Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An In Vitro Model to Study Endometrial Inflammation at Post-Insemination
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Recovering Donkey PMN from Peripheral Blood Samples
2.2. Experiment 2: Evaluation of PMN-sperm Binding (FMLP and DMSO)
2.2.1. Sperm Motility
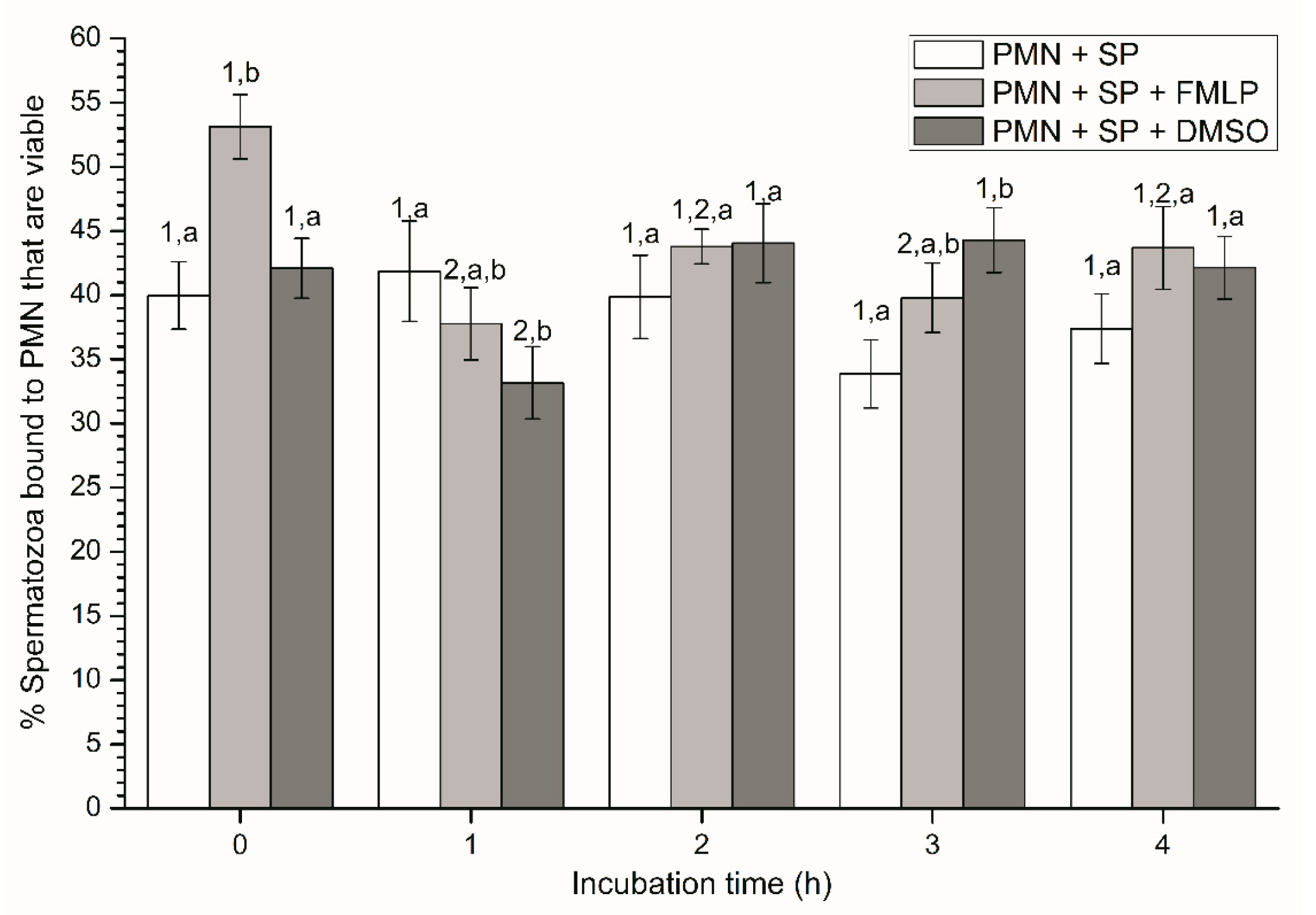
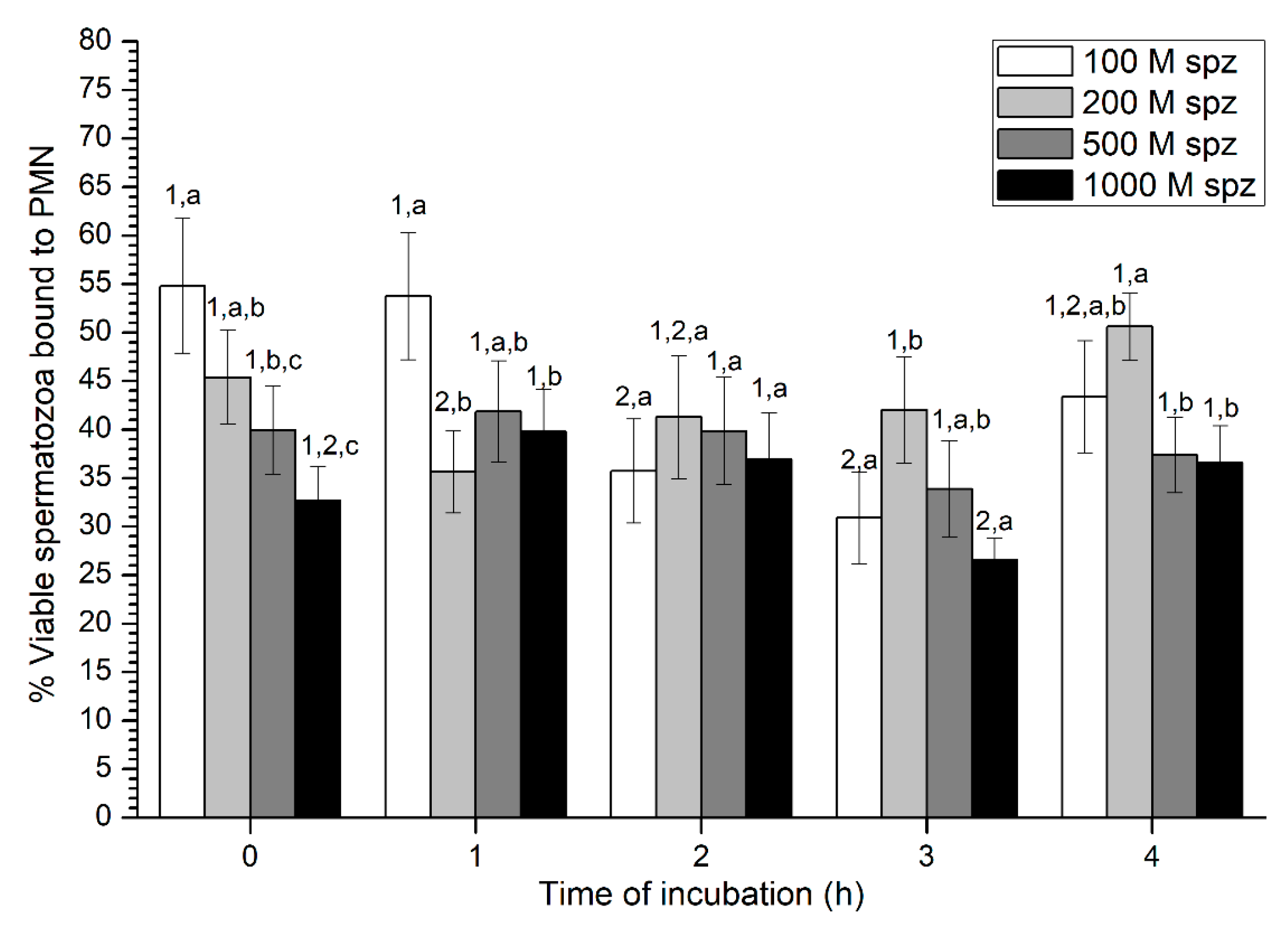
2.2.2. Sperm Viability
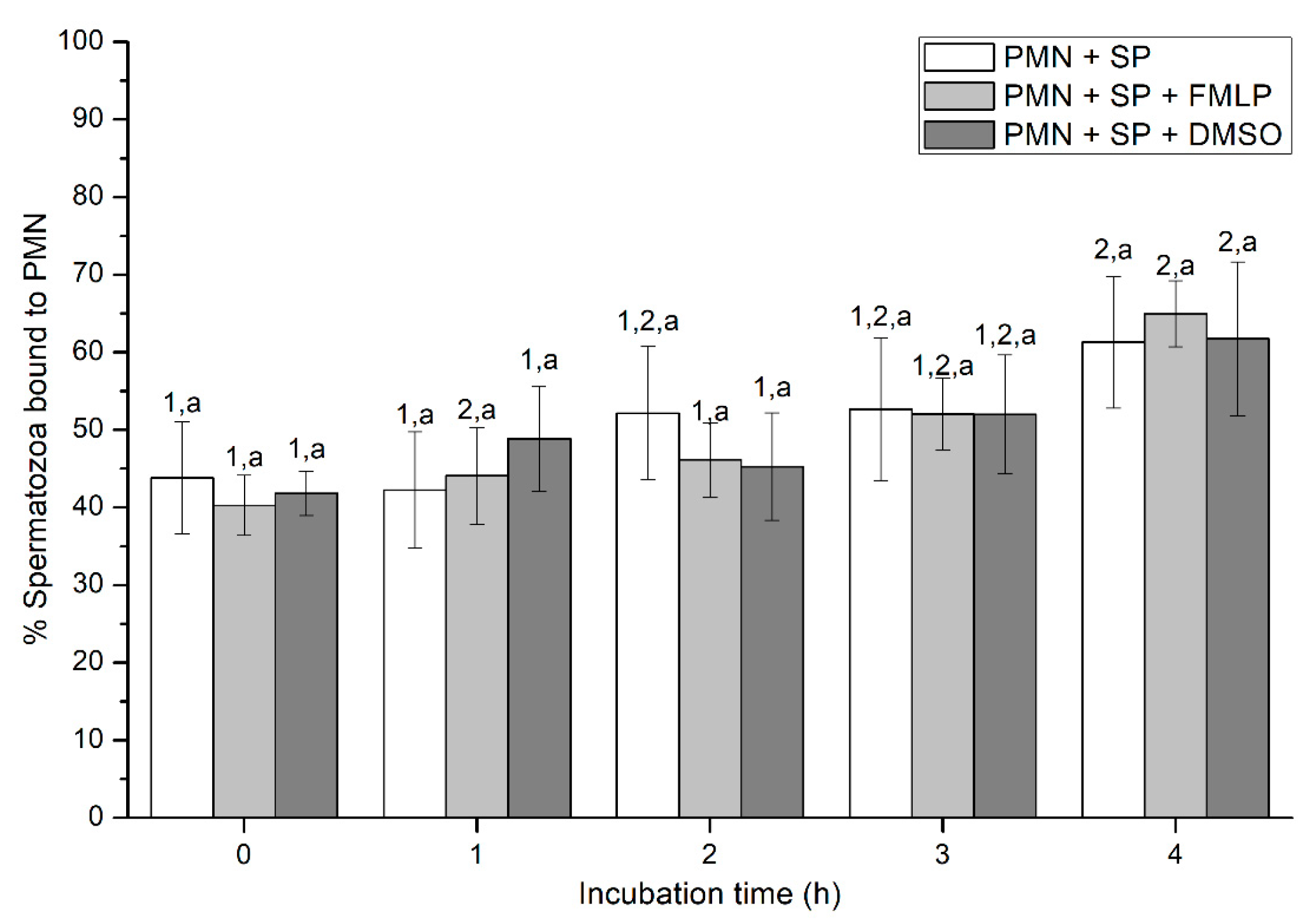
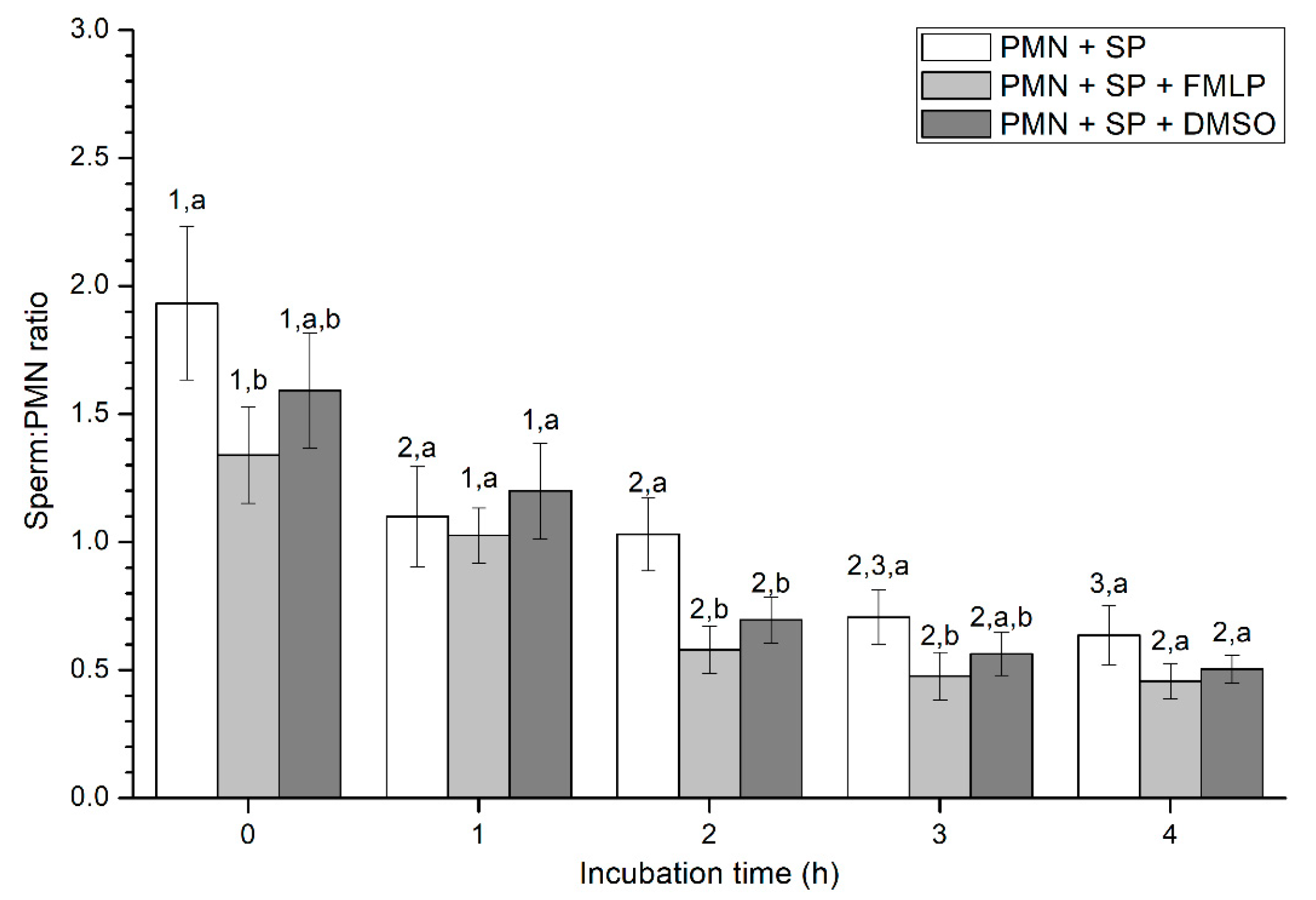
2.2.3. Sperm-PMN Binding
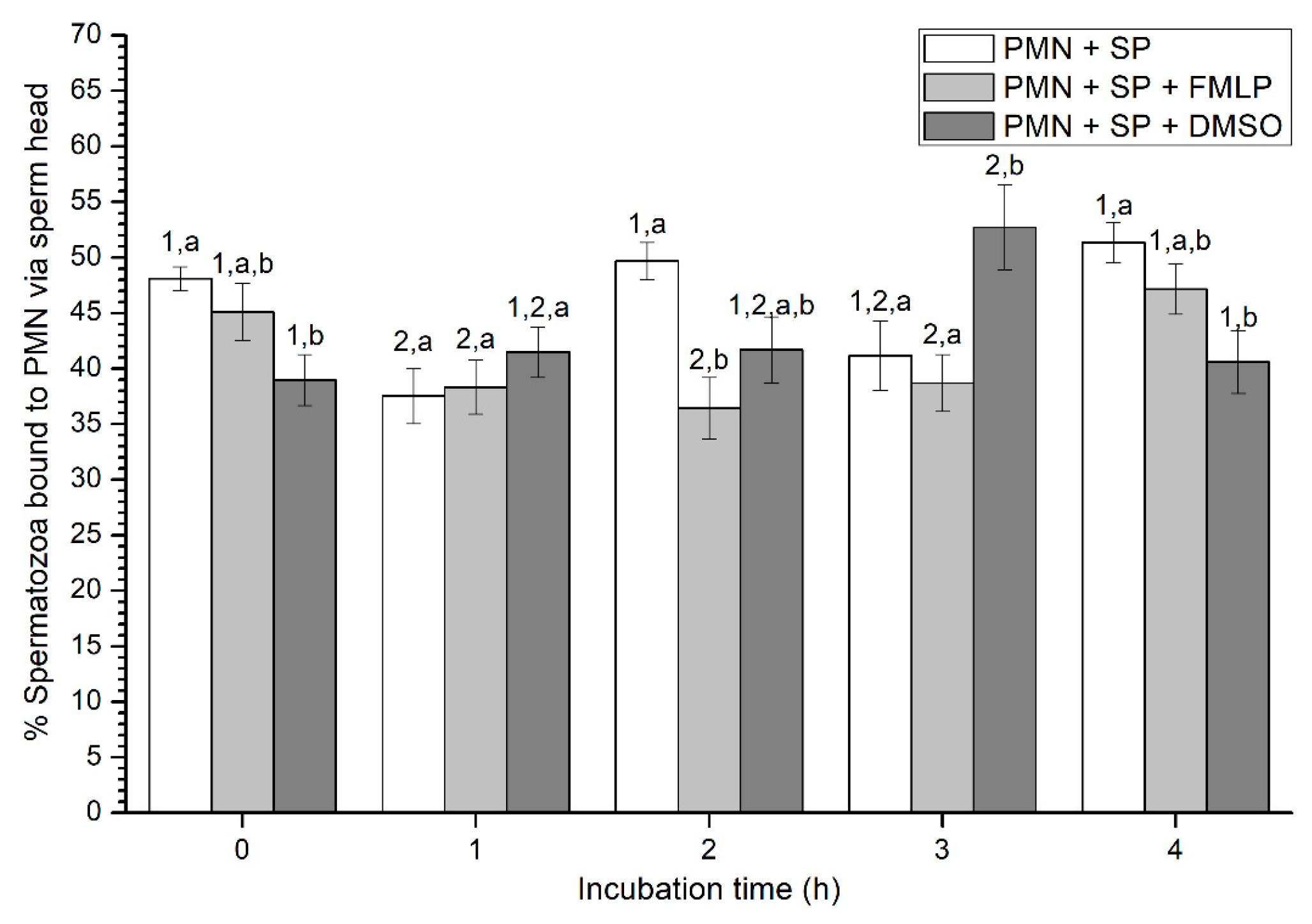
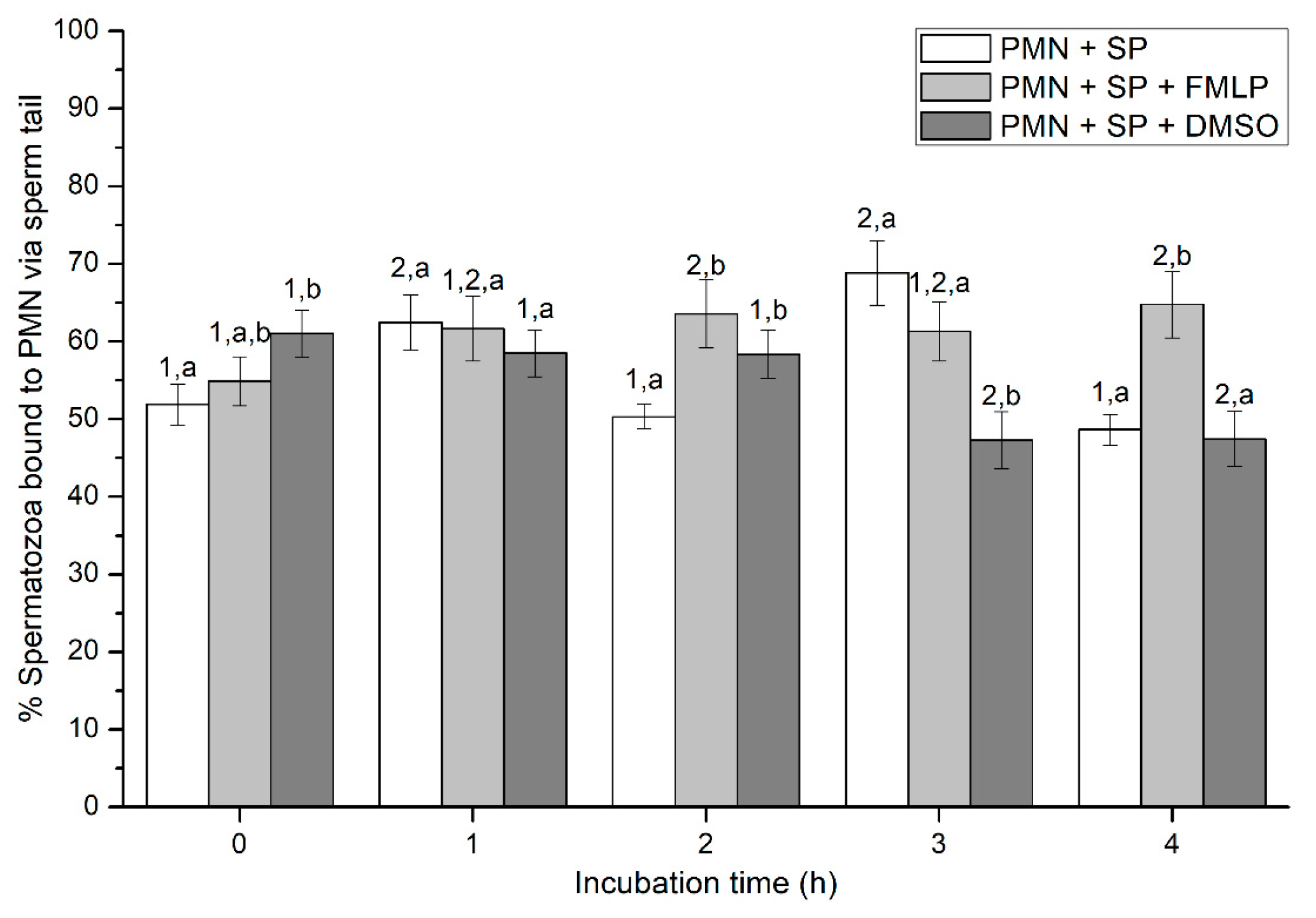
2.2.4. Sperm Attachment to PMN via Their Head or Tail
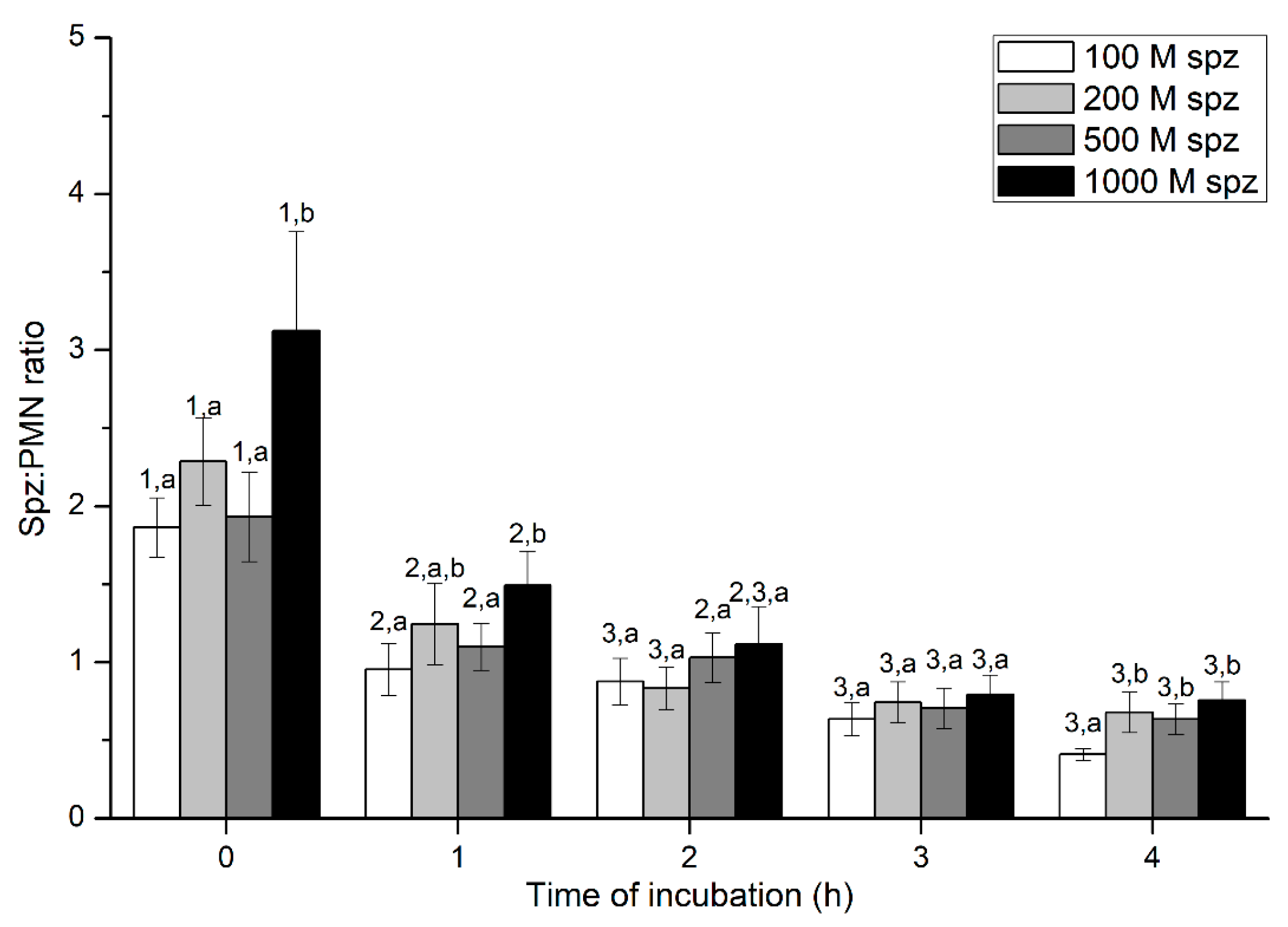
2.3. Experiment 3: Evaluation of PMN-sperm Binding (Sperm Concentration)
Sperm-PMN Binding
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experiment 1. Isolation of Donkey PMN from Peripheral Blood
4.2.1. Protocol 1
4.2.2. Protocol 2
4.2.3. Protocol 3
4.2.4. Protocol 4
4.2.5. Protocol 5
4.2.6. Protocol 6
4.2.7. Protocol 7
4.3. Experiments 2 and 3: Effects of SP and Sperm Concentration on PMN-sperm Binding
4.3.1. Semen Collection and Process
4.3.2. Treatments
4.3.3. Evaluation of Sperm Viability
4.3.4. Sperm–PMN Binding
4.3.5. Sperm Motility
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial insemination |
| ALH | Amplitude of lateral head displacement |
| BCF | Frequency of head displacement |
| CASA | Computer-Assisted Sperm Analysis |
| CAT | Cathepsin |
| DMSO | Dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| ELA | Elastase |
| FMLP | Formyl-methionyl-leucyl-phenylalanine |
