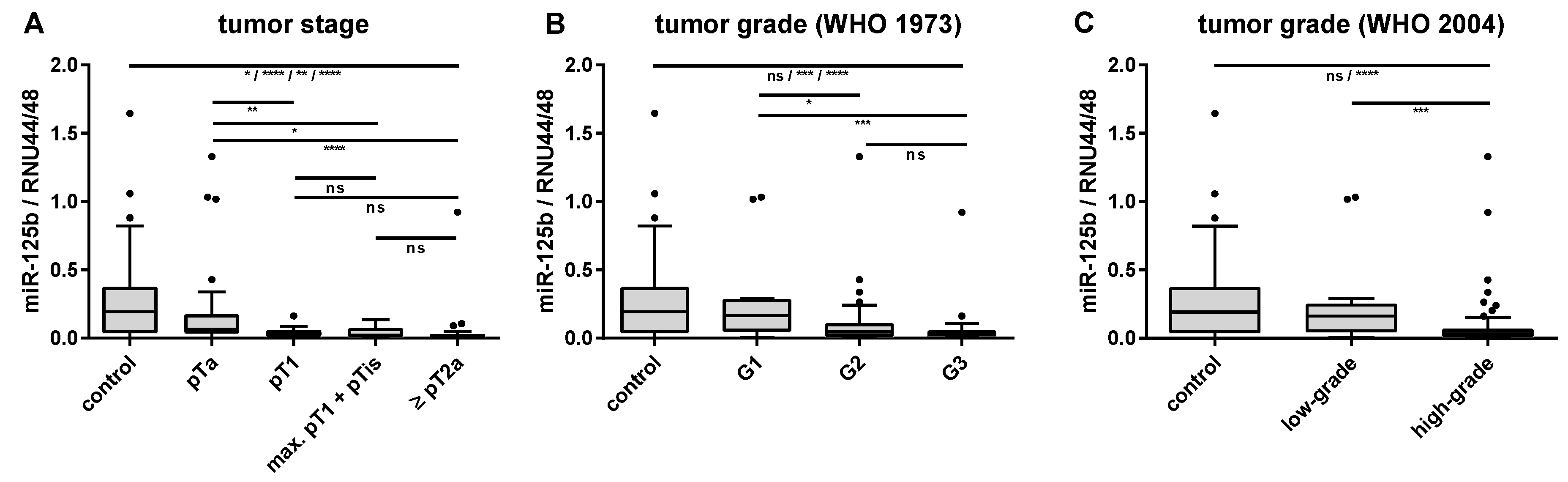
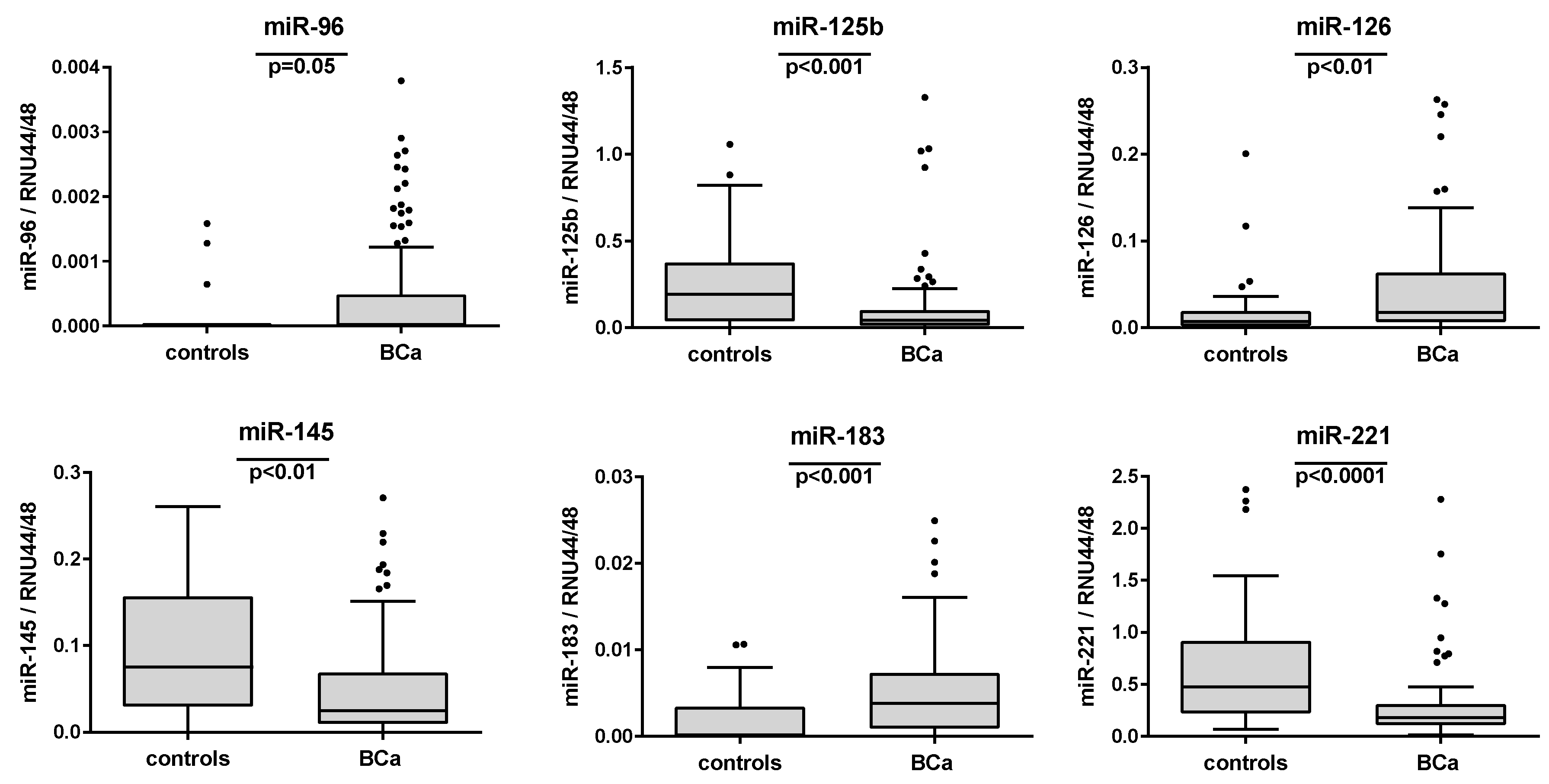
2.3. Associations of Urinary miRNA Transcript Levels and Histopathological Features
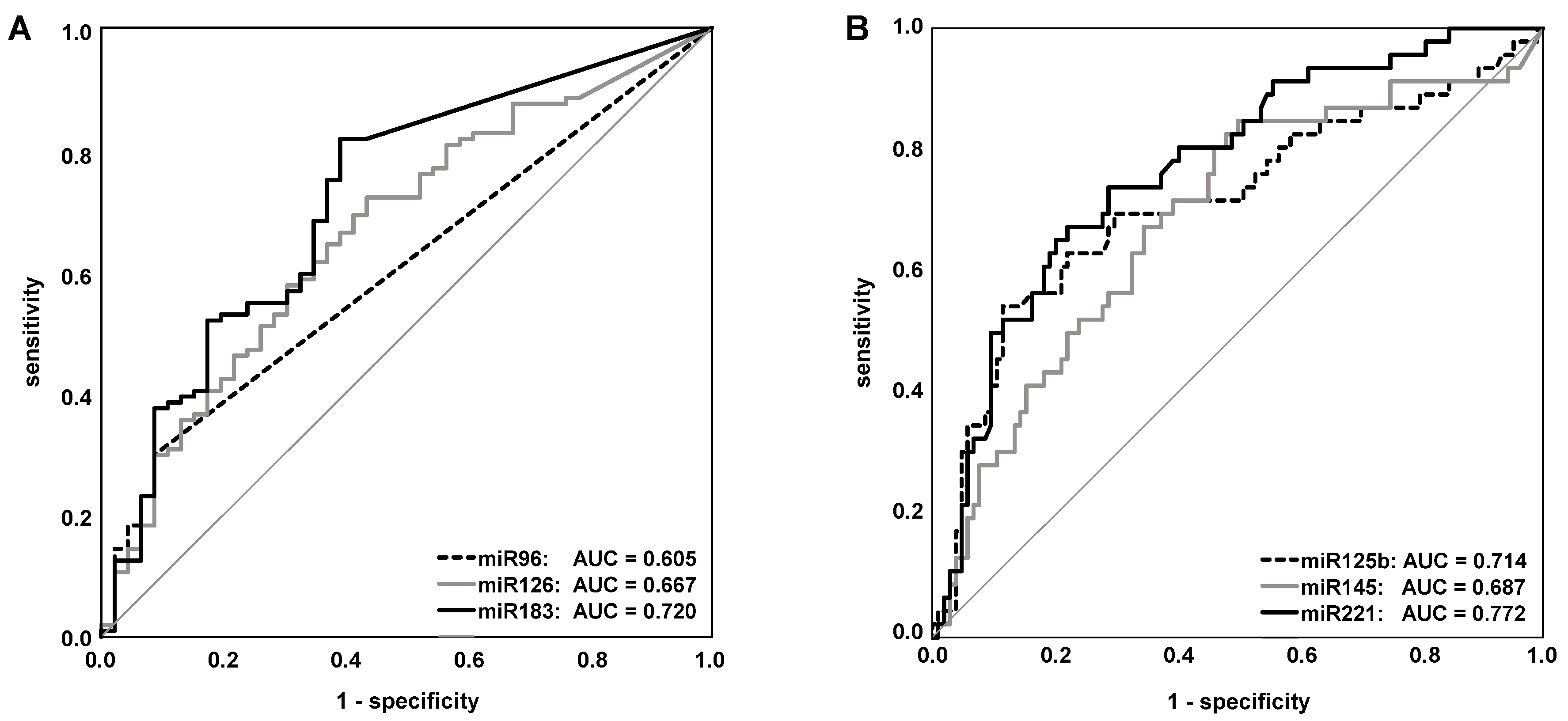
2.4. Evaluation of the Single miRNAs as Potential Markers for BCa Detection
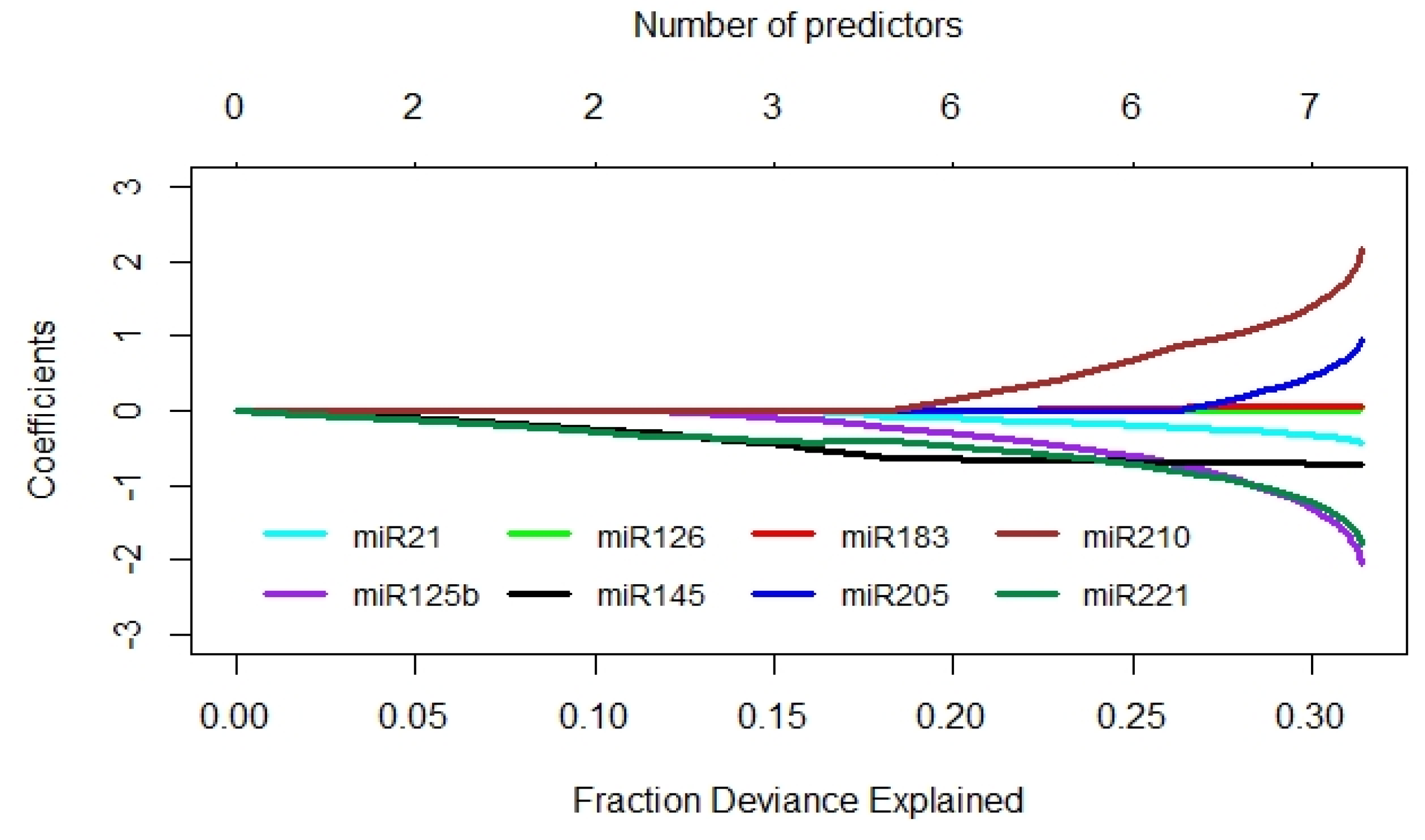
2.5. Comparison of Diagnostic Performance of miRNA Transcript Levels and Voided Urine Cytology
3. Discussion
4. Materials and Methods
4.1. Study Population, Data, and Sample Collection
4.2. Processing of Urine Samples, RNA Isolation, and cDNA Synthesis
4.3. Transcript Quantitation by Quantitative PCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | accuracy |
| AUC | area under the curve |
| BCa | bladder cancer |
| G | tumor grade |
| MIBC | muscle-invasive bladder cancer |
| miRNA or miR | microRNA |
| NGS | next-generation sequencing |
| nLR | negative likelihood ratio |
| NMIBC | non-muscle-invasive bladder cancer |
| ns | not significant |
| NPV | negative predictive value |
| pLR | positive likelihood ratio |
| PPV | positive predictive value |
| pT | pathological tumor stage |
| PUNLMP | papillary urothelial neoplasm of low malignant potential |
| qPCR | quantitative polymerase chain reaction |
| ROC | receiver operating characteristic |
| RT | reverse transcription |
| SNS | sensitivity |
| SPC | specificity |
| TUR-B | transurethral resection of the bladder |
| VUC | voided urine cytology |
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. (Basel) 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Bohle, A.; Burger, M.; Capoun, O.; Cohen, D.; Comperat, E.M.; Hernandez, V.; Kaasinen, E.; Palou, J.; Roupret, M.; et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2017, 71, 447–461. [Google Scholar] [CrossRef]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Hennenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Hollenbeck, B.K.; Holmang, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef]
- Maas, M.; Bedke, J.; Stenzl, A.; Todenhofer, T. Can urinary biomarkers replace cystoscopy? World J. Urol. 2019, 37, 1741–1749. [Google Scholar] [CrossRef]
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2017, 119, 371–380. [Google Scholar] [CrossRef]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef]
- D’Costa, J.J.; Goldsmith, J.C.; Wilson, J.S.; Bryan, R.T.; Ward, D.G. A Systematic Review of the Diagnostic and Prognostic Value of Urinary Protein Biomarkers in Urothelial Bladder Cancer. Bladder Cancer 2016, 2, 301–317. [Google Scholar] [CrossRef]
