The Role of TRM Cells in the Pathogenesis of Vitiligo—A Review of the Current State-Of-The-Art
Abstract
1. Introduction
2. Vitiligo Classification
3. The Pathogenesis of Vitiligo
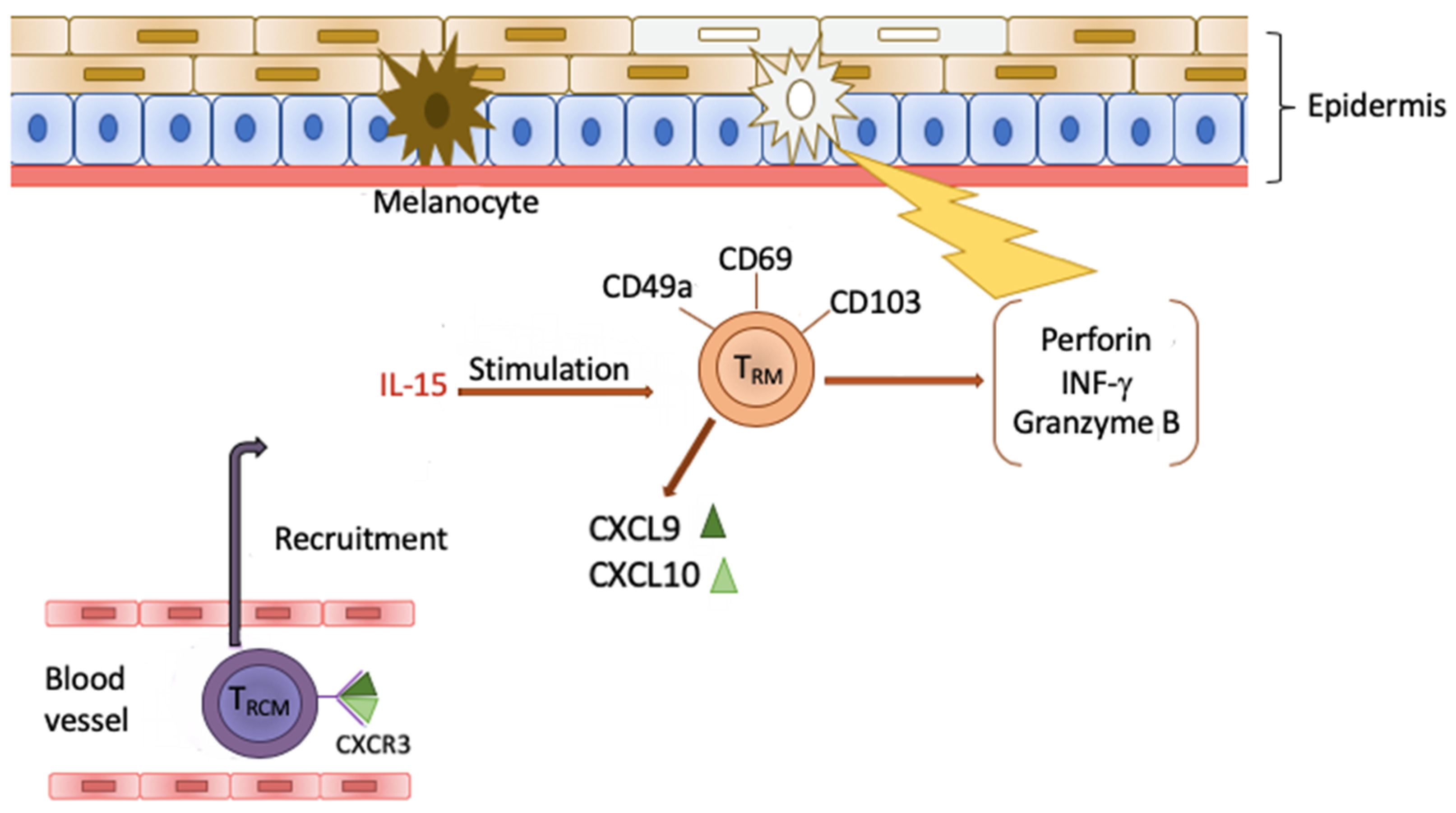
4. Tissue Resident Memory Cells (TRM)
5. TRM Cells and Vitiligo
6. Clinical Significance of TRM Cells
7. Anti-Tumor Role of TRM Cells
8. Conclusions
Funding
Conflicts of Interest
References
- Mahdi, P.; Rouzbahani, M.; Amali, A.; Khiabanlu, S.R.; Kamali, M. Audiological manifestation in vitiligo patient. Iran J. Otorhinolaryngol. 2012, 24, 35–40. [Google Scholar] [PubMed]
- Kopera, D. Historical aspects and definition of vitiligo. Clin. Dermatol. 1997, 15, 841–843. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.C.; Spritz, R.A. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kutwin, M.; Sysa-Jędrzejowska, A.; Woźnicka, A. Virtiligo and autoimmune diseases. Dermatol 2016, 103, 400–404. [Google Scholar] [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef]
- Salzes, C.; Abadie, S.; Seneschal, J.; Whitton, M.; Meurant, J.-M.; Jouary, T.; Ballanger, F.; Boralevi, F.; Taïeb, A.; Taïeb, C.; et al. The Vitiligo Impact Patient scale (VIPs): Development and validation of a vitiligo burden assessment tool. J. Investig. Dermatol 2016, 136, 52–58. [Google Scholar] [CrossRef]
- Prasad, D. A new era of vitiligo research and treatment. J. Cutan. Aesthet. Surg. 2013, 6, 63–64. [Google Scholar] [CrossRef]
- Fukazawa, K.; Sakagami, M.; Umemoto, M.; Senda, T. Development of Melanosomes and Cytochemical Observation of Tyrosinase Activity in the Inner Ear. ORL J. Otorhinolaryngol. Relat. Spec. 1994, 56, 247–252. [Google Scholar] [CrossRef]
- Creel, D.; Garber, S.R.; King, R.A.; Witkop, C.J. Auditory brainstem anomalies in human albinos. Science 1980, 209, 1253–1255. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Contreras, J.; Zurita, E.; Cediel, R.; Cantero, M.; Varela-Nieto, I.; Montoliu, L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment Cell Melanoma Res. 2010, 23, 72–83. [Google Scholar] [CrossRef]
- De Jong, M.A.; Adelman, C.; Gross, M. Hearing loss in vitiligo: Current concepts and review. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Opdecamp, K.; Nakayama, A.; Nguyen, M.T.; Hodgkinson, C.A.; Pavan, W.J.; Arnheiter, H. Melanocyte development in vivo and in neural crest cell cultures: Crucial dependence on the Mitf basic-helix-loop-helix transcription factor. Development 1997, 124, 2377–2386. [Google Scholar] [PubMed]
- Sato-Jin, K.; Nishimura, E.K.; Akasaka, E.; Huber, W.; Nakano, H.; Miller, A.; Du, J.; Wu, M.; Hanada, K.; Sawamura, D.; et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008, 22, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, Y.; Shi, M.; Jiang, S.; Cui, S.; Wu, Y.; Gao, X.-Y.; Chen, H.-D. The Prevalence of Vitiligo: A Meta-Analysis. Plos One 2016, 11, e0163806. [Google Scholar] [CrossRef] [PubMed]
- Spritz, R.A. The genetics of generalized vitiligo. Curr. Dir. Autoimmun 2008, 10, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Cellular stress and innate inflammation in organ-specific autoimmunity: Lessons learned from virtiligo. Immunol Rev 2016, 269, 11–25. [Google Scholar] [CrossRef]
