Exosomal MiRNA Transfer between Retinal Microglia and RPE
Abstract
1. Introduction
2. Results
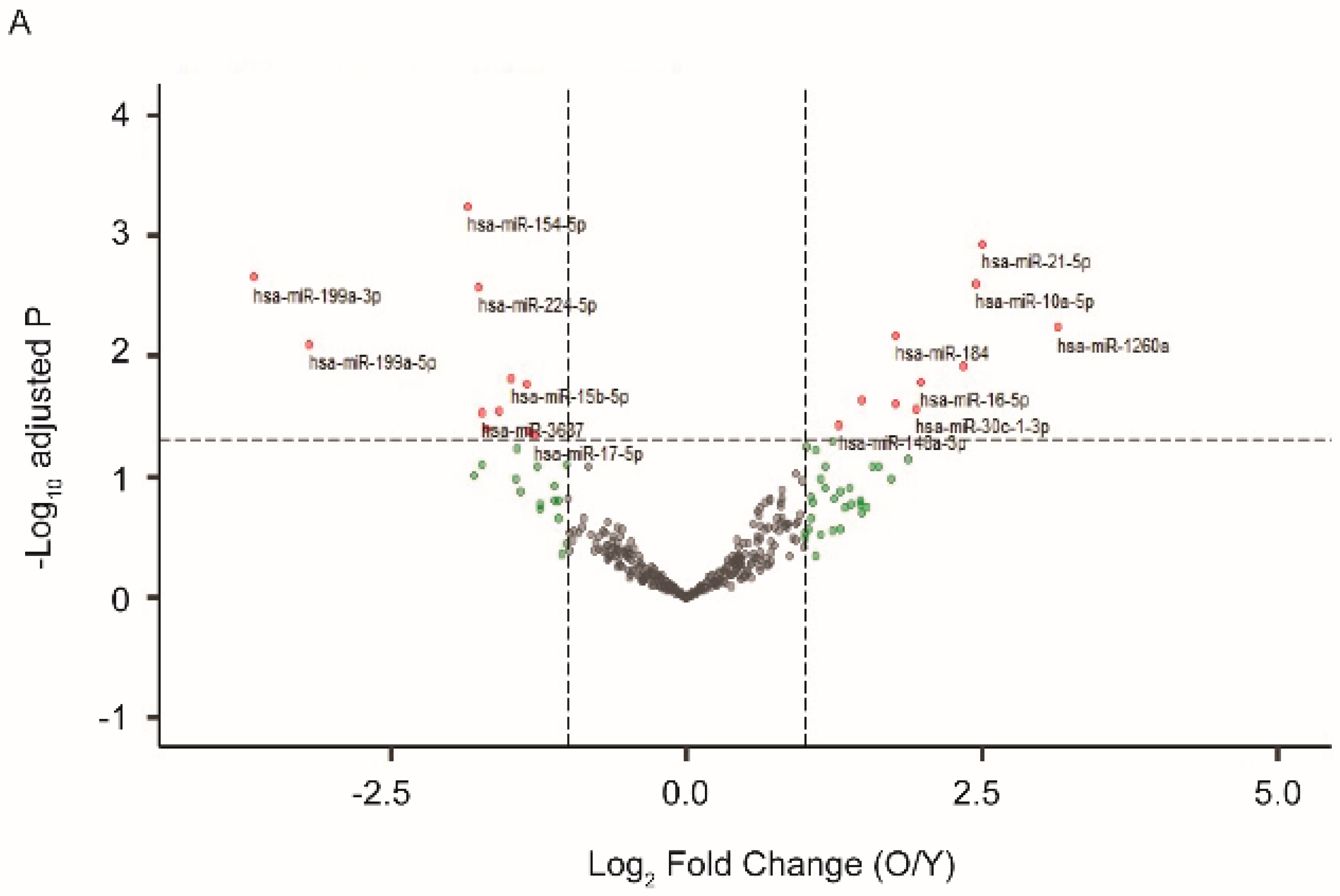
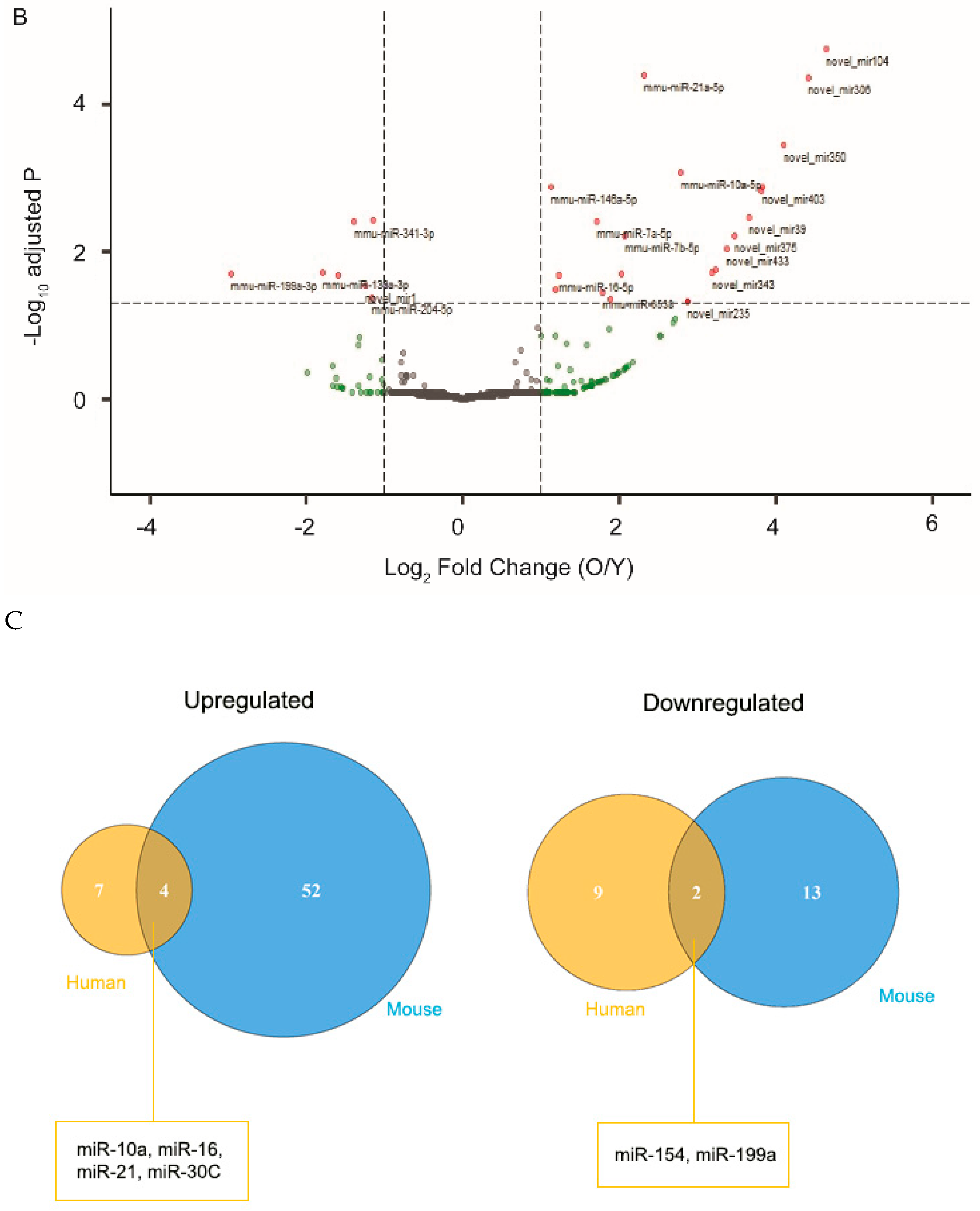
2.1. Exosomal miRNA Profiles Differ between Young and Aged RPE
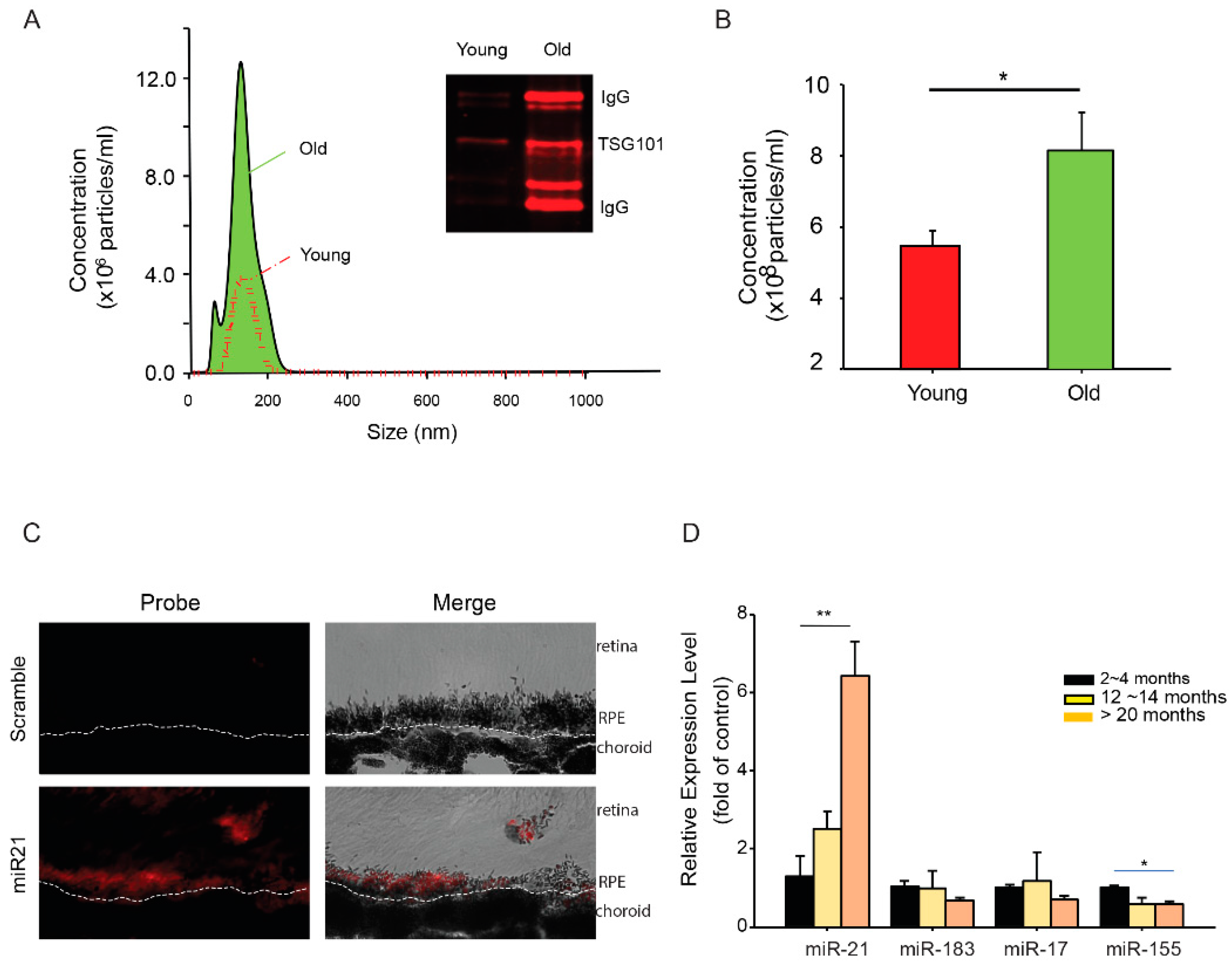
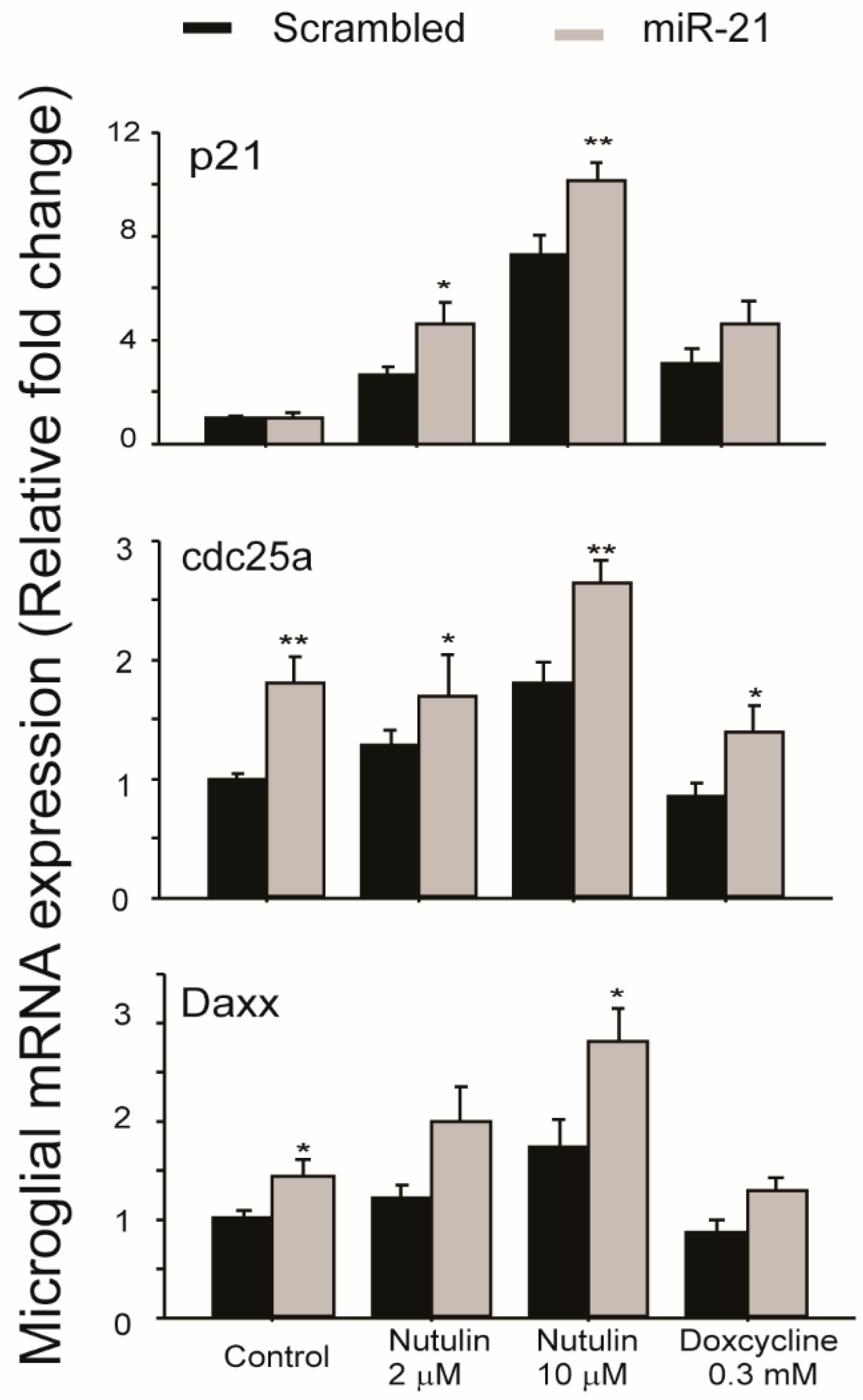
2.2. Transfer of miR-21 between RPE and Retinal Microglia
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Purification of Exosomal RNA Released by Human RPE Cells or Mice RPE-Choroid-Sclera Explant and RNA-Seq Analyses
4.3. Nanoparticle Tracking Analysis
4.4. Western Blot Analyses of EVs
4.5. Quantitation of Cellular miRNAs
4.6. Fluorescent miRNA In Situ Hybridization (ISH)
4.7. Culture of Mice Primary Retinal Microglia
4.8. Transfer of miRNA 21 from RPE to Microglia
4.9. Transfection of Microglia with miR-21 Mimics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Terman, A.; Kurz, T.; Navratil, M.; Arriaga, E.A.; Brunk, U.T. Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial-lysosomal axis theory of aging. Antioxid. Redox Signal. 2010, 12, 503–535. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P., Jr.; Jones, D.P. Oxidative damage and protection of the rpe. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Boulias, K.; Horvitz, H.R. The C. elegans microrna mir-71 acts in neurons to promote germline-mediated longevity through regulation of daf-16/foxo. Cell Metab. 2012, 15, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Castro, C.; Murfitt, K.J.; Abreu-Goodger, C.; Griffin, J.L.; Miska, E.A. Post-developmental microrna expression is required for normal physiology, and regulates aging in parallel to insulin/igf-1 signaling in C. elegans. RNA 2012, 18, 2220–2235. [Google Scholar] [CrossRef]
