C-Fiber Loss as a Possible Cause of Neuropathic Pain in Schwannomatosis
Abstract
1. Introduction
2. Results
2.1. Demographics and Course of Disease
2.2. Pain- and Personality Questionnaires
2.3. Neurophysiological Measurements
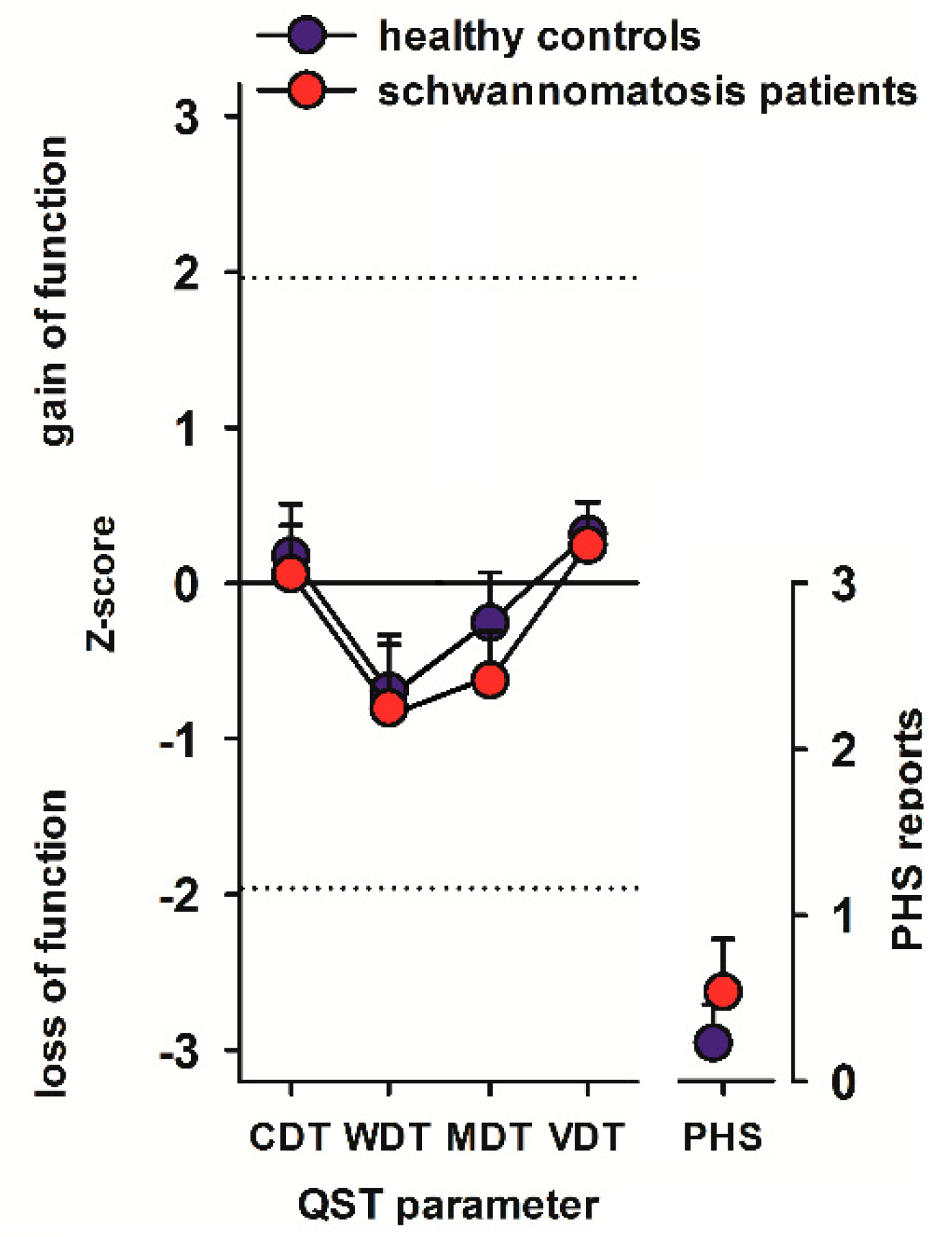
2.4. Quantitative Sensory Testing
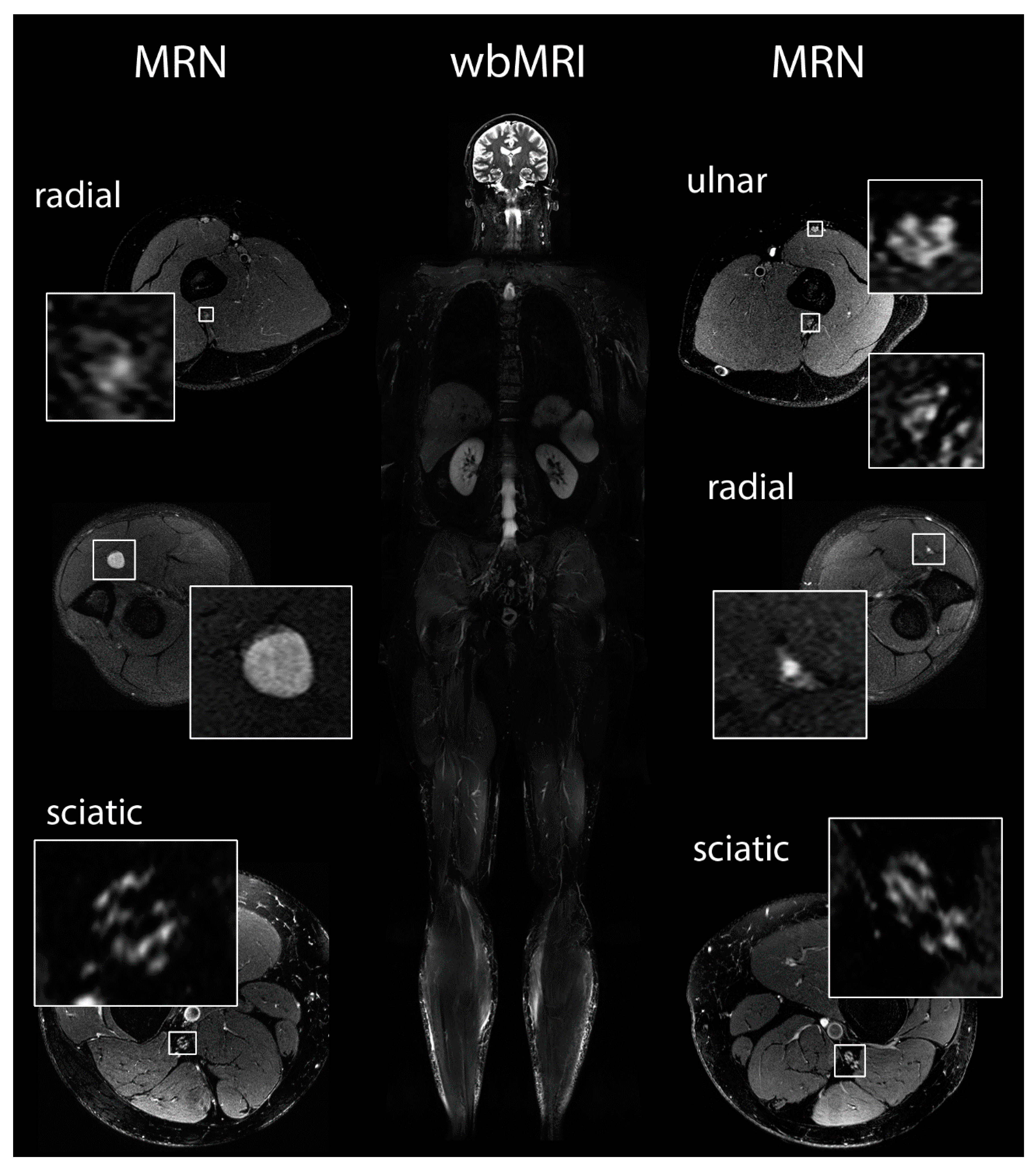
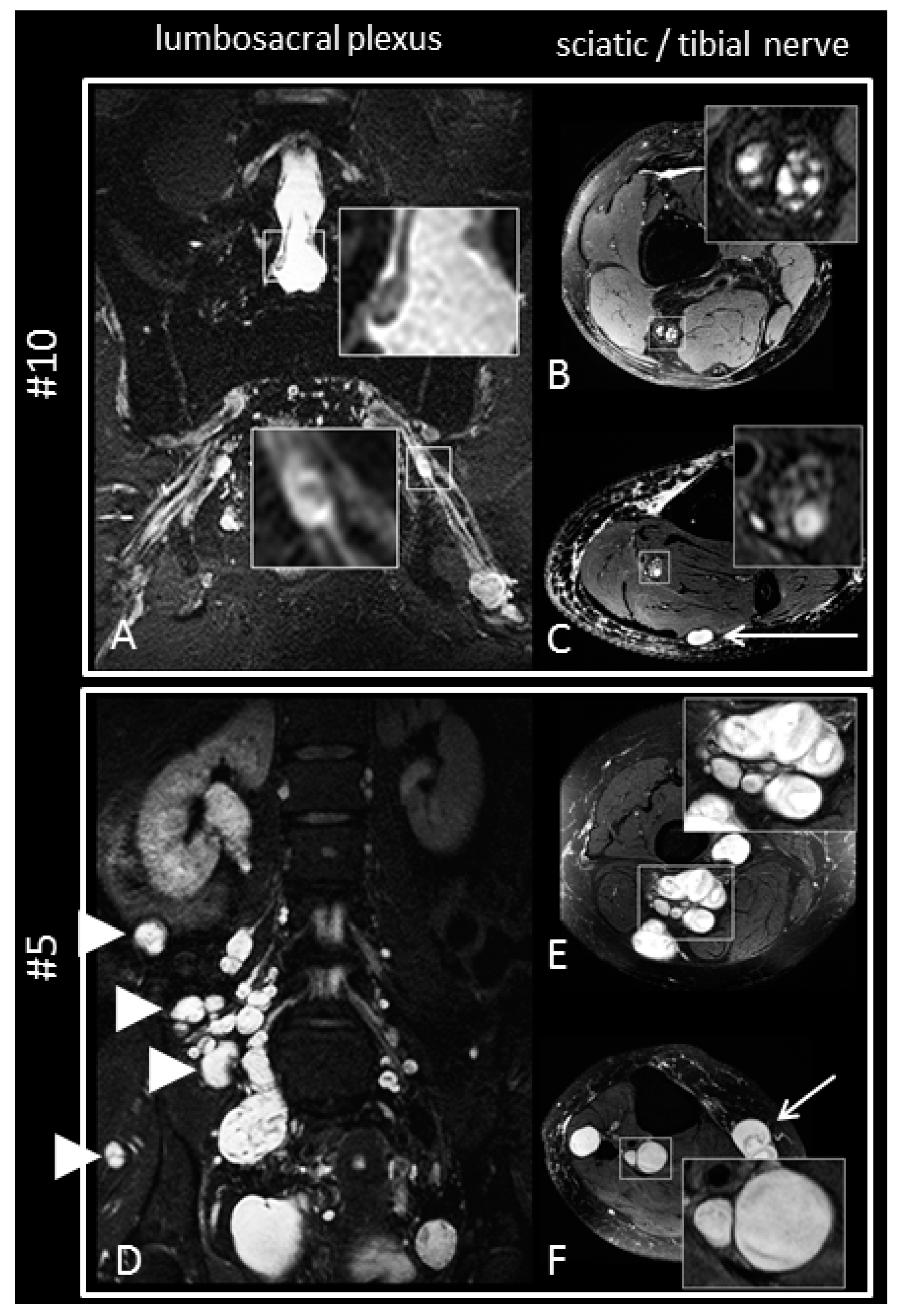
2.5. Magnetic Resonance Imaging and Magnetic Resonance Neurography
2.6. Laser Evoked Potentials
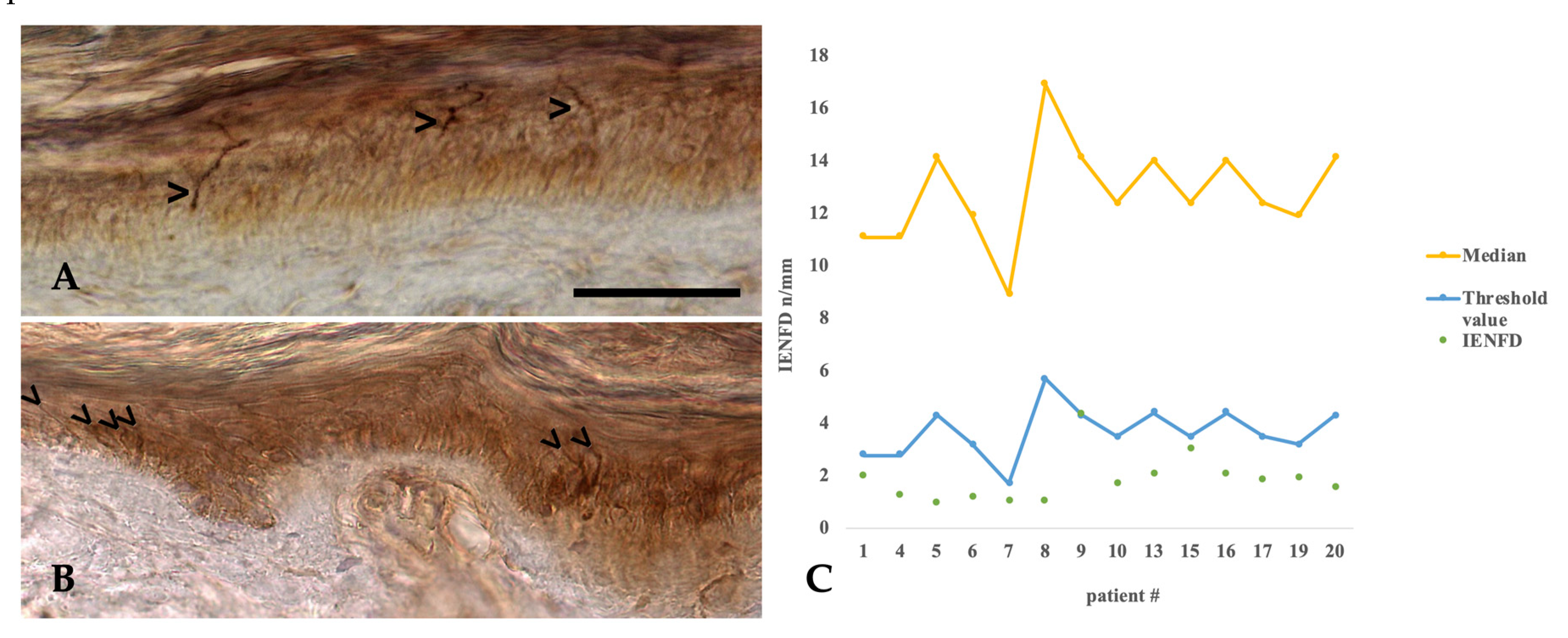
2.7. Histopathology of Intraepidermal Nerve Fiber Density
3. Subjects, Materials and Methods
3.1. Patients
3.2. Pain- and Personality-Questionnaires
3.3. Neurophysiological Measurements
3.4. Quantitative Sensory Testing (QST)
3.5. Laser Evoked Potentials (LEP)
3.6. Magnetic Resonance Imaging and Neurography
- (1)
- Manifest peripheral nerve tumors of more than 5mm in diameter;
- (2)
- Intermediate-sized nerve nodules, defined as neural caliber increase between 2 and 5 mm in diameter; and
- (3)
3.7. Skin Biopsies and Intraepidermal Nerve Fiber Density
3.8. Standard Protocol Approvals, Registrations, and Patient Consents
3.9. Data Availability Statement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CDT | Cold detection thresholds |
| CPT | Cold pain thresholds |
| DFNS | Deutscher Forschungsverbund Neuropathischer Schmerz |
| DMA | Dynamic mechanical allodynia |
| FoV | Field of view |
| FPI | Freiburger Persönlichkeitsinventar |
| HADS | The Hospital Depression and Anxiety Score |
| HPT | Heat pain thresholds |
| IENFD | Intra-epidermal nerve fiber density |
| LEP | Laser evoked potentials |
| LZTR1 | Leucine zipper like transcription regulator 1 |
| MDT | Mechanical detection threshold |
| MLPA | Multiplex ligation-dependent probe amplification |
| MPS | Mechanical pain sensitivity (stimulus-response-functions for pinprick sensitivity) |
| MPT | Mechanical pain threshold |
| MRN | Magnetic resonance neurography |
| N2/P2 | Brain responses to laser evoked potentials |
| NF1 | Neurofibromatosis type 1 |
| NF2 | Neurofibromatosis type 2 |
| NSAID | Nonsteroidal anti-inflammatory drugs |
