The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain
Abstract
1. Introduction
2. Input Systems to mPFC
3. Neuron Types and Circuitry in mPFC Cortical Layers
3.1. Pyramidal Neurons
3.2. Inhibitory Interneurons
3.2.1. Parvalbumin Interneurons
3.2.2. CCK Interneurons
3.2.3. Somatostatin Interneurons
3.2.4. VIP Interneurons
3.3. Excitatory Interneurons
3.4. Modulation by Glial Cells
4. Local Circuits and Differences Between Cortical Layers
4.1. Layer L1
4.2. Layer 2/3
4.3. Layer 5
4.4. Layer 6
5. Specific Roles of mPFC Subregions
5.1. The Anterior Cingulate Cortex (ACC)
5.2. The Prelimbic (PrL) and Infralimbic (IL) Cortices
6. Pharmacology of mPFC Neurotransmitter Systems
6.1. Glutamate
6.2. Gamma-Aminobutyric Acid (GABA)
6.3. Endogenous Opioids
6.4. Noradrenaline (NA)
6.5. Dopamine (DA)
6.6. Serotonin (5-HT)
6.7. Acetylcholine (ACh)
6.8. Endocannabinoids
7. mPFC Output Regions and Descending Modulation
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Johns, E. The pain matrix: Reloaded or reborn as we image tonic pain using arterial spin labelling. Pain 2010, 148, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.F.; Bester, H.; Besson, J.M. Involvement of the spino-parabrachio -amygdaloid and -hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog. Brain Res. 1996, 107, 243–255. [Google Scholar]
- Becerra, L.; Breiter, H.C.; Wise, R.; Gonzalez, R.G.; Borsook, D. Reward circuitry activation by noxious thermal stimuli. Neuron 2001, 32, 927–946. [Google Scholar] [CrossRef]
- Baliki, M.N.; Geha, P.Y.; Fields, H.L.; Apkarian, A.V. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron 2010, 66, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Rainville, P.; Duncan, G.H.; Price, D.D.; Carrier, B.; Bushnell, M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997, 277, 968–971. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Duncan, G.H.; Hofbauer, R.K.; Ha, B.; Chen, J.I.; Carrier, B. Pain perception: Is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. USA 1999, 96, 7705–7709. [Google Scholar] [CrossRef]
- Coghill, R.C.; Sang, C.N.; Maisog, J.M.; Iadarola, M.J. Pain intensity processing within the human brain: A bilateral, distributed mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [CrossRef]
- Runyan, J.D.; Moore, A.N.; Dash, P.K. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004, 24, 1288–1295. [Google Scholar] [CrossRef]
- Hennig, K.M.; Colombani, J.; Neufeld, T.P. TOR coordinates bulk and targeted endocytosis in the Drosophila melanogaster fat body to regulate cell growth. J. Cell Biol. 2006, 173, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Moulton, E.A.; Schmahmann, J.D.; Becerra, L.; Borsook, D. The cerebellum and pain: Passive integrator or active participator? Brain Res. Rev. 2010, 65, 14–27. [Google Scholar] [CrossRef]
- Westlund, K.N. Pain Pathways: Peripheral, Spinal, Ascending, and Descending Pathways. In Practical Management of Pain, 5th ed.; Mosby: Philadelphia, PA, USA, 2014. [Google Scholar]
- Foilb, A.R.; Flyer-Adams, J.G.; Maier, S.F.; Christianson, J.P. Posterior insular cortex is necessary for conditioned inhibition of fear. Neurobiol. Learn. Mem. 2016, 134, 317–327. [Google Scholar] [CrossRef]
- Condes-Lara, M.; Omana Zapata, I.; Leon-Olea, M.; Sanchez-Alvarez, M. Dorsal raphe and nociceptive stimulations evoke convergent responses on the thalamic centralis lateralis and medial prefrontal cortex neurons. Brain Res. 1989, 499, 145–152. [Google Scholar] [CrossRef]
- Hardy, S.G. Analgesia elicited by prefrontal stimulation. Brain Res. 1985, 339, 281–284. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Moayedi, M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. J. Pain 2017, 18, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Geha, P.Y.; Apkarian, A.V.; Chialvo, D.R. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008, 28, 1398–1403. [Google Scholar] [CrossRef]
- Bloem, B.; Poorthuis, R.B.; Mansvelder, H.D. Cholinergic modulation of the medial prefrontal cortex: The role of nicotinic receptors in attention and regulation of neuronal activity. Front. Neural. Circuits 2014, 8, 17. [Google Scholar] [CrossRef]
- Fields, H.L.; Bry, J.; Hentall, I.; Zorman, G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J. Neurosci. 1983, 3, 2545–2552. [Google Scholar] [CrossRef]
- Ji, G.; Neugebauer, V. CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model. Eur. J. Neurosci. 2014, 39, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Manders, T.R.; Eberle, S.E.; Su, C.; D’Amour, J.; Yang, R.; Lin, H.Y.; Deisseroth, K.; Froemke, R.C.; Wang, J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 2015, 35, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Sosa, Y.; Sonty, S.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Gitelman, D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004, 24, 10410–10415. [Google Scholar] [CrossRef] [PubMed]