- Boehm, M.; Slack, F. A developmental timing microrna and its target regulate life span in C. elegans. Science 2005, 310, 1954–1957. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mrnas are conserved targets of micrornas. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microrna targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Mendell, J.T.; Olson, E.N. Micrornas in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef]
- Bartel, D.P. Micrornas: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Maes, O.C.; An, J.; Sarojini, H.; Wang, E. Murine micrornas implicated in liver functions and aging process. Mech. Ageing Dev. 2008, 129, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A.; Rasmussen, B.B. Aging and microrna expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genom. 2011, 43, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Persengiev, S.; Kondova, I.; Otting, N.; Koeppen, A.H.; Bontrop, R.E. Genome-wide analysis of mirna expression reveals a potential role for mir-144 in brain aging and spinocerebellar ataxia pathogenesis. Neurobiol. Aging 2011, 32, e2316–e2317. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H., 3rd; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K. Age-related changes in microrna levels in serum. Aging (Albany Ny) 2013, 5, 725–740. [Google Scholar] [CrossRef]
- Smith-Vikos, T.; Slack, F.J. Micrornas and their roles in aging. J. Cell Sci. 2012, 125, 7–17. [Google Scholar] [CrossRef]
- Krol, J.; Busskamp, V.; Markiewicz, I.; Stadler, M.B.; Ribi, S.; Richter, J.; Duebel, J.; Bicker, S.; Fehling, H.J.; Schubeler, D.; et al. Characterizing light-regulated retinal micrornas reveals rapid turnover as a common property of neuronal micrornas. Cell 2010, 141, 618–631. [Google Scholar] [CrossRef]