| PGP 9.5 | Protein gene product 9.5 |
| PHS | Paradoxical heat sensations |
| PPT | Pressure pain threshold |
| QST | Quantitative sensory testing |
| SCL-90-R | SYMPTOM Checklist-90-R |
| SD | Standard deviation |
| SF-36 | Short form health 36 |
| SMARCB1 | SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 |
| TE | Echo time |
| TI | Inversion time |
| TR | Repetition time |
| TSE | Turbo spin echo |
| TSL | Thermal sensory limen |
| VDT | Vibration detection threshold |
| wbMRI | Whole-body Magnetic Resonance Imaging |
| WDT | Warm detection thresholds |
| WUR | Wind-up ratio (pain summation to repetitive pinprick stimuli) |
References
- MacCollin, M.; Chiocca, E.A.; Evans, D.G.; Friedman, J.M.; Horvitz, R.; Jaramillo, D.; Lev, M.; Mautner, V.F.; Niimura, M.; Plotkin, S.R.; et al. Diagnostic criteria for schwannomatosis. Neurology 2005, 64, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Bowers, N.L.; Tobi, S.; Hartley, C.; Wallace, A.J.; King, A.T.; Lloyd, S.K.W.; Rutherford, S.A.; Hammerbeck-Ward, C.; Pathmanaban, O.N.; et al. Schwannomatosis: A genetic and epidemiological study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Merker, V.L.; Esparza, S.; Smith, M.J.; Stemmer-Rachamimov, A.; Plotkin, S.R. Clinical features of schwannomatosis: A retrospective analysis of 87 patients. Oncologist 2012, 17, 1317–1322. [Google Scholar] [CrossRef]
- MacCollin, M.; Woodfin, W.; Kronn, D.; Short, M.P. Schwannomatosis: A clinical and pathologic study. Neurology 1996, 46, 1072–1079. [Google Scholar] [CrossRef]
- Baser, M.E.; Friedman, J.M.; Evans, D.G.R. Increasing the specificity of diagnostic criteria for schwannomatosis. Neurology 2006, 66, 730–732. [Google Scholar] [CrossRef]
- Hulsebos, T.J.M.; Plomp, A.S.; Wolterman, R.A.; Robanus-Maandag, E.C.; Baas, F.; Wesseling, P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am. J. Hum. Genet. 2007, 80, 805–810. [Google Scholar] [CrossRef]
- Smith, M.J.; Walker, J.A.; Shen, Y.; Stemmer-Rachamimov, A.; Gusella, J.F.; Plotkin, S.R. Expression of SMARCB1 (INI1) mutations in familial schwannomatosis. Hum. Mol. Genet. 2012, 21, 5239–5245. [Google Scholar] [CrossRef]