- Cavalieé, M.; Ezzedine, K.; Fontas, E.; Montaudieé, H.; Castela, E.; Bohadoran, P.; Taïeb, A.; Lacour, J.P.; Passeron, T. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: A randomized, double blind, placebo-controlled study. J. Investig. Derm. 2015, 135, 970–974. [Google Scholar] [CrossRef]
- Wu, H.; Liao, W.; Li, Q.; Long, H.; Yin, H.; Zhao, M.; Chan, V.; Lau, C.S.; Lu, Q. Pathogenic role of tissue resident memory T cells in autoimmune diseases. Autoimmun. Rev. 2018, 17, 906–911. [Google Scholar] [CrossRef]
- Topham, D.J.; Reilly, E.C. Tissue-Resident Memory CD8+ T Cells: From Phenotype to Function. Front. Immunol. 2018, 9, 515. [Google Scholar] [CrossRef]
- Ezzedine, K.; Lim, H.W.; Suzuki, T.; Katayama, I.; Hamzavi, I.; Lan, C.C.; Goh, B.K.; Anbar, T.; Silva de Castro, C.; Lee, A.Y.; et al. Vitiligo Global Issue Consensus Conference Panelists. Revised classification/nomenclature of vitiligo and related issues: The Vitiligo Global Issues Consensus Conference. Pigment. Cell Melanoma Res. 2012, 25, E1–E13. [Google Scholar] [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Ezzedine, K.; Gauthier, Y.; Léauté-Labrèze, C.; Marquez, S.; Bouchtnei, S.; Jouary, T.; Taïeb, A. Segmental vitiligo associated with generalized vitiligo (mixed vitiligo): A retrospective case series of 19 patients. J. Am. Acad. Dermatol. 2011, 65, 965–971. [Google Scholar] [CrossRef]
- Gauthier, Y.; Cario Andre, M.; Taïeb, A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res. 2003, 16, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Van Geel, N.A.; Mollet, I.G.; De Schepper, S.; Tjin, E.P.M.; Vermaelen, K.; Clark, R.A.; Kupper, T.S.; Luiten, R.M.; Lambert, J. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 2010, 23, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, A.; Picardo, M. The definition and assessment of vitiligo: A consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007, 20, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nejad, S.B.; Qadim, H.H.; Nazeman, L.; Fadaii, R.; Goldust, M. Frequency of autoimmune diseases in those suffering from vitiligo in comparison with normal population. Pak. J. Biol. Sci. 2013, 16, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Ingordo, V.; Cazzaniga, S.; Raone, B.; Digiuseppe, M.D.; Musumeci, M.L.; Fai, D.; Pellegrino, M.; Pezzarossa, E.; Di Lemia, V.; Battarra, V.C.; et al. Circulating autoantibodies and autoimmune comorbidities in vitiligo patients: A multicenter Italian study. Dermatology 2014, 228, 240–249. [Google Scholar] [CrossRef]
- Narita, T.; Oiso, N.; Fukai, K.; Kabashima, K.; Kawada, A.; Suzuki, T. Generalized vitiligo and associated autoimmune diseases in Japanese patients and their families. Allergol. Int. 2011, 60, 505–508. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Stetler, G.L.; Old, W.; Talbert, J.; Uhlhorn, C.; Taylor, M.; Fox, A.; Miller, C.; Dills, D.G.; Ridgway, E.C.; et al. Mapping of an autoimmunity susceptibility locus (AlS1) to chromoseome 1p31.3-p32.2. Hum. Mol. Genet 2002, 11, 661–667. [Google Scholar] [CrossRef]
- Spritz, R.A.; Andersen, G.H.L. Genetics of vitiligo. Dermatol. Clin. 2017, 35, 245–255. [Google Scholar] [CrossRef]
- Li, Z.; Ren, J.; Niu, X.; Xu, Q.; Wang, X.; Liu, Y.; Xiao, S. Meta-analysis of the association between vitiligo and human leukocyte antigen-A. BioMed. Res. Int. 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zar, A.R.; Malik, A.; Mahmood, A.; Naseer, Q.A.; Yumei, L. Pathogenesis and the emerging therapy of virtiligo. Arch. Clin. Biomed. Res. 2019, 3, 361–373. [Google Scholar] [CrossRef]
