(+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells
Abstract
1. Introduction
2. Results
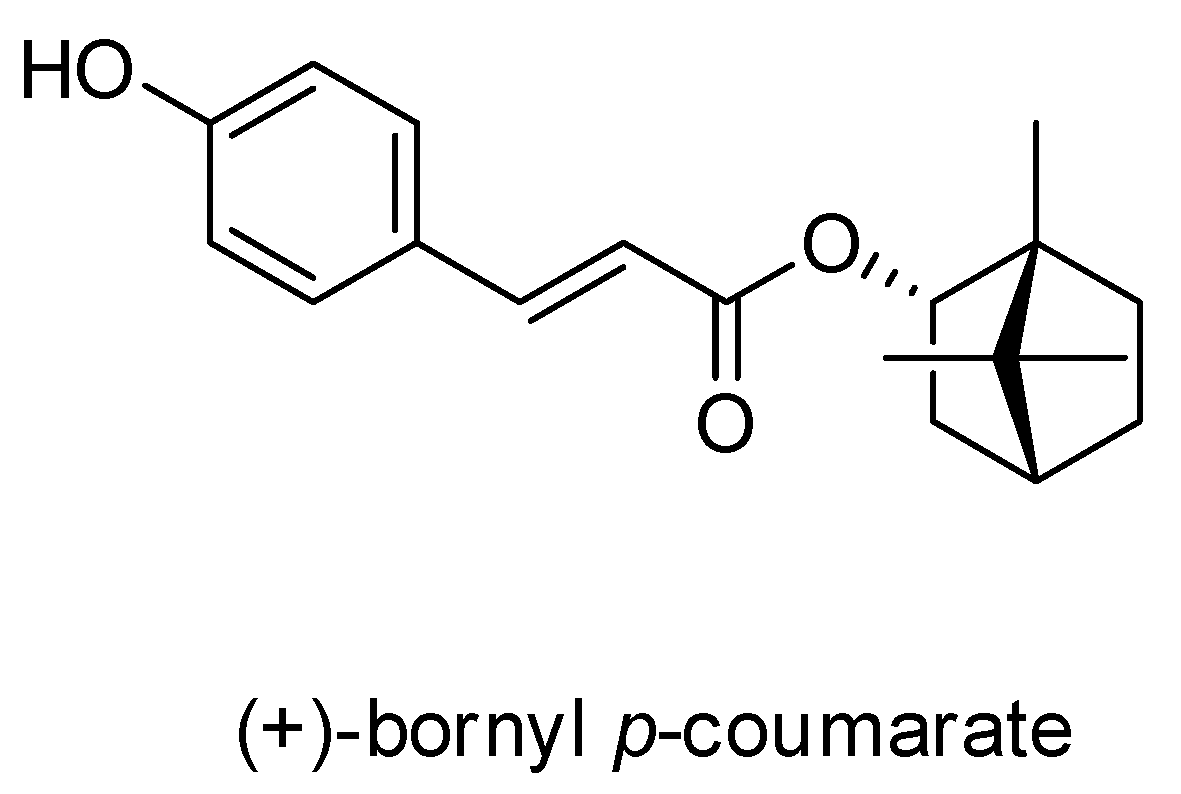
2.1. Characterization of the Constituents of the EA Fraction of the Piper Betle Stem
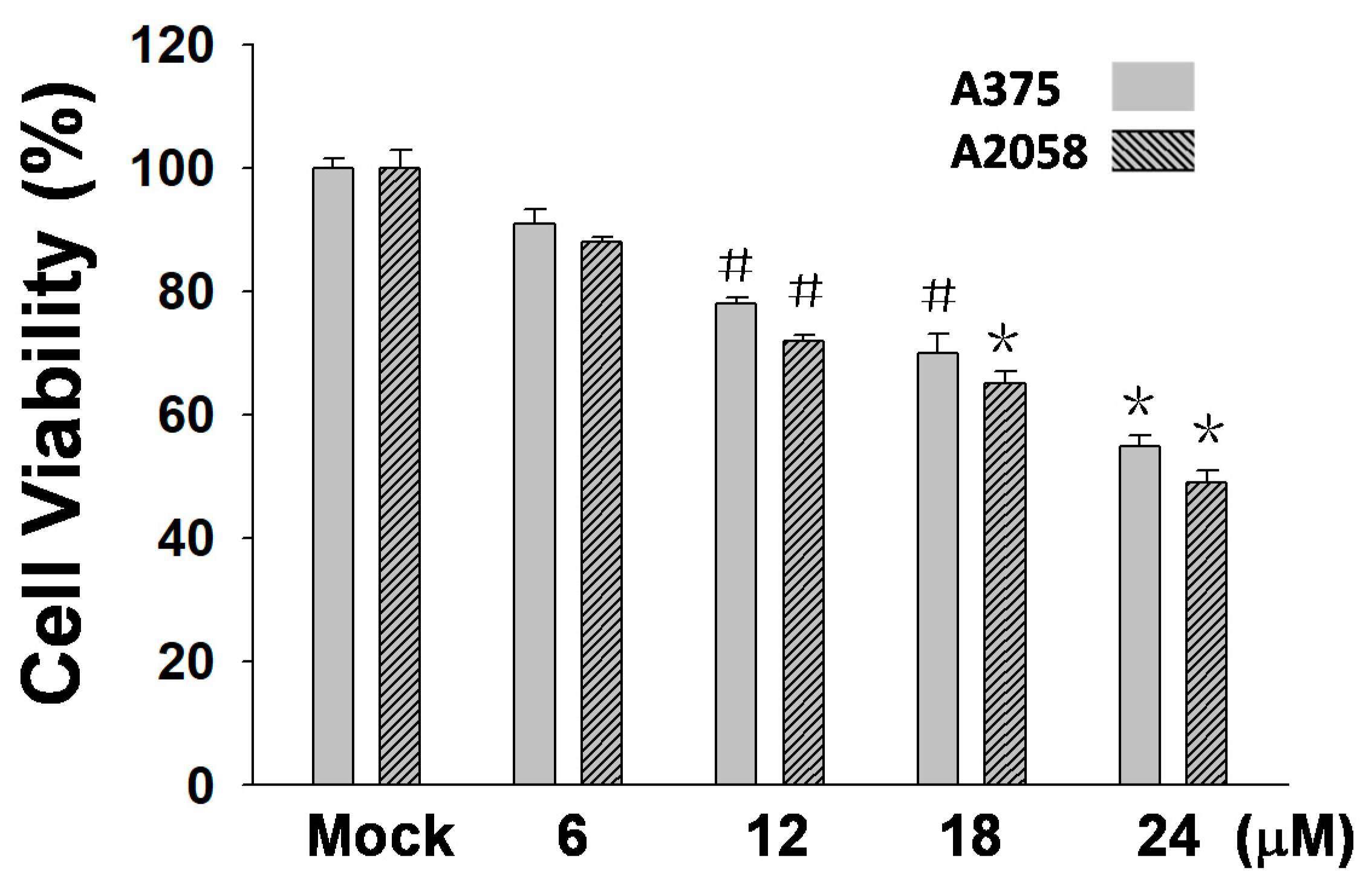
2.2. (+)-Bornyl p-Coumarate Inhibits Melanocyte Cancer Cell Proliferation
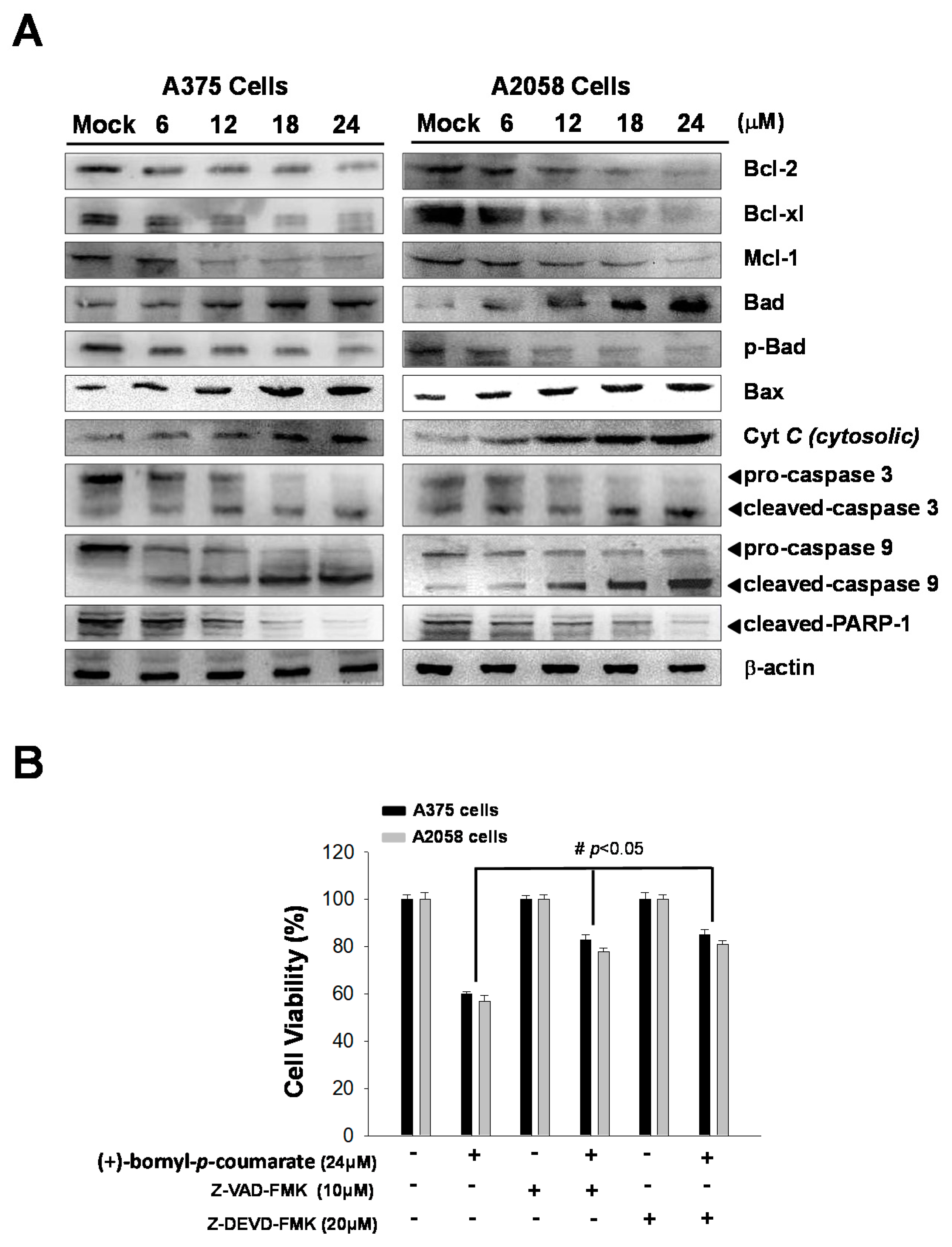
2.3. (+)-Bornyl p-Coumarate Induced Apoptosis in Melanoma Cells
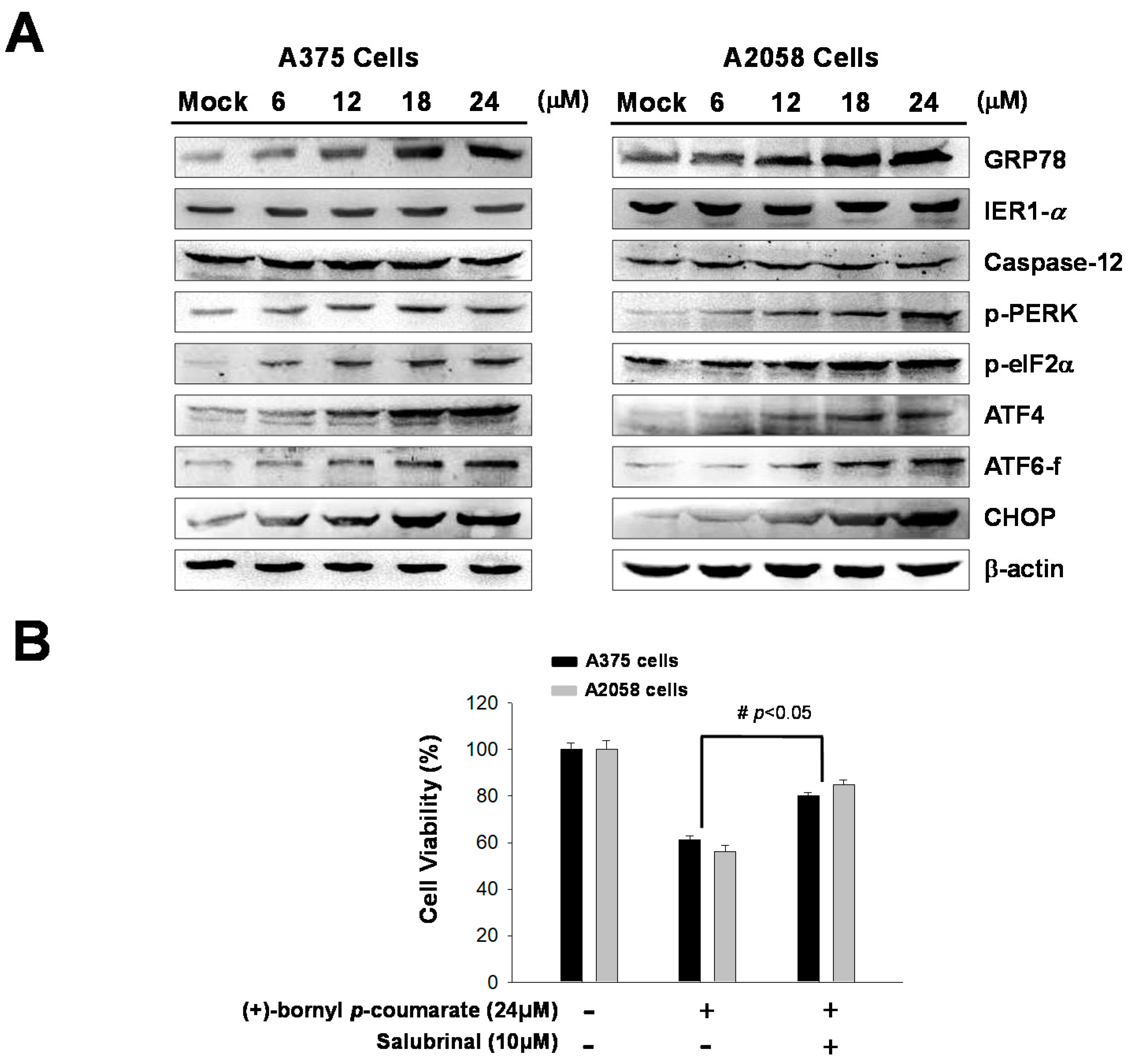
2.4. (+)-Bornyl p-Coumarate Treatment Induced the Endoplasmic Reticulum (ER) Stress Pathway
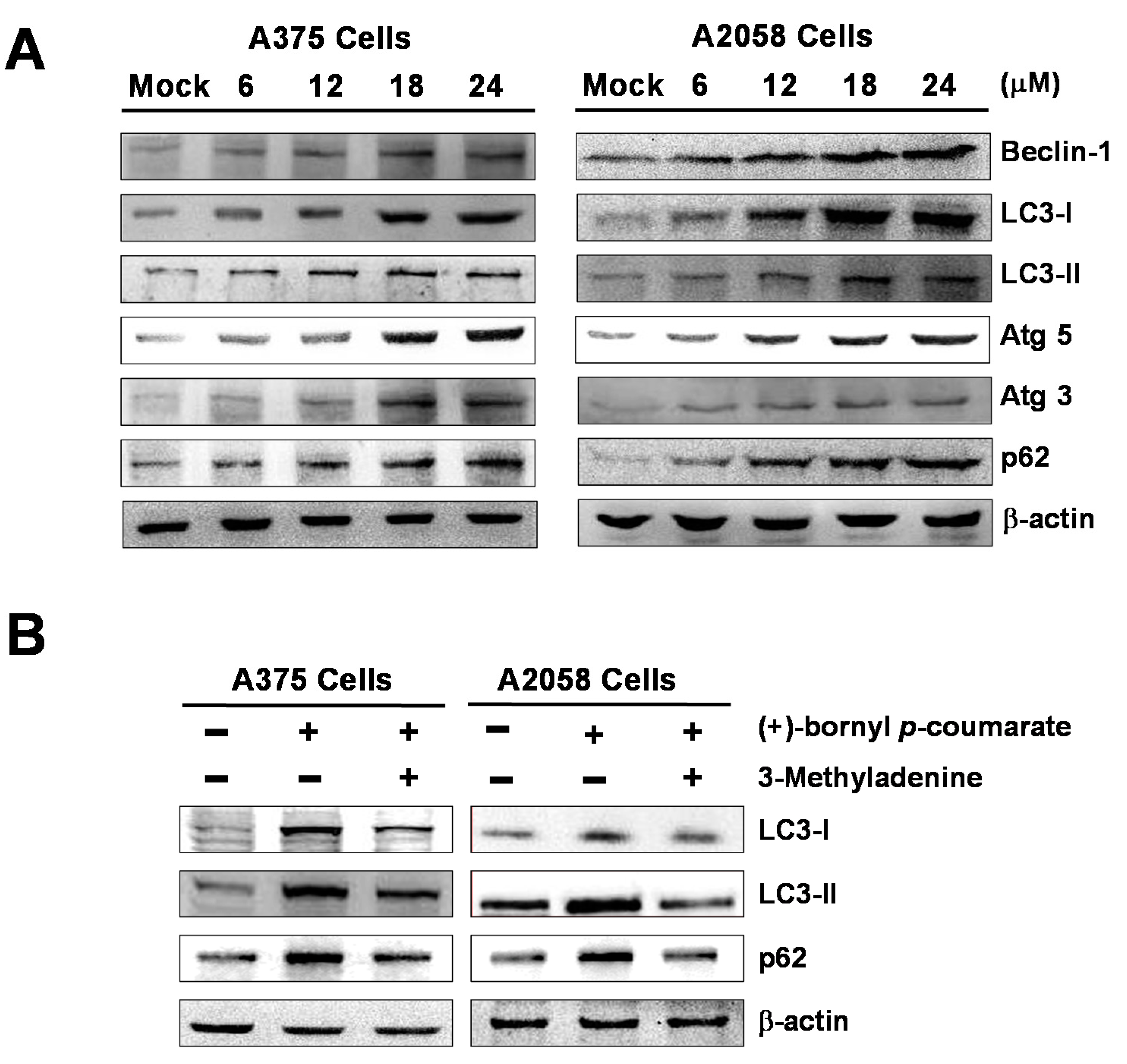
2.5. (+)-Bornyl p-Coumarate Triggers Autophagy in Melanoma Cells
3. Discussion
4. Materials and Methods
4.1. General Instrumental Operation for the Isolation and Identification of Compounds
4.2. Preparation of P. betle Stem Extract
4.3. Reagents
4.4. Cell Culture and Drug Treatment
4.5. Cell Viability Assay
4.6. Flow Cytometric Assessment of Apoptosis
4.7. Immunofluorescence Microscopy
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erdei, E.; Torres, S.M. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, H.; Drakensjö, I.R.T.; Girnita, A. Personalized medicine in malignant melanoma: Towards patient tailored treatment. Front. Oncol. 2018, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Lortet-Tieulent, J.; Schüz, J.; Zeeb, H.; Greinert, R.; Breitbart, E.W.; Bray, F. International trends in the incidence of malignant melanoma 1953–2008—Are recent generations at higher or lower risk? Int. J. Cancer 2013, 132, 385–400. [Google Scholar]