| LIN | Linearity |
| MPO | Myeloperoxidase |
| NETs | Neutrophil Extracellular Traps |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| PMN | Polymorphonuclear Neutrophils |
| SEM | Standard error of the mean |
| SP | Seminal plasma |
| STR | Straightness |
| VAP | Average path velocity |
References
- Canisso, I.F.; Panzani, D.; Miró, J.; Ellerbrock, R.E. Key Aspects of Donkey and Mule Reproduction. Vet. Clin. N. Am. Equine Pract. 2019, 35, 607–642. [Google Scholar] [CrossRef]
- Quintero-Moreno, A.; Miró, J.; Teresa Rigau, A.; Rodríguez-Gil, J.E. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 2003, 59, 1973–1990. [Google Scholar] [CrossRef]
- Miró, J.; Lobo, V.; Quintero-Moreno, A.; Medrano, A.; Peña, A.; Rigau, T. Sperm motility patterns and metabolism in Catalonian donkey semen. Theriogenology 2005, 63, 1706–1716. [Google Scholar] [CrossRef]
- Flores, E.; Taberner, E.; Rivera, M.M.; Peña, A.; Rigau, T.; Miró, J.; Rodríguez-Gil, J.E. Effects of freezing/thawing on motile sperm subpopulations of boar and donkey ejaculates. Theriogenology 2008, 70, 936–945. [Google Scholar] [CrossRef]
- Rota, A.; Magelli, C.; Panzani, D.; Camillo, F. Effect of extender, centrifugation and removal of seminal plasma on cooled-preserved Amiata donkey spermatozoa. Theriogenology 2008, 69, 176–185. [Google Scholar] [CrossRef]
- Sabatini, C.; Mari, G.; Mislei, B.; Love, C.; Panzani, D.; Camillo, F.; Rota, A. Effect of post-thaw addition of seminal plasma on motility, viability and chromatin integrity of cryopreserved donkey jack (Equus asinus) spermatozoa. Reprod. Domest. Anim. 2014, 49, 989–994. [Google Scholar] [CrossRef]