- Mengual, L.; Ribal, M.J.; Lozano, J.J.; Ingelmo-Torres, M.; Burset, M.; Fernandez, P.L.; Alcaraz, A. Validation study of a noninvasive urine test for diagnosis and prognosis assessment of bladder cancer: Evidence for improved models. J. Urol. 2014, 191, 261–269. [Google Scholar] [CrossRef]
- Ribal, M.J.; Mengual, L.; Lozano, J.J.; Ingelmo-Torres, M.; Palou, J.; Rodriguez-Faba, O.; Witjes, J.A.; Van der Heijden, A.G.; Medina, R.; Conde, J.M.; et al. Gene expression test for the non-invasive diagnosis of bladder cancer: A prospective, blinded, international and multicenter validation study. Eur. J. Cancer 2016, 54, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. Urinary mRNA biomarker panel for the detection of urothelial carcinoma. Oncotarget 2016, 7, 38731–38740. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. microRNA involvement in human cancer. Carcinogenesis 2012, 33, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Frixa, T.; Donzelli, S.; Blandino, G. Oncogenic MicroRNAs: Key Players in Malignant Transformation. Cancers (Basel) 2015, 7, 2466–2485. [Google Scholar] [CrossRef]
- Dong, F.; Xu, T.; Shen, Y.; Zhong, S.; Chen, S.; Ding, Q.; Shen, Z. Dysregulation of miRNAs in bladder cancer: Altered expression with aberrant biogenesis procedure. Oncotarget 2017, 8, 27547–27568. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Wu, Y.; Wu, Q.; Wang, Q.; Yang, Z.; Li, L. MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget 2017, 8, 32370–32379. [Google Scholar] [CrossRef]
- Matullo, G.; Naccarati, A.; Pardini, B. MicroRNA expression profiling in bladder cancer: The challenge of next-generation sequencing in tissues and biofluids. Int. J. Cancer 2016, 138, 2334–2345. [Google Scholar] [CrossRef]
- Ouyang, H.; Zhou, Y.; Zhang, L.; Shen, G. Diagnostic Value of MicroRNAs for Urologic Cancers: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015, 94, e1272. [Google Scholar] [CrossRef]
- Schubert, M.; Junker, K.; Heinzelmann, J. Prognostic and predictive miRNA biomarkers in bladder, kidney and prostate cancer: Where do we stand in biomarker development? J. Cancer Res. Clin. Oncol. 2016, 142, 1673–1695. [Google Scholar] [CrossRef]
- Enokida, H.; Yoshino, H.; Matsushita, R.; Nakagawa, M. The role of microRNAs in bladder cancer. Investig. Clin. Urol. 2016, 57, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Canturk, K.M.; Ozdemir, M.; Can, C.; Oner, S.; Emre, R.; Aslan, H.; Cilingir, O.; Ciftci, E.; Celayir, F.M.; Aldemir, O.; et al. Investigation of key miRNAs and target genes in bladder cancer using miRNA profiling and bioinformatic tools. Mol. Biol. Rep. 2014, 41, 8127–8135. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larre, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009, 69, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Deng, X.; Yang, X.; Li, P.; Zhang, X.; Li, P.; Tao, J.; Lu, Q.; Wang, Z. Urine microRNAs as biomarkers for bladder cancer: A diagnostic meta-analysis. Onco Targets Ther. 2015, 8, 2089–2096. [Google Scholar] [CrossRef]
- Ding, M.; Li, Y.; Wang, H.; Lv, Y.; Liang, J.; Wang, J.; Li, C. Diagnostic value of urinary microRNAs as non-invasive biomarkers for bladder cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15432–15440. [Google Scholar]
- Dyrskjot, L.; Ostenfeld, M.S.; Bramsen, J.B.; Silahtaroglu, A.N.; Lamy, P.; Ramanathan, R.; Fristrup, N.; Jensen, J.L.; Andersen, C.L.; Zieger, K.; et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009, 69, 4851–4860. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; Jiang, Z.; Zhang, Z.; Yang, R.; Chen, J.; et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS ONE 2011, 6, e18286. [Google Scholar] [CrossRef]
- Itesako, T.; Seki, N.; Yoshino, H.; Chiyomaru, T.; Yamasaki, T.; Hidaka, H.; Yonezawa, T.; Nohata, N.; Kinoshita, T.; Nakagawa, M.; et al. The microRNA expression signature of bladder cancer by deep sequencing: The functional significance of the miR-195/497 cluster. PLoS ONE 2014, 9, e84311. [Google Scholar] [CrossRef]
- Mengual, L.; Lozano, J.J.; Ingelmo-Torres, M.; Gazquez, C.; Ribal, M.J.; Alcaraz, A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int. J. Cancer 2013, 133, 2631–2641. [Google Scholar] [CrossRef]
- Sapre, N.; Macintyre, G.; Clarkson, M.; Naeem, H.; Cmero, M.; Kowalczyk, A.; Anderson, P.D.; Costello, A.J.; Corcoran, N.M.; Hovens, C.M. A urinary microRNA signature can predict the presence of bladder urothelial carcinoma in patients undergoing surveillance. Br. J. Cancer 2016, 114, 454–462. [Google Scholar] [CrossRef]
- Zaravinos, A.; Radojicic, J.; Lambrou, G.I.; Volanis, D.; Delakas, D.; Stathopoulos, E.N.; Spandidos, D.A. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial-mesenchymal transition and activation of metastasis in bladder cancer. J. Urol. 2012, 188, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Z.; Lau, K.M.; Chan, E.S.; Wang, G.; Szeto, C.C.; Wong, K.; Choy, R.K.; Ng, C.F. Cell-free urinary microRNA-99a and microRNA-125b are diagnostic markers for the non-invasive screening of bladder cancer. PLoS ONE 2014, 9, e100793. [Google Scholar] [CrossRef]