- Langley, A.; Levesque, L.; Baetz, T.; Asai, Y. Brief report: Increase in melanoma incidence in Ontario. J. Cutan. Med. Surg. 2018, 22, 476–478. [Google Scholar] [CrossRef]
- Linos, E.; Swetter, S.M.; Cockburn, M.G.; Colditz, G.A.; Clarke, C.A. Increasing burden of melanoma in the United States. J. Investig. Dermatol. 2009, 129, 1666–1674. [Google Scholar] [CrossRef]
- Balch, C.M.; Buzaid, A.C.; Soong, S.-J.; Atkins, M.B.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A., Jr.; Kirkwood, J.M. Final version of the american joint committee on cancer staging system for cutaneous melanoma. J. Clin. Oncol. 2001, 19, 3635–3648. [Google Scholar] [CrossRef]
- Vijuk, G.; Coates, A. Survival of patients with visceral metastatic melanoma from an occult primary lesion: A retrospective matched cohort study. Ann. Oncol. 1998, 9, 419–422. [Google Scholar] [CrossRef]
- Soengas, M.S.; Lowe, S.W. Apoptosis and melanoma chemoresistance. Oncogene 2003, 22, 3138–3151. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277. [Google Scholar] [CrossRef]
- Copetti, T.; Bertoli, C.; Dalla, E.; Demarchi, F.; Schneider, C. P65/rela modulates becn1 transcription and autophagy. Mol. Cell. Biol. 2009, 29, 2594–2608. [Google Scholar] [CrossRef]