- Taberner, E.; Morató, R.; Mogas, T.; Miró, J. Ability of Catalonian donkey sperm to penetrate zona pellucida-free bovine oocytes matured in vitro. Anim. Reprod. Sci. 2010, 118, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Vidament, M.; Vincent, P.; Martin, F.X.; Magistrini, M.; Blesbois, E. Differences in ability of jennies and mares to conceive with cooled and frozen semen containing glycerol or not. Anim. Reprod. Sci. 2009, 112, 22–35. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.V.; Oliveira, P.V.D.L.F.; E Oña, C.M.M.; Guasti, P.N.; Monteiro, G.A.; Da Silva, Y.F.R.S.; Papa, P.D.M.; Alvarenga, M.A.; Junior, J.A.D.; Papa, F.O.; et al. Strategies to improve the fertility of fresh and frozen donkey semen. Theriogenology 2016, 85, 1267–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotilainen, T.; Huhtinen, M.; Katila, T. Sperm-induced leukocytosis in the equine uterus. Theriogenology 1994, 41, 629–636. [Google Scholar] [CrossRef]
- Troedsson, M.H.T.; Loset, K.; Alghamdi, A.M.; Dahms, B.; Crabo, B.G. Interaction between equine semen and the endometrium: The inflammatory response to semen. Anim. Reprod. Sci. 2001, 68, 273–278. [Google Scholar] [CrossRef]
- Rozeboom, K.J.; Rocha-Chavez, G.; Troedsson, M.H. Inhibition of neutrophil chemotaxis by pig seminal plasma in vitro: A potential method for modulating post-breeding inflammation in sows. Reproduction 2001, 121, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Matthijs, A.; Engel, B.; Woelders, H. Neutrophil recruitment and phagocytosis