- Metz, A.E.; Yau, H.J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Neugebauer, V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J. Neurophysiol. 2011, 106, 2642–2652. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, M.; Weissman-Fogel, I.; Crawley, A.P.; Goldberg, M.B.; Freeman, B.V.; Tenenbaum, H.C.; Davis, K.D. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage 2011, 55, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Obara, I.; Goulding, S.P.; Gould, A.T.; Lominac, K.D.; Hu, J.H.; Zhang, P.W.; von Jonquieres, G.; Dehoff, M.; Xiao, B.; Seeburg, P.H.; et al. Homers at the Interface between Reward and Pain. Front. Psychiatry 2013, 4, 39. [Google Scholar] [CrossRef]
- Hung, K.L.; Wang, S.J.; Wang, Y.C.; Chiang, T.R.; Wang, C.C. Upregulation of presynaptic proteins and protein kinases associated with enhanced glutamate release from axonal terminals (synaptosomes) of the medial prefrontal cortex in rats with neuropathic pain. Pain 2014, 155, 377–387. [Google Scholar] [CrossRef]
- Kucyi, A.; Moayedi, M.; Weissman-Fogel, I.; Goldberg, M.B.; Freeman, B.V.; Tenenbaum, H.C.; Davis, K.D. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 2014, 34, 3969–3975. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier/Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Elsevier/Academic Press: New York, NY, USA, 2008. [Google Scholar]
- Laubach, M.; Amarante, L.M.; Swanson, K.; White, S.R. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.M.; Shyu, B.C. Electrophysiological study of the connection between medial thalamus and anterior cingulate cortex in the rat. Neuroreport 1997, 8, 2701–2707. [Google Scholar] [CrossRef]
- Kung, J.C.; Shyu, B.C. Potentiation of local field potentials in the anterior cingulate cortex evoked by the stimulation of the medial thalamic nuclei in rats. Brain Res. 2002, 953, 37–44. [Google Scholar] [CrossRef]
- Yang, J.W.; Shih, H.C.; Shyu, B.C. Intracortical circuits in rat anterior cingulate cortex are activated by nociceptive inputs mediated by medial thalamus. J. Neurophysiol. 2006, 96, 3409–3422. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Wake, H.; Watanabe, M.; Ishibashi, H.; Noda, M.; Yanagawa, Y.; Nabekura, J. Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J. Neurosci. 2011, 31, 7631–7636. [Google Scholar] [CrossRef]
- Delevich, K.; Tucciarone, J.; Huang, Z.J.; Li, B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J. Neurosci. 2015, 35, 5743–5753. [Google Scholar] [CrossRef]
- Han, S.; Soleiman, M.T.; Soden, M.E.; Zweifel, L.S.; Palmiter, R.D. Elucidating an Affective Pain Circuit that Creates a Threat Memory. Cell 2015, 162, 363–374. [Google Scholar] [CrossRef]
- Hoover, W.B.; Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007, 212, 149–179. [Google Scholar] [CrossRef]
- Woolf, N.J.; Eckenstein, F.; Butcher, L.L. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Res. Bull. 1984, 13, 751–784. [Google Scholar] [CrossRef]
- Bloem, B.; Schoppink, L.; Rotaru, D.C.; Faiz, A.; Hendriks, P.; Mansvelder, H.D.; Van de Berg, W.D.; Wouterlood, F.G. Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J. Neurosci. 2014, 34, 16234–16246. [Google Scholar] [CrossRef] [PubMed]
- Gielow, M.R.; Zaborszky, L. The Input-Output Relationship of the Cholinergic Basal Forebrain. Cell Rep. 2017, 18, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Coira, I.; Martin-Cortecero, J.; Nunez, A.; Rodrigo-Angulo, M.L. Basal Forebrain Nuclei Display Distinct Projecting Pathways and Functional Circuits to Sensory Primary and Prefrontal Cortices in the Rat. Front. Neuroanat. 2018, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Ahrlund-Richter, S.; Xuan, Y.; Van Lunteren, J.A.; Kim, H.; Ortiz, C.; Pollak Dorocic, I.; Meletis, K.; Carlen, M. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat. Neurosci. 2019, 22, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.; Keller, S.M. Cholinergic neuronal lesions in the medial septum and vertical limb of the diagonal bands of Broca induce contextual fear memory generalization and impair acquisition of fear extinction. Hippocampus 2016, 26, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ang, S.T.; Ariffin, M.Z.; Khanna, S. The forebrain medial septal region and nociception. Neurobiol. Learn. Mem. 2017, 138, 238–251. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Shao, S.; Zhang, Y.; Zheng, J.; Chen, X.; Cui, S.; Liu, F.Y.; Wan, Y.; Yi, M. Neural pathways in medial septal cholinergic modulation of chronic pain: Distinct contribution of the anterior cingulate cortex and ventral hippocampus. Pain 2018, 159, 1550–1561. [Google Scholar] [CrossRef]