- Gozuacik, D.; Kimchi, A. Dapk protein family and cancer. Autophagy 2006, 2, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Vessoni, A.; Filippi-Chiela, E.; Menck, C.F.; Lenz, G. Autophagy and genomic integrity. Cell Death Differ. 2013, 20, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Jin, S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2006, 2, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Mariño, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Arico, S.; Petiot, A.; Bauvy, C.; Dubbelhuis, P.F.; Meijer, A.J.; Codogno, P.; Ogier-Denis, E. The tumor suppressor pten positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase b pathway. J. Biol. Chem. 2001, 276, 35243–35246. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Shah, B. Antimicrobial, antioxidative and antihemolytic activity of piper betel leaf extracts. Int. J. Pharm. Pharm. Sci. 2011, 3, 192–199. [Google Scholar]
- Kumar, N.; Misra, P.; Dube, A.; Bhattacharya, S.; Dikshit, M.; Ranade, S. Piper betle linn. A maligned pan-asiatic plant with an array of pharmacological activities and prospects for drug discovery. Curr. Sci. 2010, 99, 922–932. [Google Scholar]
- Rai, M.P.; Thilakchand, K.R.; Palatty, P.L.; Rao, P.; Rao, S.; Bhat, H.P.; Baliga, M.S. Piper betel linn (betel vine), the maligned southeast Asian medicinal plant possesses cancer preventive effects: Time to reconsider the wronged opinion. Asian Pac. J. Cancer Prev. 2011, 12, 2149–2156. [Google Scholar]