of boar spermatozoa after artificial insemination of sows, and the effects of inseminate volume, sperm dose and specific additives in the extender. Reproduction 2003, 125, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Eisenbach, M. Why are sperm cells phagocytosed by leukocytes in the female genital tract? Med. Hypotheses 2003, 60, 590–592. [Google Scholar] [CrossRef]
- Görgens, A.; Leibold, W.; Klug, E.; Schuberth, H.J.; Martinsson, G.; Zerbe, H. Inseminate components are modulating the chemotactic activity of uterine polymorphonuclear granulocytes (PMN) of mares. Anim. Reprod. Sci. 2005, 89, 308–310. [Google Scholar] [PubMed]
- Vilés, K.; Rabanal, R.; Rodríguez-Prado, M.; Miró, J. Influence of seminal plasma on leucocyte migration and amount of COX-2 protein in the jenny endometrium after insemination with frozen-thawed semen. Anim. Reprod. Sci. 2013, 143, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Papas, M. Post–Artificial Insemination Endometrial Inflammation and Its Control in Donkeys. J. Equine Vet. Sci. 2018, 65, 38–43. [Google Scholar] [CrossRef]
- Miró, J.; Taberner, E.; Rivera, M.; Peña, A.; Medrano, A.; Rigau, T.; Peñalba, A. Effects of dilution and centrifugation on the survival of spermatozoa and the structure of motile sperm cell subpopulations in refrigerated Catalonian donkey semen. Theriogenology 2009, 72, 1017–1022. [Google Scholar] [CrossRef]