- Ang, S.T.; Lee, A.T.; Foo, F.C.; Ng, L.; Low, C.M.; Khanna, S. GABAergic neurons of the medial septum play a nodal role in facilitation of nociception-induced affect. Sci. Rep. 2015, 5, 15419. [Google Scholar] [CrossRef]
- Ariffin, M.Z.; Ibrahim, K.M.; Lee, A.T.; Lee, R.Z.; Poon, S.Y.; Thong, H.K.; Liu, E.H.C.; Low, C.M.; Khanna, S. Forebrain medial septum sustains experimental neuropathic pain. Sci. Rep. 2018, 8, 11892. [Google Scholar] [CrossRef]
- Van De Werd, H.J.; Rajkowska, G.; Evers, P.; Uylings, H.B. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct. Funct. 2010, 214, 339–353. [Google Scholar] [CrossRef]
- Fuster, J. The Prefrontal Cortex; Academic Press: London, UK, 2015. [Google Scholar]
- Wood, J.N.; Grafman, J. Human prefrontal cortex: Processing and representational perspectives. Nat. Rev. Neurosci. 2003, 4, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Uylings, H.B.; Groenewegen, H.J.; Kolb, B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003, 146, 3–17. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, T.; Descalzi, G.; Koga, K.; Qiu, S.; Zhuo, M. Characterization of neuronal intrinsic properties and synaptic transmission in layer I of anterior cingulate cortex from adult mice. Mol. Pain 2012, 8, 53. [Google Scholar] [CrossRef]
- Little, J.P.; Carter, A.G. Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J. Neurosci. 2012, 32, 12808–12819. [Google Scholar] [CrossRef] [PubMed]
- McGarry, L.M.; Carter, A.G. Inhibitory Gating of Basolateral Amygdala Inputs to the Prefrontal Cortex. J. Neurosci. 2016, 36, 9391–9406. [Google Scholar] [CrossRef] [PubMed]
- Anastasiades, P.G.; Marlin, J.J.; Carter, A.G. Cell-Type Specificity of Callosally Evoked Excitation and Feedforward Inhibition in the Prefrontal Cortex. Cell Rep. 2018, 22, 679–692. [Google Scholar] [CrossRef]
- Collins, D.P.; Anastasiades, P.G.; Marlin, J.J.; Carter, A.G. Reciprocal Circuits Linking the Prefrontal Cortex with Dorsal and Ventral Thalamic Nuclei. Neuron 2018, 98, 366–379 e364. [Google Scholar] [CrossRef]
- Wu, L.J.; Li, X.; Chen, T.; Ren, M.; Zhuo, M. Characterization of intracortical synaptic connections in the mouse anterior cingulate cortex using dual patch clamp recording. Mol. Brain 2009, 2, 32. [Google Scholar] [CrossRef]
- Beneyto, M.; Meador-Woodruff, J.H. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 2008, 33, 2175–2186. [Google Scholar] [CrossRef]
- Arion, D.; Corradi, J.P.; Tang, S.; Datta, D.; Boothe, F.; He, A.; Cacace, A.M.; Zaczek, R.; Albright, C.F.; Tseng, G.; et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol. Psychiatry 2015, 20, 1397–1405. [Google Scholar] [CrossRef]
- Shrestha, P.; Mousa, A.; Heintz, N. Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors. Elife 2015, 4. [Google Scholar] [CrossRef]
- Varodayan, F.P.; Sidhu, H.; Kreifeldt, M.; Roberto, M.; Contet, C. Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 2018, 133, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, A.; Djekidel, M.N.; Chen, R.; Chen, W.; Tuesta, L.M.; Zhang, Y. Cell type-specific transcriptional programs in mouse prefrontal cortex during adolescence and addiction. Nat. Commun. 2019, 10, 4169. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Xu, H.; Wu, L.J.; Li, X.Y.; Chen, T.; Zhuo, M. Characterization of intrinsic properties of cingulate pyramidal neurons in adult mice after nerve injury. Mol. Pain 2009, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Li, X.; Chen, T.; Steenland, H.W.; Descalzi, G.; Zhuo, M. In vivo whole-cell patch-clamp recording of sensory synaptic responses of cingulate pyramidal neurons to noxious mechanical stimuli in adult mice. Mol. Pain 2010, 6, 62. [Google Scholar] [CrossRef]
- Wang, J.Y.; Luo, F.; Zhang, H.T.; Chang, J.Y.; Woodward, D.J.; Han, J.S. Nociceptive responses of anterior cingulate cortical ensembles in behaving rats. Beijing Da Xue Xue Bao Yi Xue Ban 2004, 36, 47–51. [Google Scholar]
- Becerra, L.; Navratilova, E.; Porreca, F.; Borsook, D. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J. Neurophysiol. 2013, 110, 1221–1226. [Google Scholar] [CrossRef]
- Cordeiro Matos, S.; Zhang, Z.; Seguela, P. Peripheral Neuropathy Induces HCN Channel Dysfunction in Pyramidal Neurons of the Medial Prefrontal Cortex. J. Neurosci. 2015, 35, 13244–13256. [Google Scholar] [CrossRef]
- Yang, Z.; Tan, Q.; Cheng, D.; Zhang, L.; Zhang, J.; Gu, E.W.; Fang, W.; Lu, X.; Liu, X. The Changes of Intrinsic Excitability of Pyramidal Neurons in Anterior Cingulate Cortex in Neuropathic Pain. Front. Cell Neurosci. 2018, 12, 436. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, H.; Huang, L.; Xie, Z.; Wang, J.; Gan, W.B.; Yang, G. Neuropathic Pain Causes Pyramidal Neuronal Hyperactivity in the Anterior Cingulate Cortex. Front. Cell Neurosci. 2018, 12, 107. [Google Scholar] [CrossRef]