- Nagori, K.; Singh, M.K.; Alexander, A.; Kumar, T.; Dewangan, D.; Badwaik, H.; Tripathi, D. Piper betlel: A review on its ethnobotany, phytochemistry, pharmacological profile and profiling by new hyphenated technique dart-ms (direct analysis in real time mass spectrometry). J. Pharm. Res. 2011, 4, 2991–2997. [Google Scholar]
- Chan, L.-P.; Tseng, Y.-P.; Ding, H.-Y.; Pan, S.-M.; Chiang, F.-Y.; Wang, L.-F.; Chou, T.-H.; Lien, P.-J.; Liu, C.; Kuo, P.-L. Tris (8-hydroxyquinoline) iron induces apoptotic cell death via oxidative stress and by activating death receptor signaling pathway in human head and neck carcinoma cells. Phytomedicine 2019, 63, 153005. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, H.; Zhang, S.; Dai, W.; Zhang, Y.; Hong, L.; Chen, F.; Cao, J. Activation of reactive oxygen species-mediated mitogen-activated protein kinases pathway regulates both extrinsic and intrinsic apoptosis induced by arctigenin in hep g2. J. Pharm. Pharmacol. 2020, 72, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 25935–25938. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, J.M.; Alford, S.E.; Sakurikar, N.; Chambers, T.C. Molecular analysis of functional redundancy among anti-apoptotic bcl-2 proteins and its role in cancer cell survival. Exp. Cell Res. 2014, 322, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Gunadharini, D.; Senthilkumar, K.; Banudevi, S.; Arunkumar, R.; Benson, C.; Sharmila, G.; Arunakaran, J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012, 215, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The