- Levandowski, C.B.; Mailloux, C.M.; Ferrara, T.M.; Gowan, K.; Ben, S.; Jin, Y.; McFann, K.K.; Holland, P.J.; Fain, P.R.; Dinarello, C.A.; et al. NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1beta processing via the NLRP1 inflammasome. Proc. Natl. Acad. Sci. USA 2013, 110, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Gao, J.; Sheng, Y.; Dou, J.; Zhou, F.; Zheng, X.; Ko, R.; Tang, X.; Zhu, C.; Yin, X.; et al. Genetic Susceptibility to Vitiligo: GWAS Approaches for Identifying Vitiligo Susceptibility Genes and Loci. Front. Genet. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Jakobs, C.; Hagen, C.; Renn, M.; Luiten, R.M.; Melief, C.J.; Tuting, T.; Garbi, N.; Hartmann, G.; Homung, V. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity 2016, 44, 1406–1421. [Google Scholar] [CrossRef]
- Jin, Y.; Birlea, S.A.; Fain, P.R.; Spritz, R.A. Genetic Variations in NALP1 Are Associated with Generalized Vitiligo in a Romanian Population. J. Investig. Dermatol. 2007, 127, 2558–2562. [Google Scholar] [CrossRef]
- Puri, N.; Mojamdar, M.; Ramaiah, A. In Vitro Growth Characteristics of Melanocytes Obtained From Adult Normal and Vitiligo Subjects. J. Investig. Dermatol. 1987, 88, 434–438. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In Vivo and In Vitro Evidence for Hydrogen Peroxide (H2O2) Accumulation in the Epidermis of Patients with Vitiligo and its Successful Removal by a UVB-Activated Pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef]
- Richmond, J.M.; Frisoli, M.L.; Harris, J.E. Innate immune mechanisms in vitiligo: Danger from within. Curr. Opin. Immunol. 2013, 25, 676–682. [Google Scholar] [CrossRef]
- Toosi, S.; Orlow, S.J.; Manga, P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J. Investig. Dermatol. 2012, 132, 2601–2609. [Google Scholar] [CrossRef]
- Kirnbauer, R.; Charvat, B.; Schauer, E.; Köck, A.; Urbanski, A.; Förster, E.; Neuner, P.; Assmann, I.; Luger, T.A.; Schwarz, T. Modulation of intercellular adhesion molecule-1 expression on human melanocytes and melanoma cells: Evidence for a regulatory role of IL-6, IL-7, TNF beta, and UVB light. J. Investig. Dermatol. 1992, 98, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Piperi, C.; Ziakas, P.D.; Apostolopoulos, N.V.; Makrilakis, K.; Syriou, V.; Diamanti-Kandarakis, E.; Kaltsas, G.; Kalofoutis, A. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: Associations with low-grade systemic inflammation. J. Clin. Immunol. 2008, 28, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Girasole, G.; Pedrazzoni, M.; Passeri, M.; Giuliani, N.; Minelli, R.; Braverman, L.E.; Roti, E. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. J. Clin. Endocrinol. Metab. 1996, 81, 2976–2979. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, D.; Yang, Y.; Jian, Z.; Guo, S.; Dai, W.; Shi, Q.; Ge, R.; Ma, J.; Liu, L.; et al. Oxidative stress drives CD8(+) T-cell skin trafficking in patients with vitiligo through CXCL16 upregulation by activating the unfolded protein response in keratinocytes. J. Allergy Clin. Immunol. 2017, 140, 177–189. [Google Scholar] [CrossRef]