- Piotrowski, A.; Xie, J.; Liu, Y.F.; Poplawski, A.B.; Gomes, A.R.; Madanecki, P.; Fu, C.; Crowley, M.R.; Crossman, D.K.; Armstrong, L.; et al. Germline loss-of-function mutations in LZTR1 predispose to an inherited disorder of multiple schwannomas. Nat. Genet. 2014, 46, 182–187. [Google Scholar] [CrossRef]
- Hutter, S.; Piro, R.M.; Reuss, D.E.; Hovestadt, V.; Sahm, F.; Farschtschi, S.; Kehrer-Sawatzki, H.; Wolf, S.; Lichter, P.; von Deimling, A.; et al. Whole exome sequencing reveals that the majority of schwannomatosis cases remain unexplained after excluding SMARCB1 and LZTR1 germline variants. Acta Neuropathol. 2014, 128, 449–452. [Google Scholar] [CrossRef]
- Kehrer-Sawatzki, H.; Farschtschi, S.; Mautner, V.-F.; Cooper, D.N. The molecular pathogenesis of schwannomatosis, a paradigm for the co-involvement of multiple tumour suppressor genes in tumorigenesis. Hum. Genet. 2017, 136, 129–148. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Blakeley, J.O.; Evans, D.G.; Hanemann, C.O.; Hulsebos, T.J.M.; Hunter-Schaedle, K.; Kalpana, G.V.; Korf, B.; Messiaen, L.; Papi, L.; et al. Update from the 2011 International Schwannomatosis Workshop: From genetics to diagnostic criteria. Am. J. Med. Genet. 2013, 161, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Lauria, G.; Bakkers, M.; Schmitz, C.; Lombardi, R.; Penza, P.; Devigili, G.; Smith, A.G.; Hsieh, S.-T.; Mellgren, S.I.; Umapathi, T.; et al. Intraepidermal nerve fiber density at the distal leg: A worldwide normative reference study. J. Peripher. Nerv. Syst. 2010, 15, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Farschtschi, S.C.; Kluwe, L.; Schön, G.; Friedrich, R.E.; Matschke, J.; Glatzel, M.; Weis, J.; Hagel, C.; Mautner, V.-F. Distinctive low epidermal nerve fiber density in schwannomatosis patients provides a major parameter for diagnosis and differential diagnosis. Brain Pathol. 2020, 30, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Freynhagen, R.; Baron, R.; Gockel, U.; Tölle, T.R. painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006, 22, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Fahrenberg, J.; Hampel, R. Das Freiburger Persönlichkeitsinventar (FPI). In Revidierte Fassung. Handanweisung, 7th ed.; Hogrefe: Göttingen, Germany, 2001. [Google Scholar]
