APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF
Abstract
:1. Introduction
2. Results
2.1. APE1 Stimulates GFRα1 Expression to Promote Pancreatic Cancer Cell Proliferation
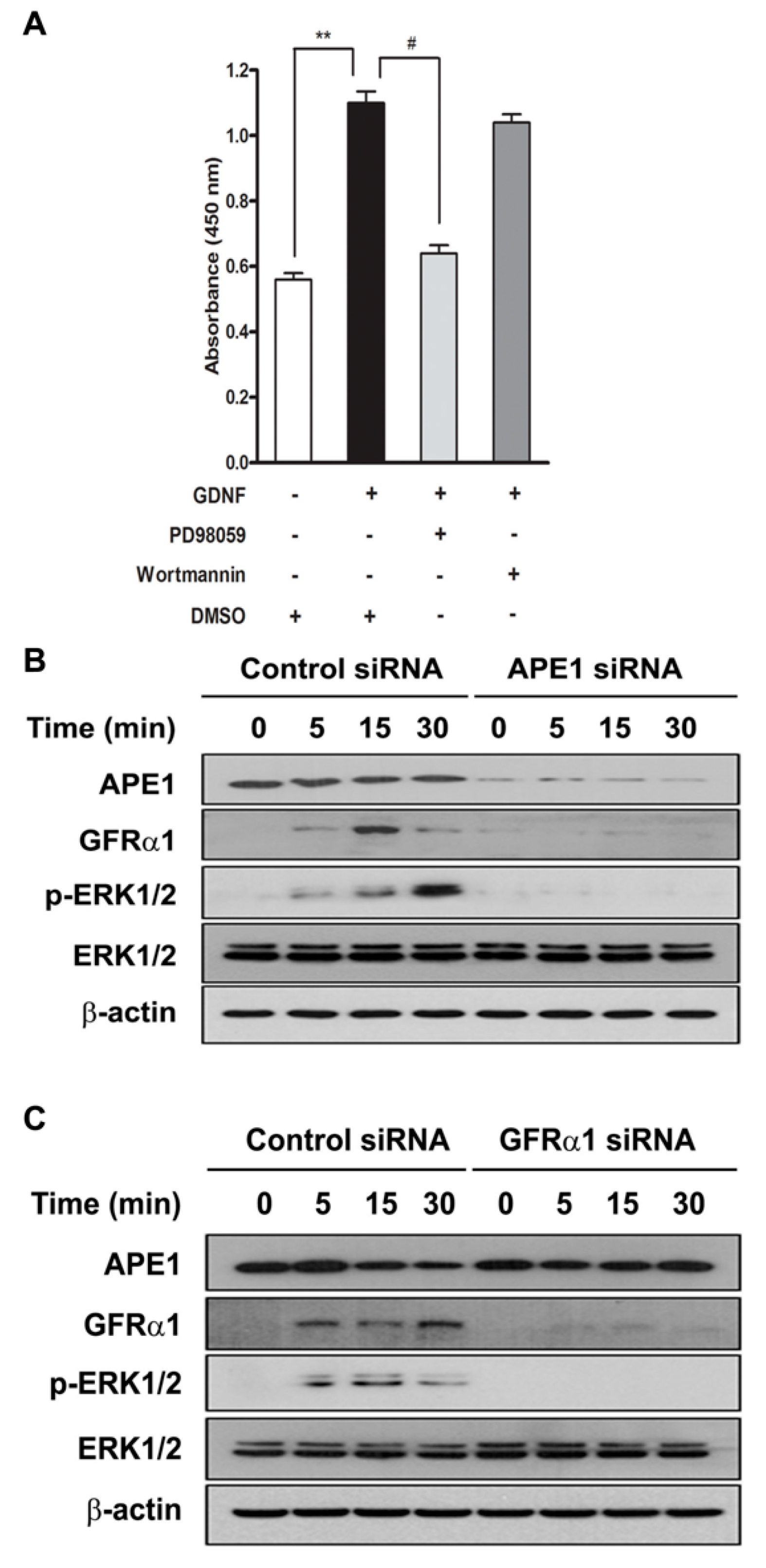
2.2. APE1 Promotes Pancreatic Cancer Cell Proliferation Via a GDNF/GFRα1/ERK Signaling Pathway
2.3. APE1-Mediated Pancreatic Cancer Cell Proliferation Requires Activation of Src for Initiation of ERK Signaling
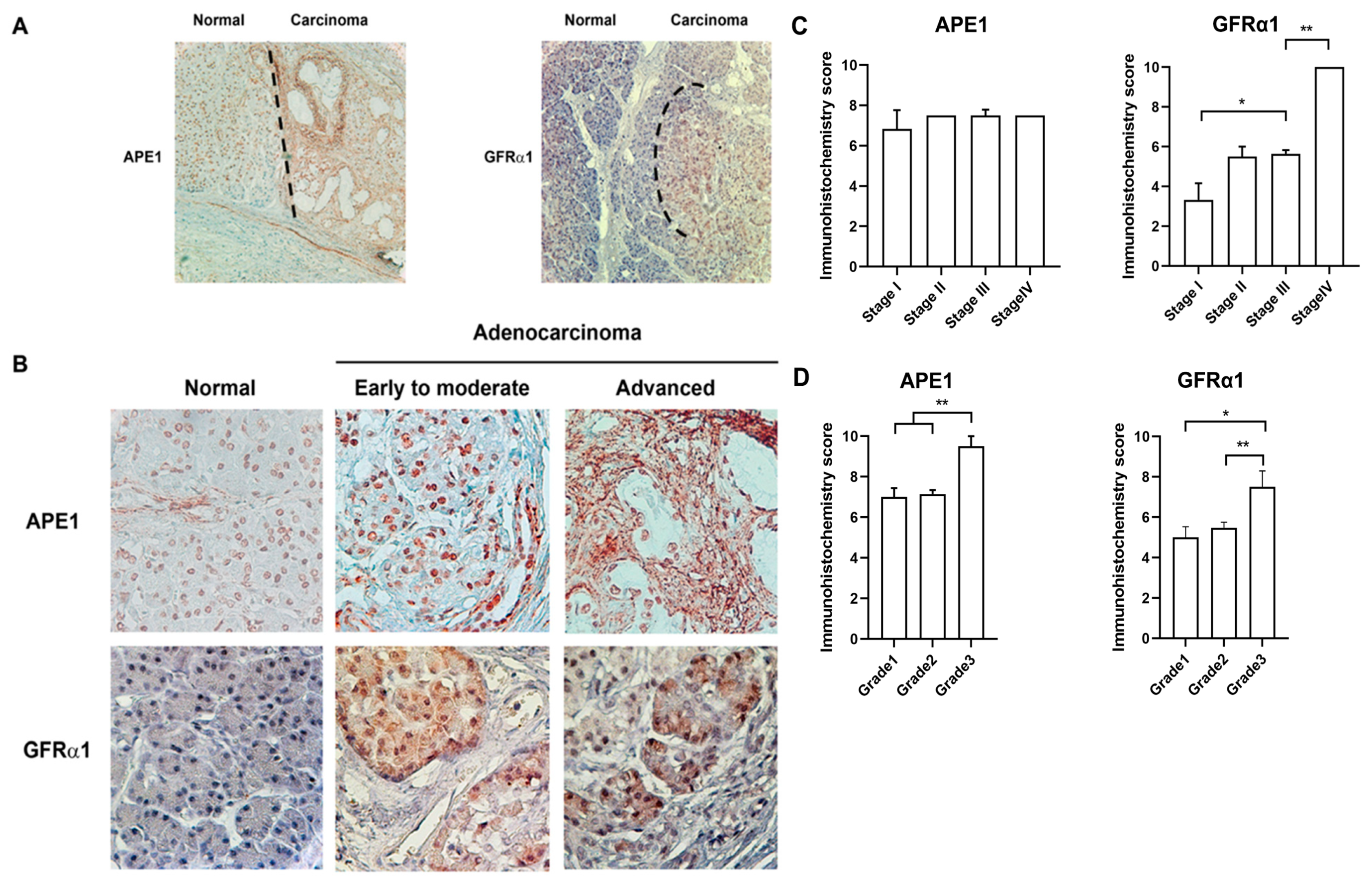
2.4. Coexpression of APE1 and GFRα1 Protein is Elevated in Aggressive Pancreatic Cancer
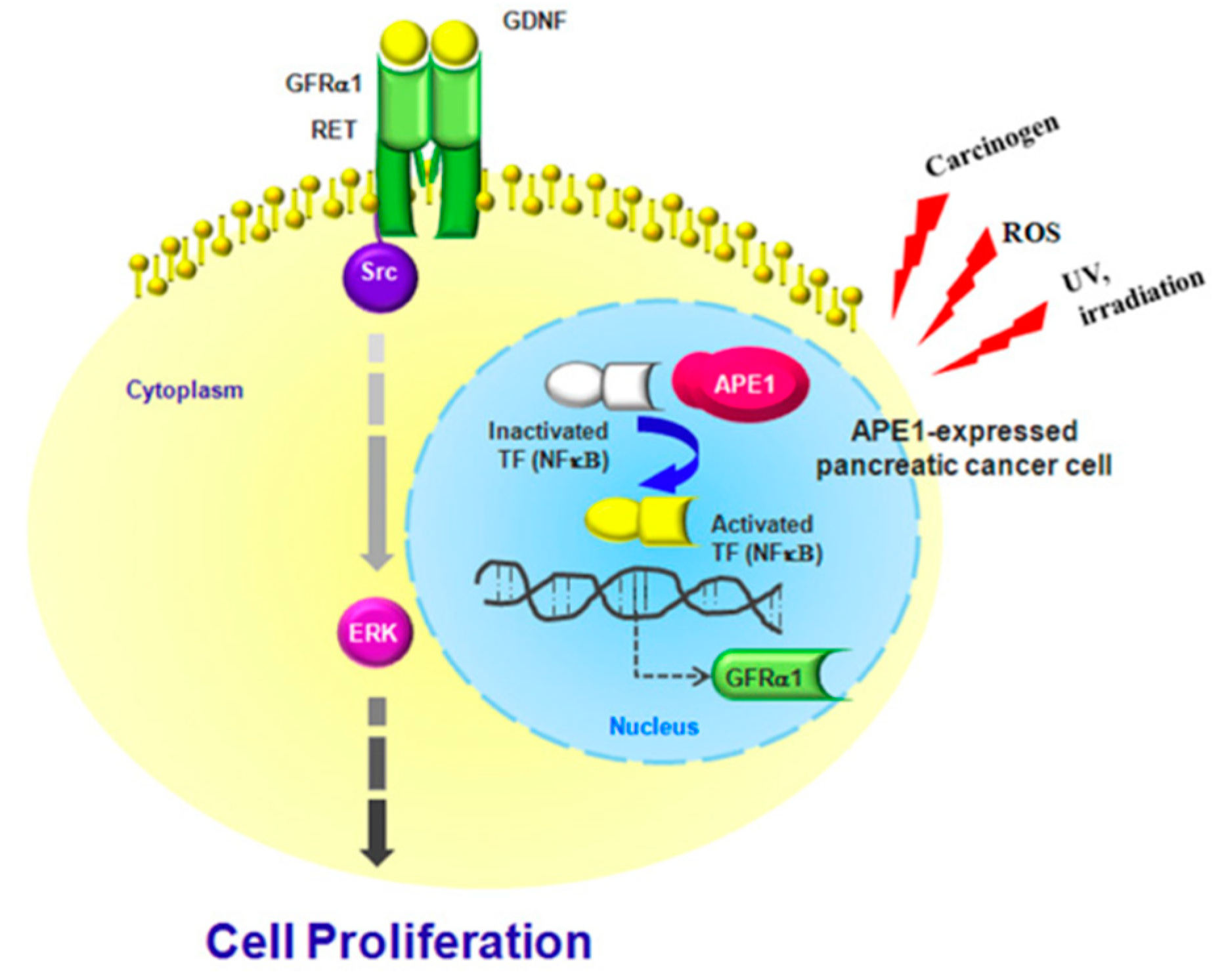
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Small Interfering RNA (siRNA)-Based Experiments
4.4. Lentiviral Construction of GFP or GFRα1
4.5. Cell Proliferation Assay
4.6. Western Blot Analysis
4.7. Immunofluorescence
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APE1 | Apurinic/apyrimidinic endodeoxyribonuclease 1 |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFRα1 | Glial factor receptor α1 |
| Src | Src proto-oncogene, Non-receptor Tyrosine Kinase |
| NTN | Neurturin |
| ART | Artemin |
| PSP | Persephin |
| RT-PCR | Reverse transcription polymerase chain reaction |
| GFP | Green fluorescent protein |
| BrdU | 5-bromo-2′-deoxyuridine |
References
- Cardoso, A.A.; Jiang, Y.; Luo, M.; Reed, A.M.; Shahda, S.; He, Y.; Maitra, A.; Kelley, M.R.; Fishel, M.L. APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3 dual-targeting effectively inhibits pancreatic cancer cell survival. PLoS ONE 2012, 7, e47462. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.R.; Limp-Foster, M.; Kelley, M.R. Going APE over ref-1. Mutat. Res. 2000, 461, 83–108. [Google Scholar] [CrossRef]