- Snowdon, J.; Boag, S.; Feilotter, H.; Izard, J.; Siemens, D.R. A pilot study of urinary microRNA as a biomarker for urothelial cancer. Can. Urol. Assoc. J. 2013, 7, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Pazourkova, E.; Horinek, A.; Brisuda, A.; Svobodova, I.; Soukup, V.; Hrbacek, J.; Capoun, O.; Hanus, T.; Mares, J.; et al. MicroRNAs in urine supernatant as potential non-invasive markers for bladder cancer detection. Neoplasma 2016, 63, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Dip, N.; Reis, S.T.; Srougi, M.; Dall’Oglio, M.F.; Leite, K.R. Expression profile of microrna-145 in urothelial bladder cancer. Int. Braz. J. Urol. 2013, 39, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Jeong, P.; Kim, W.T.; Kim, T.H.; Lee, Y.S.; Song, P.H.; Choi, Y.H.; Kim, I.Y.; Moon, S.K.; Kim, W.J. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int. J. Oncol. 2012, 41, 1871–1878. [Google Scholar] [CrossRef]
- Mearini, E.; Poli, G.; Cochetti, G.; Boni, A.; Egidi, M.G.; Brancorsini, S. Expression of urinary miRNAs targeting NLRs inflammasomes in bladder cancer. Onco Targets Ther. 2017, 10, 2665–2673. [Google Scholar] [CrossRef]
- Dambal, S.; Shah, M.; Mihelich, B.; Nonn, L. The microRNA-183 cluster: The family that plays together stays together. Nucleic Acids Res. 2015, 43, 7173–7188. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, A.J.; Fan, Y.P.; Huang, Y.R.; Zhao, X.M.; Sun, Y.; Chen, X.F. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget 2016, 7, 42805–42825. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Sun, H.M.; Zheng, R.Z.; Li, Y.C.; Zhang, Q.; Cheng, P.; Tang, Z.H.; Huang, F. Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene 2013, 527, 26–32. [Google Scholar] [CrossRef]
- Yamada, Y.; Enokida, H.; Kojima, S.; Kawakami, K.; Chiyomaru, T.; Tatarano, S.; Yoshino, H.; Kawahara, K.; Nishiyama, K.; Seki, N.; et al. MiR-96 and miR-183 detection in urine serve as potential tumor markers of urothelial carcinoma: Correlation with stage and grade, and comparison with urinary cytology. Cancer Sci. 2011, 102, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Habib, H.; Ali, E.; Kotb, Y. Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med. Oncol. 2015, 32, 413. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Essawy, N.O.; Kotb, Y.M. Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumour Biol. 2015, 36, 9545–9552. [Google Scholar] [CrossRef]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016, 7, 86290–86299. [Google Scholar] [CrossRef]