- Franke, G.H. SCL-90-R, Symptom-Checkliste von L.R. In Derogatis, Deutsche Version. Manual, 2nd ed.; Beltz Test GmbH: Göttingen, Germany, 2002. [Google Scholar]
- Bullinger, M. German translation and psychometric testing of the SF-36 Health Survey: Preliminary results from the IQOLA Project. International Quality of Life Assessment. Soc. Sci. Med. 1995, 41, 1359–1366. [Google Scholar] [CrossRef]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.-D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Magerl, W.; Krumova, E.K.; Baron, R.; Tolle, T.; Treede, R.-D.; Maier, C. Reference data for quantitative sensory testing (QST): Refined stratification for age and a novel method for statistical comparison of group data. Pain 2010, 151, 598–605. [Google Scholar] [CrossRef]
- Hauck, M.; Schröder, S.; Meyer-Hamme, G.; Lorenz, J.; Friedrichs, S.; Nolte, G.; Gerloff, C.; Engel, A.K. Acupuncture analgesia involves modulation of pain-induced gamma oscillations and cortical network connectivity. Sci. Rep. 2017, 7, 16307. [Google Scholar] [CrossRef]
- Mautner, V.-F.; Asuagbor, F.A.; Dombi, E.; Fünsterer, C.; Kluwe, L.; Wenzel, R.; Widemann, B.C.; Friedman, J.M. Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol. 2008, 10, 593–598. [Google Scholar] [CrossRef]
- Bäumer, P.; Mautner, V.F.; Bäumer, T.; Schuhmann, M.U.; Tatagiba, M.; Heiland, S.; Kaestel, T.; Bendszus, M.; Pham, M. Accumulation of non-compressive fascicular lesions underlies NF2 polyneuropathy. J. Neurol. 2013, 260, 38–46. [Google Scholar] [CrossRef]
- Lauria, G.; Hsieh, S.T.; Johansson, O.; Kennedy, W.R.; Leger, J.M.; Mellgren, S.I.; Nolano, M.; Merkies, I.S.J.; Polydefkis, M.; Smith, A.G.; et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur. J. Neurol. 2010, 17, 903–912. [Google Scholar] [CrossRef]