bcl-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Göke, A.; Göke, R.; Ofner, A.; Herbst, A.; Lankat-Buutgereit, B. The fgfr inhibitor nvp-bgj398 induces nsclc cell death by activating caspase-dependent pathways as well as caspase-independent apoptosis. Anticancer. Res. 2015, 35, 5873–5879. [Google Scholar]
- Arnoult, D.; Gaume, B.; Karbowski, M.; Sharpe, J.C.; Cecconi, F.; Youle, R.J. Mitochondrial release of aif and endog requires caspase activation downstream of bax/bak-mediated permeabilization. EMBO J. 2003, 22, 4385–4399. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Green, D.R. How do bcl-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008, 18, 157–164. [Google Scholar] [CrossRef]
- McIlwain, D.; Berger, T.; Mak, T. Caspase functions in cell death and disease. Cold Spring Harb. Pperspect. Biol. 2013, 5, A008656. [Google Scholar] [CrossRef]
- Goan, Y.-G.; Wu, W.-T.; Liu, C.-I.; Neoh, C.-A.; Wu, Y.-J. Involvement of mitochondrial dysfunction, endoplasmic reticulum stress, and the pi3k/akt/mtor pathway in nobiletin-induced apoptosis of human bladder cancer cells. Molecules 2019, 24, 2881. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-Y.; Wu, Y.-J.; Chang, C.-I.; Chiu, C.-C.; Wu, M.-L. The effect of bornyl cis-4-hydroxycinnamate on melanoma cell apoptosis is associated with mitochondrial dysfunction and endoplasmic reticulum stress. Int. J. Mol. Sci. 2018, 19, 1370. [Google Scholar] [CrossRef] [PubMed]