- Pardini, B.; Cordero, F.; Naccarati, A.; Viberti, C.; Birolo, G.; Oderda, M.; Di Gaetano, C.; Arigoni, M.; Martina, F.; Calogero, R.A.; et al. microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018, 9, 20658–20669. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.; Hoefig, K.; Merz, H.; Feller, A.C.; Kausch, I.; Jocham, D.; Warnecke, J.M.; Sczakiel, G. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol. Oncol. 2010, 28, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Hegazy, M.G.; Kotb, Y.M.; Essawy, N.O. Evaluation of urinary microRNA panel in bladder cancer diagnosis: Relation to bilharziasis. Transl. Res. 2015, 165, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Long, J.D.; Sullivan, T.B.; Humphrey, J.; Logvinenko, T.; Summerhayes, K.A.; Kozinn, S.; Harty, N.; Summerhayes, I.C.; Libertino, J.A.; Holway, A.H.; et al. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015, 7, 2500–2509. [Google Scholar]
- Matsuzaki, K.; Fujita, K.; Jingushi, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Ueda, Y.; Tanigawa, G.; Yoshioka, I.; Ueda, K.; et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget 2017, 8, 24668–24678. [Google Scholar] [CrossRef]
- Michailidi, C.; Hayashi, M.; Datta, S.; Sen, T.; Zenner, K.; Oladeru, O.; Brait, M.; Izumchenko, E.; Baras, A.; VandenBussche, C.; et al. Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use. Cancer Prev. Res. (Phila) 2015, 8, 208–221. [Google Scholar] [CrossRef]
- Wang, G.; Chan, E.S.; Kwan, B.C.; Li, P.K.; Yip, S.K.; Szeto, C.C.; Ng, C.F. Expression of microRNAs in the urine of patients with bladder cancer. Clin. Genitourin. Cancer 2012, 10, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Choi, C.; Kwon, D.D. Urinary microRNAs as potential biomarkers for differentiating the “atypical urothelial cells” category of the Paris system for reporting urine cytology. Int. J. Clin. Exp. Pathol. 2017, 10, 8303–8313. [Google Scholar] [PubMed]
- Fendler, A.; Stephan, C.; Yousef, G.M.; Jung, K. MicroRNAs as regulators of signal transduction in urological tumors. Clin. Chem. 2011, 57, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Gulia, C.; Baldassarra, S.; Signore, F.; Rigon, G.; Pizzuti, V.; Gaffi, M.; Briganti, V.; Porrello, A.; Piergentili, R. Role of Non-Coding RNAs in the Etiology of Bladder Cancer. Genes (Basel) 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leao, R.; Maximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics (Basel) 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Valenberg, F.; Hiar, A.M.; Wallace, E.; Bridge, J.A.; Mayne, D.J.; Beqaj, S.; Sexton, W.J.; Lotan, Y.; Weizer, A.Z.; Jansz, G.K.; et al. Prospective Validation of an