- Gray, M.J.; Zhang, J.; Ellis, L.M.; Semenza, G.L.; Evans, D.B.; Watowich, S.S.; Gallick, G.E. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene 2005, 24, 3110–3120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Kim, H.B.; Acharya, S.; Sohn, H.M.; Jun, J.Y.; Chang, I.Y.; You, H.J. Ape1/Ref-1 induces glial cell-derived neurotropic factor (GDNF) responsiveness by upregulating GDNF receptor alpha1 expression. Mol. Cell Biol. 2009, 29, 2264–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.H.; Kim, H.B.; Yoon, S.P.; Lim, S.C.; Cha, M.J.; Jeon, Y.J.; Park, S.G.; Chang, I.Y.; You, H.J. Colon cancer progression is driven by APEX1-mediated upregulation of Jagged. J. Clin. Invest. 2013, 123, 3211–3230. [Google Scholar] [CrossRef] [Green Version]
- Sak, S.C.; Harnden, P.; Johnston, C.F.; Paul, A.B.; Kiltie, A.E. APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clin. Cancer Res. 2005, 11, 6205–6211. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, F.; Aprile, G.; Minisini, A.M.; Barbone, F.; Cataldi, P.; Tell, G.; Kelley, M.R.; Damante, G.; Beltrami, C.A.; Di Loreto, C. Prognostic significance of Ape1/ref-1 subcellular localization in non-small cell lung carcinomas. Anticancer Res. 2001, 21, 4041–4049. [Google Scholar]
- Yang, Z.Z.; Li, M.X.; Zhang, Y.S.; Xiang, D.B.; Dai, N.; Zeng, L.L.; Li, Z.P.; Wang, G.; Wang, D. Knock down of the dual functional protein apurinic /apyrimidinic endonuclease 1 enhances the killing effect of hematoporphrphyrin derivative-mediated photodynamic therapy on non-small cell lung cancer cells in vitro and in a xenograft model. Cancer Sci. 2010, 101, 180–187. [Google Scholar] [CrossRef]
- Bobola, M.S.; Blank, A.; Berger, M.S.; Stevens, B.A.; Silber, J.R. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin. Cancer Res. 2001, 7, 3510–3518. [Google Scholar]
- Lau, J.P.; Weatherdon, K.L.; Skalski, V.; Hedley, D.W. Effects of gemcitabine on APE/ref-1 endonuclease activity in pancreatic cancer cells, and the therapeutic potential of antisense oligonucleotides. Br. J. Cancer 2004, 91, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Fritz, G.; Grosch, S.; Tomicic, M.; Kaina, B. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology 2003, 193, 67–78. [Google Scholar] [CrossRef]