- Backonja, M.M.; Attal, N.; Baron, R.; Bouhassira, D.; Drangholt, M.; Dyck, P.J.; Edwards, R.R.; Freeman, R.; Gracely, R.; Haanpaa, M.H.; et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013, 154, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Farschtschi, S.; Mautner, V.-F.; Pham, M.; Nguyen, R.; Kehrer-Sawatzki, H.; Hutter, S.; Friedrich, R.E.; Schulz, A.; Morrison, H.; Jones, D.T.W.; et al. Multifocal nerve lesions and LZTR1 germline mutations in segmental schwannomatosis. Ann. Neurol. 2016, 80, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.-D.; Lorenz, J.; Baumgärtner, U. Clinical usefulness of laser-evoked potentials. Neurophysiol. Clin. 2003, 33, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Domnick, C.; Hauck, M.; Casey, K.L.; Engel, A.K.; Lorenz, J. C-fiber-related EEG-oscillations induced by laser radiant heat stimulation of capsaicin-treated skin. J. Pain Res. 2009, 2, 49–56. [Google Scholar] [CrossRef][Green Version]
- Beydoun, A.; Dyke, D.B.; Morrow, T.J.; Casey, K.L. Topical capsaicin selectively attenuates heat pain and A delta fiber-mediated laser-evoked potentials. Pain 1996, 65, 189–196. [Google Scholar] [CrossRef]
- Hauck, M.; Lorenz, J.; Zimmermann, R.; Debener, S.; Scharein, E.; Engel, A.K. Duration of the cue-to-pain delay increases pain intensity: A combined EEG and MEG study. Exp. Brain Res. 2007, 180, 205–215. [Google Scholar] [CrossRef]
- Lacomis, D. Small-fiber neuropathy. Muscle Nerve 2002, 26, 173–188. [Google Scholar] [CrossRef]
- Sommer, C.; Üçeyler, N. Small-Fiber-Neuropathien. Fortschr. Neurol. Psychiatr. 2018, 86, 509–518. [Google Scholar] [CrossRef]
- Hilton, D.A.; Hanemann, C.O. Schwannomas and Their Pathogenesis. Brain Pathol. 2014, 24, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Baser, M.E.; Friedman, J.M.; Wallace, A.J.; Ramsden, R.T.; Joe, H.; Evans, D.G.R. Evaluation of clinical diagnostic criteria for neurofibromatosis 2. Neurology 2002, 59, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A.Y.W. Primary Pigmented Nodular Adrenocortical Disease and Its Associated Conditions; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1992; Volume 2. [Google Scholar]