mRNA-based Urine Test for Surveillance of Patients with Bladder Cancer. Eur. Urol. 2019, 75, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Morote, J.; Cornel, E.B.; Gakis, G.; van Valenberg, F.J.P.; Lozano, F.; Sternberg, I.A.; Willemsen, E.; Hegemann, M.L.; Paitan, Y.; et al. Performance of the Bladder EpiCheck Methylation Test for Patients Under Surveillance for Non-muscle-invasive Bladder Cancer: Results of a Multicenter, Prospective, Blinded Clinical Trial. Eur. Urol. Oncol. 2018, 1, 307–313. [Google Scholar] [CrossRef]
- Trenti, E.; Pycha, S.; Mian, C.; Schwienbacher, C.; Hanspeter, E.; Kafka, M.; Spedicato, G.A.; Vjaters, E.; Degener, S.; Pycha, A.; et al. Comparison of 2 new real-time polymerase chain reaction-based urinary markers in the follow-up of patients with non-muscle-invasive bladder cancer. Cancer Cytopathol. 2020, 128, 341–347. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Butaney, M.; Weight, C.J.; Kumar, R.; Konety, B.R. Urinary Biomarkers in the Evaluation of Primary Hematuria: A Systematic Review and Meta-Analysis. Bladder Cancer 2018, 4, 353–363. [Google Scholar] [CrossRef]
- Van Kessel, K.E.; Beukers, W.; Lurkin, I.; Ziel-van der Made, A.; van der Keur, K.A.; Boormans, J.L.; Dyrskjot, L.; Marquez, M.; Orntoft, T.F.; Real, F.X.; et al. Validation of a DNA Methylation-Mutation Urine Assay to Select Patients with Hematuria for Cystoscopy. J. Urol. 2017, 197, 590–595. [Google Scholar] [CrossRef]
- Sasaki, H.; Yoshiike, M.; Nozawa, S.; Usuba, W.; Katsuoka, Y.; Aida, K.; Kitajima, K.; Kudo, H.; Hoshikawa, M.; Yoshioka, Y.; et al. Expression Level of Urinary MicroRNA-146a-5p Is Increased in Patients With Bladder Cancer and Decreased in Those After Transurethral Resection. Clin. Genitourin. Cancer 2016, 14, e493–e499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Liu, X.; Fang, A.; Wang, J.; Yang, Y.; Wang, L.; Du, L.; Wang, C. Direct quantitative detection for cell-free miR-155 in urine: A potential role in diagnosis and prognosis for non-muscle invasive bladder cancer. Oncotarget 2016, 7, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- TNM Classification of Malignant Tumours; Sobin, L.H., Gospodarowicz, M.K., Wittekind, C.E., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Smith, S.C.; Reuter, V.E.; Epstein, J.I.; Grignon, D.J.; Hansel, D.E.; Lin, O.; McKenney, J.K.; Montironi, R.; Paner, G.P.; et al. Update for the practicing pathologist: The International Consultation On Urologic Disease-European association of urology consultation on bladder cancer. Mod. Pathol. 2015, 28, 612–630. [Google Scholar] [CrossRef]