- Xu, H.; Wu, L.J.; Wang, H.; Zhang, X.; Vadakkan, K.I.; Kim, S.S.; Steenland, H.W.; Zhuo, M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J. Neurosci. 2008, 28, 7445–7453. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Cortical excitation and chronic pain. Trends Neurosci. 2008, 31, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.R.; Cao, F.L.; He, Y.; Gao, C.Y.; Wang, D.D.; Li, H.; Zhang, F.K.; An, Y.Y.; Lin, Q.; Chen, J. Enhanced excitatory and reduced inhibitory synaptic transmission contribute to persistent pain-induced neuronal hyper-responsiveness in anterior cingulate cortex. Neuroscience 2010, 171, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Niikura, K.; Furuya, M.; Narita, M.; Torigoe, K.; Kobayashi, Y.; Takemura, Y.; Yamazaki, M.; Horiuchi, H.; Enomoto, T.; Iseki, M.; et al. Enhancement of glutamatergic transmission in the cingulate cortex in response to mild noxious stimuli under a neuropathic pain-like state. Synapse 2011, 65, 424–432. [Google Scholar] [CrossRef]
- Blom, S.M.; Pfister, J.P.; Santello, M.; Senn, W.; Nevian, T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J. Neurosci. 2014, 34, 5754–5764. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Seminowicz, D.A. Neuroimaging of pain in animal models: A review of recent literature. Pain Rep. 2019, 4, e732. [Google Scholar] [CrossRef]
- Rymar, V.V.; Sadikot, A.F. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J. Comp. Neurol. 2007, 501, 369–380. [Google Scholar] [CrossRef]
- Beierlein, M.; Connors, B.W. Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J. Neurophysiol. 2002, 88, 1924–1932. [Google Scholar] [CrossRef]
- Ding, C.; Emmenegger, V.; Schaffrath, K.; Feldmeyer, D. Layer-specific inhibitory microcircuits of layer 6 interneurons in rat prefrontal cortex. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mitric, M.; Seewald, A.; Moschetti, G.; Sacerdote, P.; Ferraguti, F.; Kummer, K.K.; Kress, M. Layer- and subregion-specific electrophysiological and morphological changes of the medial prefrontal cortex in a mouse model of neuropathic pain. Sci. Rep. 2019, 9, 9479. [Google Scholar] [CrossRef]
- Kim, H.; Ahrlund-Richter, S.; Wang, X.; Deisseroth, K.; Carlen, M. Prefrontal Parvalbumin Neurons in Control of Attention. Cell 2016, 164, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Sparta, D.R.; Hovelso, N.; Mason, A.O.; Kantak, P.A.; Ung, R.L.; Decot, H.K.; Stuber, G.D. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J. Neurosci. 2014, 34, 3699–3705. [Google Scholar] [CrossRef]
- Marek, R.; Jin, J.; Goode, T.D.; Giustino, T.F.; Wang, Q.; Acca, G.M.; Holehonnur, R.; Ploski, J.E.; Fitzgerald, P.J.; Lynagh, T.; et al. Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat. Neurosci. 2018, 21, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Stemkowski, P.L.; Zamponi, G.W. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 2015, 12, 752–759. [Google Scholar] [CrossRef]
- Shiers, S.; Pradhan, G.; Mwirigi, J.; Mejia, G.; Ahmad, A.; Kroener, S.; Price, T. Neuropathic Pain Creates an Enduring Prefrontal Cortex Dysfunction Corrected by the Type II Diabetic Drug Metformin but Not by Gabapentin. J. Neurosci. 2018, 38, 7337–7350. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dumitrescu, A.S.; Kruijssen, D.L.H.; Taylor, S.E.; Grubb, M.S. Rapid Modulation of Axon Initial Segment Length Influences Repetitive Spike Firing. Cell Rep. 2015, 13, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Venkatesan, S.; Binko, M.; Bang, J.Y.; Cajanding, J.D.; Briggs, C.; Sargin, D.; Imayoshi, I.; Lambe, E.K.; Kim, J.C. Cholecystokinin-Expressing Interneurons of the Medial Prefrontal Cortex Mediate Working Memory Retrieval. J. Neurosci. 2020, 40, 2314–2331. [Google Scholar] [CrossRef]
- Czeh, B.; Vardya, I.; Varga, Z.; Febbraro, F.; Csabai, D.; Martis, L.S.; Hojgaard, K.; Henningsen, K.; Bouzinova, E.V.; Miseta, A.; et al. Long-Term Stress Disrupts the Structural and Functional Integrity of GABAergic Neuronal Networks in the Medial Prefrontal Cortex of Rats. Front. Cell Neurosci. 2018, 12, 148. [Google Scholar] [CrossRef]
- Abbas, A.I.; Sundiang, M.J.M.; Henoch, B.; Morton, M.P.; Bolkan, S.S.; Park, A.J.; Harris, A.Z.; Kellendonk, C.; Gordon, J.A. Somatostatin Interneurons Facilitate Hippocampal-Prefrontal Synchrony and Prefrontal Spatial Encoding. Neuron 2018, 100, 926–939 e923. [Google Scholar] [CrossRef]
- Scheggia, D.; Manago, F.; Maltese, F.; Bruni, S.; Nigro, M.; Dautan, D.; Latuske, P.; Contarini, G.; Gomez-Gonzalo, M.; Requie, L.M.; et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat. Neurosci. 2020, 23, 47–60. [Google Scholar] [CrossRef]