- Papas, M.; Arroyo, L.; Bassols, A.; Catalán, J.; Bonilla-Correal, S.; Gacem, S.; Yeste, M.; Miró, J. Activities of antioxidant seminal plasma enzymes (SOD, CAT, GPX and GSR) are higher in jackasses than in stallions and are correlated with sperm motility in jackasses. Theriogenology 2019, 140, 180–187. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Lovaas, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Species-specific interaction of seminal plasma on sperm-neutrophil binding. Anim. Reprod. Sci. 2009, 114, 331–344. [Google Scholar] [CrossRef]
- Aloé, S.; Weber, F.; Behr, B.; Sauter-Louis, C.; Zerbe, H. Modulatory effects of bovine seminal plasma on uterine inflammatory processes. Reprod. Domest. Anim. 2012, 47, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Miró, J.; Vilés, K.; García, W.; Jordana, J.; Yeste, M. Effect of donkey seminal plasma on sperm movement and sperm-polymorphonuclear neutrophils attachment in vitro. Anim. Reprod. Sci. 2013, 140, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Vilés, K.; Rabanal, R.; Rodríguez-Prado, M.; Miró, J. Effect of ketoprofen treatment on the uterine inflammatory response after AI of jennies with frozen semen. Theriogenology 2013, 79, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.A.; Kaeberle, M.L. Isolation of neutrophils and eosinophils from the peripheral blood of cattle and comparison of their functional activities. J. Immunol. Methods 1981, 45, 153–164. [Google Scholar] [CrossRef]
- Siemsen, D.W.; Schepetkin, I.A.; Kirpotina, L.N.; Lei, B.; Quinn, M.T. Neutrophil isolation from nonhuman species. Methods Mol. Biol. 2007, 412, 21–34. [Google Scholar]
- Baumber, J.; Vo, A.; Sabeur, K.; Ball, B.A. Generation of reactive oxygen species by equine neutrophils and their effect on motility of equine spermatozoa. Theriogenology 2002, 57, 1025–1033. [Google Scholar] [CrossRef]