- Gorlin, R.J.; Koutlas, I.G. Multiple schwannomas, multiple nevi, and multiple vaginal leiomyomas: A new dominant syndrome. Am. J. Med. Genet. 1998, 78, 76–81. [Google Scholar] [CrossRef]
- Ahlawat, S.; Blakeley, J.; Montgomery, E.; Subramaniam, R.M.; Belzberg, A.; Fayad, L.M. Schwannoma in neurofibromatosis type 1: A pitfall for detecting malignancy by metabolic imaging. Skelet. Radiol. 2013, 42, 1317–1322. [Google Scholar] [CrossRef]
- Heckmann, J.G.; Dutsch, M.; Buslei, R. Hereditary neuropathy with liability to pressure palsy combined with schwannomas of the median and medial plantar nerves. Muscle Nerve 2007, 35, 122–124. [Google Scholar] [CrossRef]
- Schulz, A.; Grafe, P.; Hagel, C.; Bäumer, P.; Morrison, H.; Mautner, V.F.; Farschtschi, S. Neuropathies in the setting of Neurofibromatosis tumor syndromes: Complexities and opportunities. Exp. Neurol. 2018, 299, 334–344. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Levine, J.; Barbaro, N.M. Pain as a symptom of peripheral nerve sheath tumors: Clinical significance and future therapeutic directions. J. Brachial Plex. Peripher. Nerve Inj. 2008, 3, e136–e140. [Google Scholar] [CrossRef]
- Wagner, R.; Myers, R.R. Schwann cells produce tumor necrosis factor alpha: Expression in injured and non-injured nerves. Neuroscience 1996, 73, 625–629. [Google Scholar] [CrossRef]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; DeMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef]
- Naruse, K. Schwann Cells as Crucial Players in Diabetic Neuropathy. Adv. Exp. Med. Biol. 2019, 1190, 345–356. [Google Scholar] [CrossRef]
- Tyner, T.R.; Parks, N.; Faria, S.; Simons, M.; Stapp, B.; Curtis, B.; Sian, K.; Yamaguchi, K.T. Effects of collagen nerve guide on neuroma formation and neuropathic pain in a rat model. Am. J. Surg. 2007, 193, e1–e6. [Google Scholar] [CrossRef] [PubMed]
| PAT_ID | SEX | AGE | FAMILY HISTORY | LZTR1 | SMARC B1 | NF2 | SYMPTOMS | LOCATION Of TUMORS | NUMBER Of TUMORS (wb-MRI) | MRN: MICROLESIONS/RIGHT LEG OVERALL SCORE | NEUROPHYSIOLOGY | QST HANDS | QST FEET | LEP N2 LATENCY LEFT FOOT | PAINDETECT | IENFD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | Yes | n.d. | n.d. | n.d. | Pain, local tumor growth | Peripheral | 10 | 1/1 | normal | MFN | normal | ↑ | ++ | ↓↓ |