- Lee, S.; Kruglikov, I.; Huang, Z.J.; Fishell, G.; Rudy, B. A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat. Neurosci. 2013, 16, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.J.; Hangya, B.; Kvitsiani, D.; Sanders, J.I.; Huang, Z.J.; Kepecs, A. Cortical interneurons that specialize in disinhibitory control. Nature 2013, 503, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Karnani, M.M.; Jackson, J.; Ayzenshtat, I.; Hamzehei Sichani, A.; Manoocheri, K.; Kim, S.; Yuste, R. Opening Holes in the Blanket of Inhibition: Localized Lateral Disinhibition by VIP Interneurons. J. Neurosci. 2016, 36, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, J.; Luchicchi, A.; Heistek, T.S.; de Kloet, S.F.; Terra, H.; Bruinsma, B.; Mnie-Filali, O.; Kortleven, C.; Galakhova, A.A.; Khalil, A.J.; et al. Prefrontal cortical ChAT-VIP interneurons provide local excitation by cholinergic synaptic transmission and control attention. Nat. Commun. 2019, 10, 5280. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.Y.; Sessle, B.J.; Dostrovsky, J.O. Role of astrocytes in pain. Neurochem. Res. 2012, 37, 2419–2431. [Google Scholar] [CrossRef]
- Fiore, N.T.; Austin, P.J. Are the emergence of affective disturbances in neuropathic pain states contingent on supraspinal neuroinflammation? Brain Behav. Immun. 2016, 56, 397–411. [Google Scholar] [CrossRef]
- Tsuda, M.; Koga, K.; Chen, T.; Zhuo, M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J. Neurochem. 2017, 141, 486–498. [Google Scholar] [CrossRef]
- Zhang, F.; Vadakkan, K.I.; Kim, S.S.; Wu, L.J.; Shang, Y.; Zhuo, M. Selective activation of microglia in spinal cord but not higher cortical regions following nerve injury in adult mouse. Mol. Pain 2008, 4, 15. [Google Scholar] [CrossRef]
- Galan-Arriero, I.; Avila-Martin, G.; Ferrer-Donato, A.; Gomez-Soriano, J.; Bravo-Esteban, E.; Taylor, J. Oral administration of the p38alpha MAPK inhibitor, UR13870, inhibits affective pain behavior after spinal cord injury. Pain 2014, 155, 2188–2198. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef]
- Gui, W.S.; Wei, X.; Mai, C.L.; Murugan, M.; Wu, L.J.; Xin, W.J.; Zhou, L.J.; Liu, X.G. Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, H.; Piirainen, S.; Chen, Z.; Kalso, E.; Pertovaara, A.; Tian, L. Spinal versus brain microglial and macrophage activation traits determine the differential neuroinflammatory responses and analgesic effect of minocycline in chronic neuropathic pain. Brain Behav. Immun. 2016, 58, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Kume, K.; Ohsawa, M. Role of microglia in mechanical allodynia in the anterior cingulate cortex. J. Pharmacol. Sci. 2017, 134, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Tang, X.H.; Pan, W.; Xie, Z.M.; Zhang, G.F.; Ji, M.H.; Yang, J.J.; Zhou, M.T.; Zhou, Z.Q. Spared Nerve Injury Increases the Expression of Microglia M1 Markers in the Prefrontal Cortex of Rats and Provokes Depression-Like Behaviors. Front. Neurosci. 2017, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Barcelon, E.E.; Cho, W.H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef]
- Diaz, A.F.; Polo, S.; Gallardo, N.; Leanez, S.; Pol, O. Analgesic and Antidepressant Effects of Oltipraz on Neuropathic Pain in Mice by Modulating Microglial Activation. J. Clin. Med. 2019, 8, 890. [Google Scholar] [CrossRef]
- Fiore, N.T.; Austin, P.J. Peripheral Nerve Injury Triggers Neuroinflammation in the Medial Prefrontal Cortex and Ventral Hippocampus in a Subgroup of Rats with Coincident Affective Behavioural Changes. Neuroscience 2019, 416, 147–167. [Google Scholar] [CrossRef]
- Georgieva, M.; Wei, Y.; Dumitrascuta, M.; Pertwee, R.; Finnerup, N.B.; Huang, W. Fatty acid suppression of glial activation prevents central neuropathic pain after spinal cord injury. Pain 2019, 160, 2724–2742. [Google Scholar] [CrossRef]
- Marrone, M.C.; Morabito, A.; Giustizieri, M.; Chiurchiu, V.; Leuti, A.; Mattioli, M.; Marinelli, S.; Riganti, L.; Lombardi, M.; Murana, E.; et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat. Commun. 2017, 8, 15292. [Google Scholar] [CrossRef]
- Narita, M.; Kuzumaki, N.; Narita, M.; Kaneko, C.; Hareyama, N.; Miyatake, M.; Shindo, K.; Miyoshi, K.; Nakajima, M.; Nagumo, Y.; et al. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J. Neurochem. 2006, 97, 1369–1378. [Google Scholar] [CrossRef]
- Kuzumaki, N.; Narita, M.; Narita, M.; Hareyama, N.; Niikura, K.; Nagumo, Y.; Nozaki, H.; Amano, T.; Suzuki, T. Chronic pain-induced astrocyte activation in the cingulate cortex with no change in neural or glial differentiation from neural stem cells in mice. Neurosci. Lett. 2007, 415, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, L.; Gao, Y.J. Pain-related aversion induces astrocytic reaction and proinflammatory cytokine expression in the anterior cingulate cortex in rats. Brain Res. Bull. 2011, 84, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Dong, Y.L.; Zhang, Z.J.; Cao, D.L.; Xu, J.; Hui, J.; Zhu, L.; Gao, Y.J. Activation of astrocytes in the anterior cingulate cortex contributes to the affective component of pain in an inflammatory pain model. Brain Res. Bull. 2012, 87, 60–66. [Google Scholar] [CrossRef]