- Loftus, J.P.; Williams, J.M.; Belknap, J.K.; Black, S.J. In vivo priming and ex vivo activation of equine neutrophils in black walnut extract-induced equine laminitis is not attenuated by systemic lidocaine administration. Vet. Immunol. Immunopathol. 2010, 138, 60–69. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Kazzaz, N.M.; Sule, G.; Knight, J.S. Intercellular interactions as regulators of netosis. Front. Immunol. 2016, 7, 453. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, D.; Kumar, S.V.; Desai, J.; Anders, H.J. Neutrophil extracellular traps in tissue pathology. Histol. Histopathol. 2017, 32, 203–213. [Google Scholar] [PubMed]
- Rebordão, M.R.; Amaral, A.; Lukasik, K.; Szóstek-Mioduchowska, A.; Pinto-Bravo, P.; Galvão, A.; Skarzynski, D.J.; Ferreira-Dias, G. Constituents of neutrophil extracellular traps induce in vitro collagen formation in mare endometrium. Theriogenology 2018, 113, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, A.S.; Foster, D.N. Seminal DNase Frees Spermatozoa Entangled in Neutrophil Extracellular Traps. Biol. Reprod. 2005, 73, 1174–1181. [Google Scholar] [CrossRef]
- Alghamdi, A.S.; Funnell, B.J.; Bird, S.L.; Lamb, G.C.; Rendahl, A.K.; Taube, P.C.; Foster, D.N. Comparative studies on bull and stallion seminal DNase activity and interaction with semen extender and spermatozoa. Anim. Reprod. Sci. 2010, 121, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, T.; Kotarski, F.; Gärtner, U.; Conejeros, I.; Hermosilla, C.; Wrenzycki, C.; Taubert, A. Bovine sperm samples induce different NET phenotypes in a NADPH oxidase-, PAD4-, and Ca++-dependent process. Biol. Reprod. 