- Kelley, M.R.; Cheng, L.; Foster, R.; Tritt, R.; Jiang, J.; Broshears, J.; Koch, M. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin. Cancer Res. 2001, 7, 824–830. [Google Scholar] [PubMed]
- Raffoul, J.J.; Banerjee, S.; Singh-Gupta, V.; Knoll, Z.E.; Fite, A.; Zhang, H.; Abrams, J.; Sarkar, F.H.; Hillman, G.G. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007, 67, 2141–2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, S.; Moore, D.H.; Michael, H.; Kelley, M.R. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: Correlations with tumor progression and platinum resistance. Clin. Cancer Res. 2003, 9, 4689–4694. [Google Scholar] [PubMed]
- Zou, G.M.; Maitra, A. Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration. Mol. Cancer Ther. 2008, 7, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Maxwell, G.D.; Reid, K.; Elefanty, A.; Bartlett, P.F.; Murphy, M. Glial cell line-derived neurotrophic factor promotes the development of adrenergic neurons in mouse neural crest cultures. Proc. Natl. Acad. Sci. USA 1996, 93, 13274–13279. [Google Scholar] [CrossRef] [Green Version]
- Funahashi, H.; Okada, Y.; Sawai, H.; Takahashi, H.; Matsuo, Y.; Takeyama, H.; Manabe, T. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J. Surg. Oncol. 2005, 91, 77–83. [Google Scholar] [CrossRef]
- Ito, Y.; Okada, Y.; Sato, M.; Sawai, H.; Funahashi, H.; Murase, T.; Hayakawa, T.; Manabe, T. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery 2005, 138, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Okada, Y.; Kazanjian, K.; Kim, J.; Hasan, S.; Hines, O.J.; Reber, H.A.; Hoon, D.S.; Eibl, G. The G691S RET polymorphism increases glial cell line-derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res. 2005, 65, 