| 2 | W | 38 | No | VUS c.1288C>T;His430Tyr | n.d. | n.d. | Pain, local tumor growth | Segmental | 2 | 2/1 | normal | normal | n/a | n/a | ++ | n/a |
| 3 | W | 53 | No | n.d. | n.d. | n.d. | Pain, local tumor growth | Peripheral | 15 | 3/2 | normal | normal | normal | n/a | + | n/a |
| 4 | M | 66 | No | n.d. | n.d. | n.d. | Pain, local tumor growth | Spinal and peripheral | 32 | 3/2 | normal | MFN | normal | 0 | 0 | ↓↓ |
| 5 | W | 52 | No | n.d. | n.d. | n.d. | Pain, local tumor growth | Spinal and peripheral | 4 | 4/4 | path. | normal | SFN | ↓ | + | ↓↓ |
| 6 | W | 61 | No | n.d. | n.d. | n.d. | Other, pain, local tumor growth | Segmental | 5 | 1/0 | normal | normal | normal | ↓ | ++ | ↓↓ |
| 7 | M | 80 | No | n.d. | n.d. | n.d. | Other, pain | Cerebral, peripheral, and spinal | 5 | 2/1 | normal | LFN | LFN | ↓ | + | ↓↓ |
| 8 | W | 46 | No | c.C2247A;p.Y749X | n.d. | n.d. | Pain | Segmental | 5 | 2/1 | normal | normal | normal | ↓ | 0 | ↓↓ |
| 9 | W | 56 | No | c.G1312T;p.E438X (Exon12) | n.d. | n.d. | Pain, local tumor growth | Spinal and peripheral | 13 | 1/1 | normal | normal | normal | ↓ | + | ↓ |
| 10 | M | 56 | No | n.d. | n.d. | n.d. | Pain, weakness/ sensory loss, local tumor growth | Spinal and peripheral | 5 | 4/3 | path. | SFN | normal | ↑ | 0 | ↓↓ |
| 11 | W | 41 | No | c.1480_1481insAGp.R494fs (Exon14) | n.d. | n.d. | Pain | Cerebral, peripheral, and spinal | 8 | n/a | normal | LFN | normal | 0 | + | n/a |
| 12 | M | 64 | No | n.p. | - | n.d. | Pain, local tumor growth | Peripheral | 3 | n/a | normal | SFN | LFN | 0 | 0 | n/a |
| 13 | M | 42 | Yes | c.978-985delCAGCTCCG (Pro326fs) | n.d. | n.d. | Pain, local tumor growth | Spinal and peripheral | 61 | 1/0 | normal | normal | SFN | ↓ | 0 | ↓↓ |
| 14 | W | 46 | No | n.d. | n.d. | n.d. | Pain | Spinal and peripheral | 25 | 4/4 | normal | MFN | LFN | ↓ | ++ | n/a |
| 15 | M | 57 | No | n.d. | n.d. | n.d. | Weakness/sensory loss, other, pain | Spinal and peripheral | 3 | 1/1 | path. | SFN | SFN | 0 | ++ | ↓↓ |