2020, 102, 902–914. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020, 100, 1077–1117. [Google Scholar] [CrossRef]
- Menegazzi, R.; Decleva, E.; Dri, P. Killing by neutrophil extracellular traps: Fact or folklore? Blood 2012, 119, 1214–1216. [Google Scholar] [CrossRef] [Green Version]
- Kenney, R.M. Minimal contamination techniques for breeding mares: Techniques and preliminary findings. Proc. Am. Assoc. Equine Pract. 1975, 21, 327–336. [Google Scholar]
- Bamba, K. Evaluation of acrosomal integrity of boar spermatozoa by bright field microscopy using an eosin-nigrosin stain. Theriogenology 1988, 29, 1245–1251. [Google Scholar] [CrossRef]
- Palm, F.; Walter, I.; Budik, S.; Kolodziejek, J.; Nowotny, N.; Aurich, C. Influence of different semen extenders and seminal plasma on PMN migration and on expression of IL-1β, IL-6, TNF-α and COX-2 mRNA in the equine endometrium. Theriogenology 2008, 70, 843–851. [Google Scholar] [CrossRef] [PubMed]
| Protocol | [PMN] (×103 cells/µL) |
|---|---|
| 1 | 8.23 ± 0.87 a |
| 2 | 6.20 ± 1.23 a,b |
| 3 | 3.37 ± 0.95 b |
| 4 | 56.16 ± 6.31 c |
| 5 | 93.70 ± 7.07 d |
| 6 | 126.07 ± 10.01 d |
| 7 | 307.07 ± 19.77 e |
| Treatment | 0 h | 1 h | 2 h | 3 h | 4 h |
|---|---|---|---|---|---|
| PMN + SP | 77.9 ± 4.7 1, a | 65.2 ± 7.0 1, 2, a | 55.8 ± 6.0 2, 3, a | 43.2 ± 4.3 3, a | 30.5 ± 3.3 4, a |
| PMN + SP + FMLP | 73.9 ± 6.8 1, a | 62.5 ± 7.1 1, 2, a | 53.6 ± 7.1 2, 3, a | 38.7 ± 4.5 3, 4, a | 28.9 ± 3.4 4, a |