- Ikeda, H.; Mochizuki, K.; Murase, K. Astrocytes are involved in long-term facilitation of neuronal excitation in the anterior cingulate cortex of mice with inflammatory pain. Pain 2013, 154, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Hamada, A.; Suhara, Y.; Kawabe, R.; Yanase, M.; Kuzumaki, N.; Narita, M.; Matsui, R.; Okano, H.; Narita, M. Astrocytic activation in the anterior cingulate cortex is critical for sleep disorder under neuropathic pain. Synapse 2014, 68, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W. Astrocyte activation in the anterior cingulate cortex and altered glutamatergic gene expression during paclitaxel-induced neuropathic pain in mice. PeerJ 2015, 3, e1350. [Google Scholar] [CrossRef]
- Alotaibi, G.; Rahman, S. Effects of glial glutamate transporter activator in formalin-induced pain behaviour in mice. Eur. J. Pain 2019, 23, 765–783. [Google Scholar] [CrossRef]
- Hestrin, S.; Armstrong, W.E. Morphology and physiology of cortical neurons in layer I. J. Neurosci. 1996, 16, 5290–5300. [Google Scholar] [CrossRef]
- Zhou, F.M.; Hablitz, J.J. Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J. Comp. Neurol. 1996, 376, 198–213. [Google Scholar] [CrossRef]
- Poorthuis, R.B.; Muhammad, K.; Wang, M.; Verhoog, M.B.; Junek, S.; Wrana, A.; Mansvelder, H.D.; Letzkus, J.J. Rapid Neuromodulation of Layer 1 Interneurons in Human Neocortex. Cell Rep. 2018, 23, 951–958. [Google Scholar] [CrossRef]
- Schuman, B.; Machold, R.P.; Hashikawa, Y.; Fuzik, J.; Fishell, G.J.; Rudy, B. Four Unique Interneuron Populations Reside in Neocortical Layer 1. J. Neurosci. 2019, 39, 125–139. [Google Scholar] [CrossRef]
- Dembrow, N.; Johnston, D. Subcircuit-specific neuromodulation in the prefrontal cortex. Front. Neural. Circuits 2014, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, S.J.; Lewis, T.J.; Connors, B.W. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 2007, 10, 462–468. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Koltzenburg, M.; Tracey, I.; Turk, D. Wall and Melzack’s Textbook of Pain; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Kim, S.K.; Kato, G.; Ishikawa, T.; Nabekura, J. Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol. Pain 2011, 7, 87. [Google Scholar] [CrossRef]
- Cichon, J.; Blanck, T.J.J.; Gan, W.B.; Yang, G. Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat. Neurosci. 2017, 20, 1122–1132. [Google Scholar] [CrossRef]
- Liu, R.J.; Aghajanian, G.K. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc. Natl. Acad. Sci. USA 2008, 105, 359–364. [Google Scholar] [CrossRef]
- Negron-Oyarzo, I.; Dagnino-Subiabre, A.; Munoz Carvajal, P. Synaptic Impairment in Layer 1 of the Prefrontal Cortex Induced by Repeated Stress During Adolescence is Reversed in Adulthood. Front. Cell Neurosci. 2015, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.H.; Wen, H.Z.; Shen, L.L.; Zhao, Y.D.; Ruan, H.Z. Activation of mGluR1 contributes to neuronal hyperexcitability in the rat anterior cingulate cortex via inhibition of HCN channels. Neuropharmacology 2016, 105, 361–377. [Google Scholar] [CrossRef]
- Van Aerde, K.I.; Feldmeyer, D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb. Cortex 2015, 25, 788–805. [Google Scholar] [CrossRef]
- Yuste, R. Dendritic spines and distributed circuits. Neuron 2011, 71, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, P.L.; Warner, T.A.; Jays, P.R.; Salway, P.; Busby, S.J. Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005, 492, 145–177. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Morishima, M.; Karube, F.; Kawaguchi, Y. Specialized cortical subnetworks differentially connect frontal cortex to parahippocampal areas. J. Neurosci. 2012, 32, 1898–1913. [Google Scholar] [CrossRef] [PubMed]
- Quiquempoix, M.; Fayad, S.L.; Boutourlinsky, K.; Leresche, N.; Lambert, R.C.; Bessaih, T. Layer 2/3 Pyramidal Neurons Control the Gain of Cortical Output. Cell Rep. 2018, 24, 2799–2807 e2794. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro Matos, S.; Zamfir, M.; Longo, G.; Ribeiro-da-Silva, A.; Seguela, P. Noradrenergic fiber sprouting and altered transduction in neuropathic prefrontal cortex. Brain Struct. Funct. 2018, 223, 1149–1164. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Zhao, M.G.; Ulzhofer, B.; Wu, L.J.; Xu, H.; Seeburg, P.H.; Sprengel, R.; Kuner, R.; Zhuo, M. Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Mol. Pain 2009, 5, 46. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, N.; Wang, Y.J.; Zuo, Z.X.; Koga, K.; Luo, F.; Zhuo, M. Long-term temporal imprecision of information coding in the anterior cingulate cortex of mice with peripheral inflammation or nerve injury. J. Neurosci. 2014, 34, 10675–10687. [Google Scholar] [CrossRef]