- Xu, S.; Witmer, P.D.; Lumayag, S.; Kovacs, B.; Valle, D. Microrna (mirna) transcriptome of mouse retina and identification of a sensory organ-specific mirna cluster. J. Biol. Chem. 2007, 282, 25053–25066. [Google Scholar] [CrossRef]
- Lumayag, S.; Haldin, C.E.; Corbett, N.J.; Wahlin, K.J.; Cowan, C.; Turturro, S.; Larsen, P.E.; Kovacs, B.; Witmer, P.D.; Valle, D.; et al. Inactivation of the microrna-183/96/182 cluster results in syndromic retinal degeneration. Proc. Natl. Acad. Sci. USA 2013, 110, E507–E516. [Google Scholar] [CrossRef]
- Saxena, K.; Rutar, M.V.; Provis, J.M.; Natoli, R.C. Identification of mirnas in a model of retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1820–1829. [Google Scholar] [CrossRef]
- Loscher, C.J.; Hokamp, K.; Kenna, P.F.; Ivens, A.C.; Humphries, P.; Palfi, A.; Farrar, G.J. Altered retinal microrna expression profile in a mouse model of retinitis pigmentosa. Genome Biol. 2007, 8, R248. [Google Scholar] [CrossRef] [PubMed]
- McArthur, K.; Feng, B.; Wu, Y.; Chen, S.; Chakrabarti, S. Microrna-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 2011, 60, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of extracellular mirnas in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Koster, K.M.; He, Y.; Zhou, Q. Mirnas as potential therapeutic targets for age-related macular degeneration. Future Med. Chem. 2012, 4, 277–287. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microrna: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F. Unidirectional transfer of microrna-loaded exosomes from t cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Provis, J.M.; Diaz, C.M.; Penfold, P.L. Microglia in human retina: A heterogeneous population with distinct ontogenies. Perspect. Dev. Neurobiol. 1996, 3, 213–222. [Google Scholar]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.B.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. Embo Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef]