11536–11544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frame, M.C. Src in cancer: Deregulation and consequences for cell behaviour. Biochim. Biophys. Acta 2002, 1602, 114–130. [Google Scholar] [CrossRef]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeatman, T.J. A renaissance for SRC. Nat. Rev. Cancer 2004, 4, 470–480. [Google Scholar] [CrossRef]
- Hunter, T. A tail of two src’s: Mutatis mutandis. Cell 1987, 49, 1–4. [Google Scholar] [CrossRef]
- Wu, W.; Sun, Z.; Wu, J.; Peng, X.; Gan, H.; Zhang, C.; Ji, L.; Xie, J.; Zhu, H.; Ren, S.; et al. Trihydrophobin 1 phosphorylation by c-Src regulates MAPK/ERK signaling and cell migration. PLoS ONE 2012, 7, e29920. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, D.C.; Zhao, H.; Li, Z.; Wang, J.X.; Zhu, D.M.; Zhou, J.; Cen, J.N. A study of the suppressive effect on human pancreatic adenocarcinoma cell proliferation and angiogenesis by stable plasmid-based siRNA silencing of c-Src gene expression. Oncol. Rep. 2012, 27, 628–636. [Google Scholar]
- Kodama, Y.; Asai, N.; Kawai, K.; Jijiwa, M.; Murakumo, Y.; Ichihara, M.; Takahashi, M. The RET proto-oncogene: A molecular therapeutic target in thyroid cancer. Cancer Sci. 2005, 96, 143–148. [Google Scholar] [CrossRef]
- Takahashi, M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001, 12, 361–373. [Google Scholar] [CrossRef]
- Tian, H.P.; Huang, B.S.; Zhao, J.; Hu, X.H.; Guo, J.; Li, L.X. Non-receptor tyrosine kinase Src is required for ischemia-stimulated neuronal cell proliferation via Raf/ERK/CREB activation in the dentate gyrus. BMC Neurosci. 2009, 10, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbotts, R.; Madhusudan, S. Human AP endonuclease 1 (APE1): From mechanistic insights to druggable target in cancer. Cancer Treat. Rev. 2010, 36, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sarkar, B.; Cholia, R.P.; Gautam, N.; Dhiman, M.; Mantha, A.K. APE1/Ref-1 as an emerging therapeutic target for various human diseases: Phytochemical modulation of its functions. Exp. Mol. Med. 2014, 46, e106. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.L.; Jiang, Y.; Rajeshkumar, N.V.; Scandura, G.; Sinn, A.L.; He, Y.; Shen, C.; Jones, D.R.; Pollok, K.E.; Ivan, M.; et al. Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth. Mol. Cancer Ther. 2011, 10, 1698–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuzillet, C.; Hammel, P.; Tijeras-Raballand, A.; Couvelard, A.; Raymond, E. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013, 32, 147–162. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [Green Version]
- Tell, G.; Fantini, D.; Quadrifoglio, F. Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell Mol. Life Sci. 2010, 67, 3589–3608. [Google Scholar] [CrossRef]
- Luo, M.; Delaplane, S.; Jiang, A.; Reed, A.; He, Y.; Fishel, M.; Nyland, R.L., 2nd; Borch, R.F.; Qiao, X.; Georgiadis, M.M.; et al. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: Small-molecule inhibition of the redox function of Ape1. Antioxid. Redox Signal 2008, 10, 1853–1867. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Saarma, M. The GDNF family: Signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef]
- He, S.; Chen, C.H.; Chernichenko, N.; He, S.; Bakst, R.L.; Barajas, F.; Deborde, S.; Allen, P.J.; Vakiani, E.; Yu, Z.; et al. GFRalpha1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc. Natl. Acad. Sci. USA 2014, 111, E2008–E2017. [Google Scholar] [CrossRef] [Green Version]
- Iwahashi, N.; Nagasaka, T.; Tezel, G.; Iwashita, T.; Asai, N.; Murakumo, Y.; Kiuchi, K.; Sakata, K.; Nimura, Y.; Takahashi, M. Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer 2002, 94, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, K.; Rao, S.; Friess, H.; Weiss, J.; Buchler, M.W.; Korc, M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin. Cancer Res. 2003, 9, 5127–5136. [Google Scholar] [PubMed]