- Hubbard, C.S.; Khan, S.A.; Xu, S.; Cha, M.; Masri, R.; Seminowicz, D.A. Behavioral, metabolic and functional brain changes in a rat model of chronic neuropathic pain: A longitudinal MRI study. Neuroimage 2015, 107, 333–344. [Google Scholar] [CrossRef]
- Wu, X.B.; He, L.N.; Jiang, B.C.; Wang, X.; Lu, Y.; Gao, Y.J. Increased CXCL13 and CXCR5 in Anterior Cingulate Cortex Contributes to Neuropathic Pain-Related Conditioned Place Aversion. Neurosci. Bull. 2019, 35, 613–623. [Google Scholar] [CrossRef]
- Tan, W.; Yao, W.L.; Zhang, B.; Hu, R.; Wan, L.; Zhang, C.H.; Zhu, C. Neuronal loss in anterior cingulate cortex in spared nerve injury model of neuropathic pain. Neurochem. Int. 2018, 118, 127–133. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Shimoyama, S.; Yamada, A.; Furukawa, T.; Nikaido, Y.; Furue, H.; Nakamura, K.; Ueno, S. Chronic inflammatory pain induced GABAergic synaptic plasticity in the adult mouse anterior cingulate cortex. Mol. Pain 2018, 14, 1744806918783478. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.S.; Huang, C.C.; Liang, Y.C.; Tsai, Y.C.; Hsu, K.S. Impairment of long-term depression in the anterior cingulate cortex of mice with bone cancer pain. Pain 2012, 153, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Liu, M.G.; Chen, T.; Ko, H.G.; Baek, G.C.; Lee, H.R.; Lee, K.; Collingridge, G.L.; Kaang, B.K.; Zhuo, M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J. Neurosci. 2012, 32, 11318–11329. [Google Scholar] [CrossRef]
- Ning, L.; Ma, L.Q.; Wang, Z.R.; Wang, Y.W. Chronic constriction injury induced long-term changes in spontaneous membrane-potential oscillations in anterior cingulate cortical neurons in vivo. Pain Physician 2013, 16, E577–E589. [Google Scholar]
- Cruikshank, S.J.; Ahmed, O.J.; Stevens, T.R.; Patrick, S.L.; Gonzalez, A.N.; Elmaleh, M.; Connors, B.W. Thalamic control of layer 1 circuits in prefrontal cortex. J. Neurosci. 2012, 32, 17813–17823. [Google Scholar] [CrossRef]
- Dembrow, N.C.; Chitwood, R.A.; Johnston, D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J. Neurosci. 2010, 30, 16922–16937. [Google Scholar] [CrossRef]
- Kelly, C.J.; Huang, M.; Meltzer, H.; Martina, M. Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Front. Cell Neurosci. 2016, 10, 133. [Google Scholar] [CrossRef]
- Gao, S.H.; Shen, L.L.; Wen, H.Z.; Zhao, Y.D.; Ruan, H.Z. Inhibition of Metabotropic Glutamate Receptor Subtype 1 Alters the Excitability of the Commissural Pyramidal Neuron in the Rat Anterior Cingulate Cortex after Chronic Constriction Injury to the Sciatic Nerve. Anesthesiology 2017, 127, 515–533. [Google Scholar] [CrossRef]
- Radzicki, D.; Pollema-Mays, S.L.; Sanz-Clemente, A.; Martina, M. Loss of M1 Receptor Dependent Cholinergic Excitation Contributes to mPFC Deactivation in Neuropathic Pain. J. Neurosci. 2017, 37, 2292–2304. [Google Scholar] [CrossRef]
- Meda, K.S.; Patel, T.; Braz, J.M.; Malik, R.; Turner, M.L.; Seifikar, H.; Basbaum, A.I.; Sohal, V.S. Microcircuit Mechanisms through which Mediodorsal Thalamic Input to Anterior Cingulate Cortex Exacerbates Pain-Related Aversion. Neuron 2019, 102, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Nevian, T. Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 2015, 86, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, W.; Dong, Y.L.; Zhang, M.M.; Wang, J.; Koga, K.; Liao, Y.H.; Li, J.L.; Budisantoso, T.; Shigemoto, R.; et al. Postsynaptic insertion of AMPA receptor onto cortical pyramidal neurons in the anterior cingulate cortex after peripheral nerve injury. Mol. Brain 2014, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Liang, B.; Gao, Y.J. The increase of intrinsic excitability of layer V pyramidal cells in the prelimbic medial prefrontal cortex of adult mice after peripheral inflammation. Neurosci. Lett. 2016, 611, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cheriyan, J.; Sheets, P.L. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J. Neurosci. 2018, 38, 4829–4839. [Google Scholar] [CrossRef]
- Huang, J.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Huang, S.; Wang, D.; Ramakrishnan, C.; Deisseroth, K.; Zhang, Z.; Zamponi, G.W. A neuronal circuit for activating descending modulation of neuropathic pain. Nat. Neurosci. 2019, 22, 1659–1668. [Google Scholar] [CrossRef]
- Kelly, C.J.; Martina, M. Circuit-selective properties of glutamatergic inputs to the rat prelimbic cortex and their alterations in neuropathic pain. Brain Struct. Funct. 2018, 223, 2627–2639. [Google Scholar] [CrossRef]
- Van Eden, C.G.; Uylings, H.B. Cytoarchitectonic development of the prefrontal cortex in the rat. J. Comp. Neurol. 1985, 241, 253–267. [Google Scholar] [CrossRef]
- Bailey, C.D.; De Biasi, M.; Fletcher, P.J.; Lambe, E.K. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 2010, 30, 9241–9252. [Google Scholar] [CrossRef]