- Yu, B.; Xu, P.; Zhao, Z.; Cai, J.; Sternberg, P.; Chen, Y. Subcellular distribution and activity of mechanistic target of rapamycin in aged retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8638–8650. [Google Scholar] [CrossRef]
- Klingeborn, M.; Stamer, W.D.; Bowes Rickman, C. Polarized exosome release from the retinal pigmented epithelium. Adv. Exp. Med. Biol. 2018, 1074, 539–544. [Google Scholar] [PubMed]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.S.; Yang, Y.; Moran, E.; Ma, J.X. Pathogenic role of microrna-21 in diabetic retinopathy through downregulation of pparalpha. Diabetes 2017, 66, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Gutsaeva, D.R.; Thounaojam, M.; Rajpurohit, S.; Powell, F.L.; Martin, P.M.; Goei, S.; Duncan, M.; Bartoli, M. Stat3-mediated activation of mir-21 is involved in down-regulation of timp3 and neovascularization in the ischemic retina. Oncotarget 2017, 8, 103568–103580. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Forrester, J. Inflammation in age-related macular degeneration: What is the evidence? In Uveitis and Immunological Disorders; Springer: Berlin/Heidelberg, Germany, 2009; pp. 61–71. [Google Scholar]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Shapiro, A.; Kosik, K.S. Microrna-21 targets a network of key tumor-suppressive pathways in glioblastoma cells. Cancer Res. 2008, 68, 8164–8172. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-box protein 1 is required to sort micrornas into exosomes in cells and in a cell-free reaction. eLife 2016, 5, e19276. [Google Scholar] [CrossRef]