- Zhen, Y.; Yao, L.; Zhong, S.; Song, Y.; Cui, Y.; Li, S. Enhanced Th1 and Th17 responses in peripheral blood in active non-segmental vitiligo. Arch. Dermatol. Res. 2016, 308, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Yssel, H.; Pene, J. Interleukin-22-producing T cells: A specialized population involved in skin inflammation. Immunol. Cell. Biol. 2009, 87, 574–576. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.-Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Wilson, N.J.; Boniface, K.; Chan, J.R.; McKenzie, B.S.; Blumenschein, W.M.; Mattson, J.D. Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat. Immunol. 2007, 8, 950–957. [Google Scholar] [CrossRef]
- Bassiouny, D.A.; Shaker, O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin. Exp. Dermatol. 2010, 36, 292–297. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.L.; Li, K.; Hamzavi, I.; Gao, T.W.; Huggins, R.H.; Lim, H.W.; Mi, Q.S. Increased circulating Th17 cells and elevated serumlevels of TGF-beta and IL-21 are correlated with humannon-segmental vi- tiligo development. Pigment Cell Melanoma Res. 2015, 28, 324–329. [Google Scholar] [CrossRef]
- Trifari, S.; Kaplan, C.D.; Tran, E.H.; Natasha, K.; Crellin, N.K.; Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from Th17, Th1 and Th2 cells. Nat. Immunol 2009, 10, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Behfarjam, F.; Jadali, Z. Vitiligo patients show significant up-regulation of aryl hydrocarbon receptor transcription factor. An. Bras. Derm. 2018, 93, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Akhtar, S.; Kurra, S.; Gupta, S.; Sharma, A. Emerging role of immune cell network in autoimmune skin disorders: An update on pemphigus, vitiligo and psoriasis. Cytokine Growth Factor Rev. 2019, 45, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lili, Y.; Yi, W.; Ji, Y.; Yue, S.; Weimin, S.; Ming, L. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Ben Ahmed, M.; Zaraa, I.; Rekik, R.; Elbeldi-Ferchiou, A.; Kourda, N.; Belhadj Hmida, N.; Abdeladhim, M.; Karoui, O.; Ben Osman, A.; Mokni, M.; et al. Functional defects of peripheral regulatory T lymphocytes in patients with progressive vitiligo. Pigment Cell Melanoma Res. 2011, 25, 99–109. [Google Scholar] [CrossRef]
- Abdallah, M.; Lotfi, R.; Othman, W.; Galal, R. Assessment of tissue FoxP3+, CD4+and CD8+T-cells in active and stable nonsegmental vitiligo. Int. J. Dermatol. 2014, 53, 940–946. [Google Scholar] [CrossRef]
- Mami-Chouaib, F.; Tartour, E. Tissue Resident Memory T cells. Front. Immunol. 2019. [Google Scholar] [CrossRef]
- Clark, R.A.; Chong, B.; Mirchandani, N.; Brinster, N.K.; Yamanaka, K.; Dowgiert, R.K.; Kupper, T.S. The vast majority of CLA+ t cells are resident in normal skin. J. Immunol. 2006, 176, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Bardawil, T.; Kurban, M.; Abbas, O. Tissue-resident memory T cells in the skin. Inflamm. Res. 2020, 69, 245–254. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Z. Tissue-resident memory T cells and their biological characteristics in the recurrence of inflammatory skin disorders. Cell. Mollecular Immunol. 2019, 17, 64–75. [Google Scholar] [CrossRef]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103+ CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1391. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Schlums, H.; Gallais Sérézal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Forkel, M.; Höög, C.; et al. CD49a expression defines tissue - resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Fraser, K.A.; Schenkel, J.M.; Moran, A.; Abt, M.C.; Beura, L.K.; Lucas, P.J.; Artis, D.; Wherry, J.; Hogquist, K.; et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012, 188, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair follicle–derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Zapata, L., Jr.; Garg, M.; Riding, R.L.; Refat, M.A.; Fan, X.; Azzolino, V.; Tovar-Garza, A.; Tsurushita, N.; et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018, 10, eaam7710. [Google Scholar] [CrossRef]
- Jiang, X.; Clark, R.A.; Liu, L.; Wagers, A.J.; Fuhlbrigge, R.C.; Kupper, T.S. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 2012, 483, 227–231. [Google Scholar] [CrossRef]
- Kragten, N.A.M.; Behr, F.M.; Vieira Braga, F.A.; Remmerswaal, E.B.M.; Wesselink, T.H.; Oja, A.E.; Hombrink, P.; Kallies, A.; Van Lier, R.A.W.; Stark, R.; et al. Blimp-1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur. J. Immunol. 2018, 48, 1644–1662. [Google Scholar] [CrossRef]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef]
- Wang, D.; Diao, H.; Getzler, A.J.; Rogal, W.; Frederick, M.A.; Milner, J.; Yu, B.; Crotty, S.; Goldrath, A.W.; Pipkin, M.E. The transcription factor Runx3 establishes chromatin accessibility of cis-regulatory landscapes that drive memory cytotoxic T lymphocyte formation. Immunity 2018, 48, 659–674. [Google Scholar] [CrossRef]
- Milner, J.J.; Toma, C.; Yu, B.; Zhang, K.; Omilusik, K.; Phan, A.T.; Wang, D.; Getzler, A.J.; Nguyen, T.; Crotty, S.; et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 2017, 552, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Mills, K.H.G. CD4 TRM Cells Following Infection and Immunization: Implications for More Effective Vaccine Design. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Park, C.O.; Fu, X.; Jiang, X.; Pan, Y.; Teague, J.E.; Collins, N.; Tian, T.; O’Malley, J.T.; Emerson, R.O.; Kim, J.H.; et al. Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 2017, 142, 647–662. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting Edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef]
- Milner, J.; Goldrath, A.W. Transcriptional programming of tissue-resident memory CD8+ T cells. Curr. Opin. Immunol. 2018, 51, 162–169. [Google Scholar] [CrossRef]
- Pizzolla, A.; Nguyen, T.H.O.; Smith, J.M.; Brooks, A.G.; Kedzieska, K.; Heath, R.W.; Reading, P.C.; Wakim, L.M. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2017, 2, eaam6970. [Google Scholar] [CrossRef]
- Zaid, A.; Mackay, L.K.; Rahimpour, A.; Braun, A.; Veldhoen, M.; Carbone, F.R.; Manton, J.H.; Heath, W.R.; Mueller, S.N. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. USA 2014, 111, 5307–5312. [Google Scholar] [CrossRef]
- Clark, R.A. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015, 7, 269rv1. [Google Scholar] [CrossRef]
- Ariotti, S.; Beltman, J.B.; Chodaczek, G.; Hoekstra, M.E.; Van Beek, A.E.; Gomez-Eerland, R.; Ritsma, L.; Van Rheenen, J.; Marée, A.F.; Zal, T.; et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. USA 2012, 109, 19739–19744. [Google Scholar] [CrossRef]