| Case No. | Gender | Age | Differentiation | TNM Stage | APE1 Expression | GFRα1 Expression |
|---|---|---|---|---|---|---|
| 1 | M | 55 | G1, Well differentiation | pT1N0Mx | ++ | ++ |
| 2 | F | 73 | G1, Well differentiation | pT1N0Mx | ++ | ++ |
| 3 | M | 61 | G1, Well differentiation | pT3N0Mx | ++ | ++ |
| 4 | F | 68 | G2, Moderate differentiation | pT3N0Mx | +++ | ++ |
| 5 | M | 62 | G2, Moderate differentiation | pT3N1bMx | +++ | +++ |
| 6 | M | 49 | G2, Moderate differentiation | pT3N1Mx | +++ | +++ |
| 7 | M | 83 | G2, Moderate differentiation | pT2N0Mx | +++ | +++ |
| 8 | M | 71 | G2, Moderate differentiation | pT3N0Mx | +++ | +++ |
| 9 | M | 64 | G2, Moderate differentiation | pT3N1bMx | +++ | +++ |
| 10 | M | 74 | G3, Poor differentiation | pT4N1Mx | ++++ | ++++ |
| 11 | F | 47 | G2, Moderately differentiation | T3N0M0 | +++ | ++ |
| 12 | M | 55 | G2, Moderately differentiation | T1N0M0 | +++ | + |
| 13 | M | 66 | G2, Moderately differentiation | T2N1M0 | +++ | ++ |
| 14 | F | 77 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 15 | M | 47 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 16 | M | 76 | G2, Moderately differentiation | T3N0M0 | +++ | ++ |
| 17 | M | 77 | G2, Moderately differentiation | T3N0M0 | +++ | ++ |
| 18 | M | 58 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 19 | M | 72 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 20 | F | 62 | G2, Moderately differentiation | T2N0M0 | +++ | ++ |
| 21 | M | 62 | G2, Moderately differentiation | T3N0M0 | +++ | ++ |
| 22 | M | 71 | G1, Well differentiation | T3N1M0 | ++ | +++ |
| 23 | F | 71 | G1, Well differentiation | T3N1M0 | ++ | +++ |
| 24 | M | 67 | G3, Poorly differentiated | T3N1M0 | ++++ | +++ |
| 25 | F | 58 | G1, Well differentiation | T1N0M0 | ++ | + |
| 26 | M | 60 | G2, Moderately differentiation | T2N1M0 | +++ | ++ |