- Xu, Y.F.; Hannafon, B.N.; Khatri, U.; Gin, A.; Ding, W.Q. The origin of exosomal mir-1246 in human cancer cells. RNA Biol. 2019, 16, 770–784. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liang, Y.; Liu, Y.; Xu, P.; Flamme-Wiese, M.J.; Sun, D.; Sun, J.; Mullins, R.F.; Chen, Y.; Cai, J. Choroidal gammadelta t cells in protection against retinal pigment epithelium and retinal injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 4903–4916. [Google Scholar]
- Li, L.; Qu, C.; Wang, F. A novel method for co-culture with muller cells and microglia in rat retina in vitro. Biomed. Rep. 2015, 3, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Saura, J.; Tusell, J.M.; Serratosa, J. High-yield isolation of murine microglia by mild trypsinization. Glia 2003, 44, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chang, Q.; Zhao, Z.; Yan, S.; Wang, L.; Cai, J.; Xu, G. Melatonin-mediated cytoprotection against hyperglycemic injury in muller cells. PLoS ONE 2012, 7, e50661. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.C.; Neil, S.E.; Quandt, J.A. High yield primary microglial cultures using granulocyte macrophage-colony stimulating factor from embryonic murine cerebral cortical tissue. J. Neuroimmunol. 2017, 307, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, Y.; Murphy, T.J.; Jones, D.P.; Sartorelli, A.C. Role of caspase activation in butyrate-induced terminal differentiation of ht29 colon carcinoma cells. Arch. Biochem. Biophys. 2004, 424, 119–127. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia responses to pro-inflammatory stimuli (lps, ifngamma+tnfalpha) and reprogramming by resolving cytokines (il-4, il-10). Front. Cell Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- O’Koren, E.G.; Yu, C.; Klingeborn, M.; Wong, A.Y.W.; Prigge, C.L.; Mathew, R.; Kalnitsky, J.; Msallam, R.A.; Silvin, A.; Kay, J.N.; et al. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity 2019, 50, 723–737.e727. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Shriver, L.P.; Dittel, B.N. Cd40 expression by microglial cells is required for their completion of a two-step activation process during central nervous system autoimmune inflammation. J. Immunol. 2006, 176, 1402–1410. [Google Scholar] [CrossRef]
- Salemi, J.; Obregon, D.F.; Cobb, A.; Reed, S.; Sadic, E.; Jin, J.; Fernandez, F.; Tan, J.; Giunta, B. Flipping the switches: Cd40 and cd45 modulation of microglial activation states in hiv associated dementia (had). Mol. Neurodegener. 2011, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.; Stepicheva, N.A.; Hose, S.; Zigler, J.S., Jr.; Sinha, D. Primary cell cultures from the mouse retinal pigment epithelium. J. Vis. Exp. Jove 2018, 133, e56997. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, D.R.; Bounds, S.E.; Liu, H.; Ding, W.-Q.; Chen, Y.; Liu, Y.; Cai, J. Exosomal MiRNA Transfer between Retinal Microglia and RPE. Int. J. Mol. Sci. 2020, 21, 3541. https://doi.org/10.3390/ijms21103541
Morris DR, Bounds SE, Liu H, Ding W-Q, Chen Y, Liu Y, Cai J. Exosomal MiRNA Transfer between Retinal Microglia and RPE. International Journal of Molecular Sciences. 2020; 21(10):3541. https://doi.org/10.3390/ijms21103541
Chicago/Turabian StyleMorris, Dorothea R., Sarah E. Bounds, Huanhuan Liu, Wei-Qun Ding, Yan Chen, Yin Liu, and Jiyang Cai. 2020. "Exosomal MiRNA Transfer between Retinal Microglia and RPE" International Journal of Molecular Sciences 21, no. 10: 3541. https://doi.org/10.3390/ijms21103541
APA StyleMorris, D. R., Bounds, S. E., Liu, H., Ding, W.-Q., Chen, Y., Liu, Y., & Cai, J. (2020). Exosomal MiRNA Transfer between Retinal Microglia and RPE. International Journal of Molecular Sciences, 21(10), 3541. https://doi.org/10.3390/ijms21103541