- Kouroku, Y.; Fujita, E.; Tanida, I.; Ueno, T.; Isoai, A.; Kumagai, H.; Ogawa, S.; Kaufman, R.; Kominami, E.; Momoi, T. Er stress (perk/eif2α phosphorylation) mediates the polyglutamine-induced lc3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007, 14, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of chop/gadd153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Ochiai, K.; Kondo, S.; Tsumagari, K.; Murakami, T.; Cavener, D.R.; Imaizumi, K. Endoplasmic reticulum stress response mediated by the perk-eif2α-atf4 pathway is involved in osteoblast differentiation induced by bmp2. J. Biol. Chem. 2011, 286, 4809–4818. [Google Scholar] [CrossRef]
- Gross, A.; Graef, M. Mechanisms of autophagy in metabolic stress response. J. Mol. Biol. 2020, 432, 28–52. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, H. Core autophagy genes and human diseases. Curr. Opin. Cell Biol. 2019, 61, 117–125. [Google Scholar] [CrossRef]
- Kelekar, A. Autophagy. Ann. N. Y. Acad. Sci. 2005, 1066, 259–271. [Google Scholar] [CrossRef]
- Høyer-Hansen, M.; Bastholm, L.; Mathiasen, I.; Elling, F.; Jäättelä, M. Vitamin d analog eb1089 triggers dramatic lysosomal changes and beclin 1-mediated autophagic cell death. Cell Death Differ. 2005, 12, 1297–1309. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wang, J.; Chang, Q.; Hu, Z.; Shen, X.; Feng, J.; Zhang, Z.; Wu, X. Total flavonoid aglycones extract in radix scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J. Ethnopharmacol. 