| PMN + SP + DMSO | 74.9 ± 7.4 1, a | 62.3 ± 8.1 1, 2, a | 54.7 ± 6.8 2, 3, a | 43.0 ± 8.3 3, 4, a | 30.7 ± 7.3 4, a |
| SP + DMSO | 74.5 ± 6.1 1, a | 64.2 ± 7.0 1, 2, a | 55.0 ± 6.8 2, 3, a | 43.3 ± 4.8 3, a | 31.2 ± 4.9 4, a |
| SP | 77.7 ± 6.3 1, a | 68.1 ± 5.3 1, 2, a | 55.5 ± 5.8 2, 3, a | 45.1 ± 6.5 3, a | 28.3 ± 4.8 4, a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miró, J.; Marín, H.; Catalán, J.; Papas, M.; Gacem, S.; Yeste, M. Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An In Vitro Model to Study Endometrial Inflammation at Post-Insemination. Int. J. Mol. Sci. 2020, 21, 3478. https://doi.org/10.3390/ijms21103478
Miró J, Marín H, Catalán J, Papas M, Gacem S, Yeste M. Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An In Vitro Model to Study Endometrial Inflammation at Post-Insemination. International Journal of Molecular Sciences. 2020; 21(10):3478. https://doi.org/10.3390/ijms21103478
Chicago/Turabian StyleMiró, Jordi, Henar Marín, Jaime Catalán, Marion Papas, Sabrina Gacem, and Marc Yeste. 2020. "Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An In Vitro Model to Study Endometrial Inflammation at Post-Insemination" International Journal of Molecular Sciences 21, no. 10: 3478. https://doi.org/10.3390/ijms21103478
APA StyleMiró, J., Marín, H., Catalán, J., Papas, M., Gacem, S., & Yeste, M. (2020). Seminal Plasma, Sperm Concentration, and Sperm-PMN Interaction in the Donkey: An In Vitro Model to Study Endometrial Inflammation at Post-Insemination. International Journal of Molecular Sciences, 21(10), 3478. https://doi.org/10.3390/ijms21103478