| 16 | M | 47 | No | n.d. | n.d. | n.d. | Other, pain | Peripheral | 8 | 3/2 | normal | normal | normal | ↓ | 0 | ↓↓ |
| 17 | M | 56 | No | n.d. | n.d. | n.d. | Pain | Spinal and peripheral | 15 | 2/2 | normal | normal | normal | ↓ | + | ↓↓ |
| 18 | M | 73 | Yes | n.d. | n.d. | n.d. | Pain, local tumor growth | Spinal and peripheral | 33 | 3/2 | normal | SFN | MFN | 0 | ++ | n/a |
| 19 | W | 60 | No | n.d. | n.d. | n.d. | Pain, other, local tumor growth | Spinal and peripheral | 3 | 0/1 | path. | normal | normal | ↓ | 0 | ↓↓ |
| 20 | W | 49 | No | n.d. | n.d. | n.d.* | Other, pain | Peripheral | 3 | 0/0 | normal | normal | normal | ↓ | + | ↓↓ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farschtschi, S.C.; Mainka, T.; Glatzel, M.; Hannekum, A.-L.; Hauck, M.; Gelderblom, M.; Hagel, C.; Friedrich, R.E.; Schuhmann, M.U.; Schulz, A.; et al. C-Fiber Loss as a Possible Cause of Neuropathic Pain in Schwannomatosis. Int. J. Mol. Sci. 2020, 21, 3569. https://doi.org/10.3390/ijms21103569
Farschtschi SC, Mainka T, Glatzel M, Hannekum A-L, Hauck M, Gelderblom M, Hagel C, Friedrich RE, Schuhmann MU, Schulz A, et al. C-Fiber Loss as a Possible Cause of Neuropathic Pain in Schwannomatosis. International Journal of Molecular Sciences. 2020; 21(10):3569. https://doi.org/10.3390/ijms21103569
Chicago/Turabian StyleFarschtschi, Said C., Tina Mainka, Markus Glatzel, Anna-Lena Hannekum, Michael Hauck, Mathias Gelderblom, Christian Hagel, Reinhard E. Friedrich, Martin U. Schuhmann, Alexander Schulz, and et al. 2020. "C-Fiber Loss as a Possible Cause of Neuropathic Pain in Schwannomatosis" International Journal of Molecular Sciences 21, no. 10: 3569. https://doi.org/10.3390/ijms21103569
APA StyleFarschtschi, S. C., Mainka, T., Glatzel, M., Hannekum, A.-L., Hauck, M., Gelderblom, M., Hagel, C., Friedrich, R. E., Schuhmann, M. U., Schulz, A., Morrison, H., Kehrer-Sawatzki, H., Luhmann, J., Gerloff, C., Bendszus, M., Bäumer, P., & Mautner, V.-F. (2020). C-Fiber Loss as a Possible Cause of Neuropathic Pain in Schwannomatosis. International Journal of Molecular Sciences, 21(10), 3569. https://doi.org/10.3390/ijms21103569