2019, 235, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Selvakumaran, M.; Amaravadi, R.K.; Vasilevskaya, I.A.; O’Dwyer, P.J. Autophagy inhibition sensitizes colon cancer cells to antiangiogenic and cytotoxic therapy. Clin. Cancer Res. 2013, 19, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Lim, C.J.; Chung, H.-J.; Kim, J.-H.; Huh, Y.H.; Park, K.; Jeong, S. Autophagy activation by crepidiastrum denticulatum extract attenuates environmental pollutant-induced damage in dermal fibroblasts. Int. J. Mol. Sci. 2019, 20, 517. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-J.; Su, T.-R.; Chang, C.-I.; Chen, C.-R.; Hung, K.-F.; Liu, C. (+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 3737. https://doi.org/10.3390/ijms21103737
Wu Y-J, Su T-R, Chang C-I, Chen C-R, Hung K-F, Liu C. (+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells. International Journal of Molecular Sciences. 2020; 21(10):3737. https://doi.org/10.3390/ijms21103737
Chicago/Turabian StyleWu, Yu-Jen, Tzu-Rong Su, Chi-I Chang, Chiy-Rong Chen, Kuo-Feng Hung, and Cheng Liu. 2020. "(+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells" International Journal of Molecular Sciences 21, no. 10: 3737. https://doi.org/10.3390/ijms21103737
APA StyleWu, Y.-J., Su, T.-R., Chang, C.-I., Chen, C.-R., Hung, K.-F., & Liu, C. (2020). (+)-Bornyl p-Coumarate Extracted from Stem of Piper betle Induced Apoptosis and Autophagy in Melanoma Cells. International Journal of Molecular Sciences, 21(10), 3737. https://doi.org/10.3390/ijms21103737