| 27 | M | 59 | G1, Well differentiation | T3N0M1 | +++ | + |
| 28 | M | 72 | G2, Moderately differentiation | T2N1M1 | +++ | ++ |
| 29 | F | 67 | G2, Moderately differentiation | T3N1M0 | ++ | + |
| 30 | M | 71 | G1, Well differentiation | T3N0M0 | +++ | ++ |
| 31 | F | 66 | G3, Poorly differentiated | T3N1M0 | ++++ | +++ |
| 32 | M | 69 | G1, Well differentiation | T3N0M0 | ++ | ++ |
| 33 | F | 63 | G1, Well differentiation | T3N1M0 | ++ | ++ |
| 34 | M | 83 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 35 | M | 71 | G3, Poorly differentiated | T3N1M0 | ++++ | +++ |
| 36 | F | 73 | G2, Moderately differentiation | T3N1M0 | +++ | ++ |
| 37 | F | 60 | G3, Poorly differentiated | T3N0M0 | ++++ | ++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-D.; Jung, J.-Y.; Baek, M.; Khan, S.; Song, P.I.; Ryu, S.; Koo, J.-Y.; Chauhan, S.C.; Tsin, A.; Choi, C.; et al. APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF. Int. J. Mol. Sci. 2020, 21, 3586. https://doi.org/10.3390/ijms21103586
Choi Y-D, Jung J-Y, Baek M, Khan S, Song PI, Ryu S, Koo J-Y, Chauhan SC, Tsin A, Choi C, et al. APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF. International Journal of Molecular Sciences. 2020; 21(10):3586. https://doi.org/10.3390/ijms21103586
Chicago/Turabian StyleChoi, Yoo-Duk, Ji-Yeon Jung, Minwoo Baek, Sheema Khan, Peter I. Song, Sunhyo Ryu, Joo-Yeon Koo, Subhash C. Chauhan, Andrew Tsin, Chan Choi, and et al. 2020. "APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF" International Journal of Molecular Sciences 21, no. 10: 3586. https://doi.org/10.3390/ijms21103586
APA StyleChoi, Y.-D., Jung, J.-Y., Baek, M., Khan, S., Song, P. I., Ryu, S., Koo, J.-Y., Chauhan, S. C., Tsin, A., Choi, C., Kim, W. J., & Kim, M. (2020). APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF. International Journal of Molecular Sciences, 21(10), 3586. https://doi.org/10.3390/ijms21103586