- Gebhardt, T.; Wakim, M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Ariotti, S.; Hogenbirk, M.A.; Dijkgraaf, F.E.; Visser, L.L.; Hoekstra, M.E.; Song, J.Y.; Jacobs, H.; Haanen, J.B.; Schumacher, T.N. T cell memory. Skin-resident memory CD8⁺ T cells trigger a state of tissue-wide pathogen alert. Science 2014, 346, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Srivastava, R.; Chentoufi, A.A.; Kritzer, E.; Cgilukuri, S.; Garg, S.; Yu, D.C.; Vahed, H.; Huang, H.L.; Syed, S.A.; et al. Bolstering the numer and function of HSV-1-specific CD8(+) effector memory T cells and tissue-resident memory T cells in latently infected trigeminal ganglia reduces recurrent ocular herpes infection and disease. J. Immunol. 2017, 199, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kupper, T.S. Metabolic reprogramming and longevity of tissue-resident memory T cells. Front. Immunol. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Owczarczyk-Saczonek, A.; Krajewska-Włodarczyk, M.; Kasprowicz-Furmańczyk, M.; Placek, W. Immunological memory of psoriatic lesions. Int. J. Mol. Sci. 2020, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Jacquemin, C.; Darrigade, A.S.; Dessarthe, B.; Martins, C.; Boukhedouni, N.; Vernisse, C.; Grasseau, A.; Thiolat, D.; Rambert, J.; et al. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J. Investig. Dermatol. 2018, 138, 355–364. [Google Scholar] [CrossRef]
- Richmond, J.M.; Strassner, J.P.; Rashighi, M.; Garwal, P.A.; Garg, M.; Essien, K.I.; Pell, L.S.; Harris, J.E. Resident Memory and Recirculating Memory T Cells Cooperate to Maintain Disease in a Mouse Model of Vitiligo. J. Investig. Dermatol. 2019, 139, 769–778. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the Murine Contact Hypersensitivity Model: Toawrd the understanding of Allergic Contact Dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef]
- Gaide, O.; Emerson, R.O.; Jiang, X.; Gulati, N.; Nizza, S.; Desmarais, C.; Robins, H.; Krueger, J.G.; Clark, R.A.; Kupper, T.S. Common clonal origin of central and resident memory T cells following skin immunization. Nat. Med. 2015, 21, 647–653. [Google Scholar] [CrossRef]
- Sacirbegovic, F.; Zhu, J.; Liu, J.; Rosenberg, S.; Shlomchik, M.J.; Schlochik, W.D. Identifying tissue-resident memory T cells in graft-versus-host disease. Blood 2016, 128, 4544. [Google Scholar] [CrossRef]
- Badri, A.M.T.A.; Todd, P.M.; Garioch, J.J.; Gudgeon, J.E.; Stewart, D.G.; Goudie, R.B. An immunohistological study of cutaneous lymphocytes in vitiligo. J. Pathol. 1993, 170, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Konijnenberg, D.; Dellemijn, T.A.; Van der Veen, J.P.; Bos, J.D.; Melief, C.J.; Vyth-Dreese, F.A.; Luiten, R.M. Autoimmune Destruction of Skin Melanocytes by Perilesional T Cells from Vitiligo Patients. J. Investig. Dermatol. 2009, 129, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Essien, K.I.; Harris, J.E. Animal models of vitiligo: Matching the model to the question. Dermatol. Sin. 2014, 32, 240–247. [Google Scholar] [CrossRef]
- Boniface, K.; Seneschal, J. Vitiligo as a skin memory disease: The need for early interventio with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp. Dermatol. 2019, 28, 656–661. [Google Scholar] [CrossRef]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 2014, 6, 223ra23. [Google Scholar] [CrossRef]