- Papanicolaou, G.N.; Marshall, V.F. URINE SEDIMENT SMEARS AS A DIAGNOSTIC PROCEDURE IN CANCERS OF THE URINARY TRACT. Science 1945, 101, 519–520. [Google Scholar] [CrossRef]
- Marano, G.; Boracchi, P.; Biganzoli, E.M.; R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Open J. Stat. 2016, 6, 3. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wienertitle, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
| Parameter | Category | Number (n) | Percentage (%) |
|---|---|---|---|
| gender | male | 83 | 79.8 |
| female | 21 | 20.2 | |
| age 1 (years) | ≤70.0 | 54 | 51.9 |
| >70.0 | 50 | 48.1 | |
| tumor stage | pTa | 50 | 48.1 |
| pT1 | 22 | 21.2 | |
| pTis | 15 | 14.4 | |
| pTis only | 0 | 0.0 | |
| pTis + pTa | 3 | 2.9 | |
| pTis + pT1 | 7 | 6.7 | |
| pTis + ≥ pT2a | 5 | 4.8 | |
| ≥ pT2a | 17 | 16.3 | |
| tumor grade (WHO 1973) | G1 | 14 | 13.5 |
| G2 | 52 | 50.0 | |
| G3 | 38 | 36.5 | |
| tumor grade (WHO 2004) multifocality | low-grade | 17 | 16.3 |
| high-grade | 87 | 83.7 | |
| unifocal | 72 | 69.2 | |
| multifocal | 32 | 30.8 | |
| voided urine | positive | 80 | 76.9 |
| cytology | negative | 24 | 23.1 |
| Parameter | Category | Number (n) | Percentage (%) |
|---|---|---|---|
| gender | male | 30 | 65.2 |
| female | 16 | 34.8 | |
| age 1 (years) | <64.5 | 23 | 50.0 |
| ≥64.5 | 23 | 50.0 | |
| diagnosis | BCa-negative TUR-B | 8 | 17.4 |
| urolithiasis | 38 | 82.6 | |
| voided urine | positive | 0 | 0.0 |
| cytology | negative | 46 | 100.0 |
| miRNA | Regulation | Mann–Whitney | ROC Curve Analysis | |
|---|---|---|---|---|
| in BCa | U Test (p-Value) | AUC | p-Value | |
| miR-21 | not different | =1.000 | 0.581 | =1.000 |
| miR-96 | up | =0.050 | 0.605 | =0.369 |
| miR-125b | down | <0.001 | 0.714 | <0.001 |
| miR-126 | up | <0.01 | 0.667 | <0.01 |
| miR-145 | down | <0.01 | 0.687 | <0.01 |
| miR-183 | up | <0.001 | 0.720 | <0.001 |
| miR-205 | not different | =1.000 | 0.537 | =1.000 |
| miR-210 | not different | =1.000 | 0.526 | =1.000 |
| miR-221 | down | <0.0001 | 0.772 | <0.0001 |
| Parameter | miR-21 | -96 | -125b | -126 | -145 | -183 | -205 | -210 | -221 | VUC |
|---|---|---|---|---|---|---|---|---|---|---|
| SNS | 0.865 | 0.298 | 0.885 | 0.885 | 0.500 | 0.817 | 0.779 | 0.663 | 0.779 | 0.769 |
| SPC | 0.304 | 0.913 | 0.543 | 0.217 | 0.848 | 0.609 | 0.435 | 0.500 | 0.674 | 1.000 |
| PPV | 0.738 | 0.886 | 0.814 | 0.719 | 0.881 | 0.825 | 0.757 | 0.750 | 0.844 | 1.000 |
| NPV | 0.500 | 0.365 | 0.676 | 0.455 | 0.429 | 0.596 | 0.465 | 0.397 | 0.574 | 0.657 |