- Harris, J.E.; Harris, T.H.; Weninger, W.; Wherry, E.J.; Hunter, C.A.; Turka, L.A. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J. Investig. Dermatol. 2012, 132, 1869–1876. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef]
- Schenkel, J.M.; Fraser, K.A.; Vezys, V.; Masopust, D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 2013, 14, 509–513. [Google Scholar] [CrossRef]
- Gálvez-Cancino, F.; López, E.; Menares, E.; Díaz, X.; Flores, C.; Cáceres, P.; Hidalgo, S.; Chovar, O.; Alcántara-Hernández, M.; Borgna, V.; et al. Vaccination-induced skin-resident memory CD8+ T cells mediate strong protection against cutaneous melanoma. OncoImmunology 2018, 7, e1442163. [Google Scholar] [CrossRef]
- Corgnac, S.; Boutet, M.; Kfoury, M.; Naltet, C.; Mami-Chouaib, F. The emerging role of CD8(+) tissue resident memory T (TRM) cells in antitumor immunity: A unique functional contribution of the CD103 integrin. Front. Immunol. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Watson, P.; DeLeeuw, R.J.; Nelson, B.H. Tumor-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2013, 20, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Milne, K.; Derocher, H.; Webb, J.R.; Nelson, B.H.; Watson, P.H. CD103 and Intratumoral Immune Response in Breast Cancer. Clin. Cancer Res. 2016, 22, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Enamorado, M.; Iborra, S.; Priego, E.; Cueto, F.J.; Quintana, J.A.; Martínez-Cano, S.; Mejías-Pérez, E.; Esteban, M.; Melero, I.; Hidalgo, A.; et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nat. Commun. 2017, 8, 16073. [Google Scholar] [CrossRef] [PubMed]
| Type | Subtypes |
|---|---|
| Non-segmented vitiligo/vitiligo | Acrofacial Generalized Universal Mucosal (more than one site) Mixed (coexistence of SV and NSV) Rare forms |
| Segmented vitiligo | Unisegmental Bisegmental Plurisegmental |
| Undetermined/unclassified vitiligo | Mucosal (one site) Focal |
| Function | |||
|---|---|---|---|
| CD4+/ CD8+ | Co-receptors of the T cell receptor (TCR) [59] | ||
| Surface marker | CD 49a+ | Secretion of granzyme B, perforin, IFN-γ and achieving high cytotoxic properties following IL-15 stimulation [63] | |
| CD 69+ | Blockade of the sphingosine 1-phosphate receptor 1 (S1PR1) [75] | ||
| CD 103+ | Participation in binding TRM cells to E-cadherin to promote retention within epithelial tissues [72] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frączek, A.; Owczarczyk-Saczonek, A.; Placek, W. The Role of TRM Cells in the Pathogenesis of Vitiligo—A Review of the Current State-Of-The-Art. Int. J. Mol. Sci. 2020, 21, 3552. https://doi.org/10.3390/ijms21103552
Frączek A, Owczarczyk-Saczonek A, Placek W. The Role of TRM Cells in the Pathogenesis of Vitiligo—A Review of the Current State-Of-The-Art. International Journal of Molecular Sciences. 2020; 21(10):3552. https://doi.org/10.3390/ijms21103552
Chicago/Turabian StyleFrączek, Alicja, Agnieszka Owczarczyk-Saczonek, and Waldemar Placek. 2020. "The Role of TRM Cells in the Pathogenesis of Vitiligo—A Review of the Current State-Of-The-Art" International Journal of Molecular Sciences 21, no. 10: 3552. https://doi.org/10.3390/ijms21103552
APA StyleFrączek, A., Owczarczyk-Saczonek, A., & Placek, W. (2020). The Role of TRM Cells in the Pathogenesis of Vitiligo—A Review of the Current State-Of-The-Art. International Journal of Molecular Sciences, 21(10), 3552. https://doi.org/10.3390/ijms21103552