| pLR | 1.244 | 3.428 | 1.938 | 1.130 | 3.286 | 2.089 | 1.378 | 1.327 | 2.388 | n.d. |
| nLR | 0.442 | 0.769 | 0.212 | 0.531 | 0.590 | 0.300 | 0.509 | 0.673 | 0.328 | 0.231 |
| ACC | 0.693 | 0.487 | 0.780 | 0.680 | 0.607 | 0.753 | 0.673 | 0.613 | 0.747 | 0.840 |
| Parameter | 6 miRs 96/125b/126/145/183/221 0-3/4-6 pos. Markers | 4 miRs 125b/145/183/221 0-2/3-4 pos. Markers | 6 miRs + VUC 96/125b/126/145/183/221 0-3/4-7 pos. Markers | 4 miRs + VUC 125b/145/183/221 0-2/3-5 pos. Markers |
|---|---|---|---|---|
| SNS | 0.731 | 0.731 | 0.808 | 0.846 |
| SPC | 0.935 | 0.957 | 0.935 | 0.957 |
| PPV | 0.962 | 0.974 | 0.966 | 0.978 |
| NPV | 0.606 | 0.611 | 0.683 | 0.733 |
| pLR | 11.205 | 16.808 | 12.385 | 19.462 |
| nLR | 0.288 | 0.281 | 0.206 | 0.161 |
| ACC | 0.793 | 0.800 | 0.847 | 0.880 |
| miRNA | Assay Name | Assay ID |
|---|---|---|
| miR-21-5p | hsa-miR-21 | 000397 |
| miR-96-5p | mmu-miR-96 (for hsa-miR-96-5p) | 000186 |
| miR-125b-5p | hsa-miR-125b | 000449 |
| miR-126-3p | hsa-miR-126 | 002228 |
| miR-145-5p | hsa-miR-145 | 002278 |
| miR-183-5p | hsa-miR-183 | 002269 |
| miR-205-5p | hsa-miR-205 | 000509 |
| miR-210-3p | hsa-miR-210 | 000512 |
| miR-221-3p | hsa-miR-221 | 000524 |
| RNU44 (NR_002750) * | RNU44 | 001094 |
| RNU48 (NR_002745) * | RNU48 | 001006 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdmann, K.; Salomo, K.; Klimova, A.; Heberling, U.; Lohse-Fischer, A.; Fuehrer, R.; Thomas, C.; Roeder, I.; Froehner, M.; Wirth, M.P.; et al. Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer. Int. J. Mol. Sci. 2020, 21, 3814. https://doi.org/10.3390/ijms21113814
Erdmann K, Salomo K, Klimova A, Heberling U, Lohse-Fischer A, Fuehrer R, Thomas C, Roeder I, Froehner M, Wirth MP, et al. Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer. International Journal of Molecular Sciences. 2020; 21(11):3814. https://doi.org/10.3390/ijms21113814
Chicago/Turabian StyleErdmann, Kati, Karsten Salomo, Anna Klimova, Ulrike Heberling, Andrea Lohse-Fischer, Romy Fuehrer, Christian Thomas, Ingo Roeder, Michael Froehner, Manfred P. Wirth, and et al. 2020. "Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer" International Journal of Molecular Sciences 21, no. 11: 3814. https://doi.org/10.3390/ijms21113814
APA StyleErdmann, K., Salomo, K., Klimova, A., Heberling, U., Lohse-Fischer, A., Fuehrer, R., Thomas, C., Roeder, I., Froehner, M., Wirth, M. P., & Fuessel, S. (2020). Urinary MicroRNAs as Potential Markers for Non-Invasive Diagnosis of Bladder Cancer. International Journal of Molecular Sciences, 21(11), 3814. https://doi.org/10.3390/ijms21113814