- Sparks, D.W.; Tian, M.K.; Sargin, D.; Venkatesan, S.; Intson, K.; Lambe, E.K. Opposing Cholinergic and Serotonergic Modulation of Layer 6 in Prefrontal Cortex. Front. Neural. Circuits 2017, 11, 107. [Google Scholar] [CrossRef]
- Devoize, L.; Alvarez, P.; Monconduit, L.; Dallel, R. Representation of dynamic mechanical allodynia in the ventral medial prefrontal cortex of trigeminal neuropathic rats. Eur. J. Pain 2011, 15, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Guillery, R.W.; Sherman, S.M. Thalamic relay functions and their role in corticocortical communication: Generalizations from the visual system. Neuron 2002, 33, 163–175. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Barbas, H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J. Neurosci. 2006, 26, 7348–7361. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M. Neocortical layer, 6, a review. Front. Neuroanat. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Jurik, A.; Auffenberg, E.; Klein, S.; Deussing, J.M.; Schmid, R.M.; Wotjak, C.T.; Thoeringer, C.K. Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain 2015, 156, 2479–2491. [Google Scholar] [CrossRef]
- Leite-Almeida, H.; Guimaraes, M.R.; Cerqueira, J.J.; Ribeiro-Costa, N.; Anjos-Martins, H.; Sousa, N.; Almeida, A. Asymmetric c-fos expression in the ventral orbital cortex is associated with impaired reversal learning in a right-sided neuropathy. Mol. Pain 2014, 10, 41. [Google Scholar] [CrossRef]
- Moriarty, O.; Gorman, C.L.; McGowan, F.; Ford, G.K.; Roche, M.; Thompson, K.; Dockery, P.; McGuire, B.E.; Finn, D.P. Impaired recognition memory and cognitive flexibility in the rat L5-L6 spinal nerve ligation model of neuropathic pain. Scand. J. Pain 2016, 10, 61–73. [Google Scholar] [CrossRef]
- Palazzo, E.; Luongo, L.; Guida, F.; Marabese, I.; Romano, R.; Iannotta, M.; Rossi, F.; D’Aniello, A.; Stella, L.; Marmo, F.; et al. D-Aspartate drinking solution alleviates pain and cognitive impairment in neuropathic mice. Amino. Acids 2016, 48, 1553–1567. [Google Scholar] [CrossRef]
- De Novellis, V.; Vita, D.; Gatta, L.; Luongo, L.; Bellini, G.; De Chiaro, M.; Marabese, I.; Siniscalco, D.; Boccella, S.; Piscitelli, F.; et al. The blockade of the transient receptor potential vanilloid type 1 and fatty acid amide hydrolase decreases symptoms and central sequelae in the medial prefrontal cortex of neuropathic rats. Mol. Pain 2011, 7, 7. [Google Scholar] [CrossRef]
- Cardoso-Cruz, H.; Sousa, M.; Vieira, J.B.; Lima, D.; Galhardo, V. Prefrontal cortex and mediodorsal thalamus reduced connectivity is associated with spatial working memory impairment in rats with inflammatory pain. Pain 2013, 154, 2397–2406. [Google Scholar] [CrossRef]
- Lee, H.; Im, J.; Won, H.; Nam, W.; Kim, Y.O.; Lee, S.W.; Lee, S.; Cho, I.H.; Kim, H.K.; Kwon, J.T.; et al. Effects of tianeptine on symptoms of fibromyalgia via BDNF signaling in a fibromyalgia animal model. Korean J. Physiol. Pharmacol. 2017, 21, 361–370. [Google Scholar] [CrossRef]
- Brederson, J.D.; Chu, K.L.; Xu, J.; Nikkel, A.L.; Markosyan, S.; Jarvis, M.F.; Edelmayer, R.; Bitner, R.S.; McGaraughty, S. Characterization and comparison of rat monosodium iodoacetate and medial meniscal tear models of osteoarthritic pain. J. Orthop. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Yen, C.T. Differential Expression of Phosphorylated ERK and c-Fos of Limbic Cortices Activities in Response to Tactile Allodynia of Neuropathic Rats. Chin. J. Physiol. 2018, 61, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, G.F.; Li, H.H.; Ji, M.H.; Zhou, Z.Q.; Li, K.Y.; Yang, J.J. Ketamine differentially restores diverse alterations of neuroligins in brain regions in a rat model of neuropathic pain-induced depression. Neuroreport 2018, 29, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yang, C.; Li, S.; Zhan, G.; Zhang, J.; Huang, N.; Du, X.; Xu, H.; Hashimoto, K.; Luo, A. Brain-derived neurotrophic factor-TrkB signaling in the medial prefrontal cortex plays a role in the anhedonia-like phenotype after spared nerve injury. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 195–205. [Google Scholar] [CrossRef]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef]
- Koga, K.; Descalzi, G.; Chen, T.; Ko, H.G.; Lu, J.; Li, S.; Son, J.; Kim, T.; Kwak, C.; Huganir, R.L.; et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015, 85, 377–389. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L.; Kaang, B.K.; Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016, 17, 485–496. [Google Scholar] [CrossRef]
- Chen, T.; Taniguchi, W.; Chen, Q.Y.; Tozaki-Saitoh, H.; Song, Q.; Liu, R.H.; Koga, K.; Matsuda, T.; Kaito-Sugimura, Y.; Wang, J.; et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018, 9, 1886. [Google Scholar] [CrossRef]
- LaGraize, S.C.; Labuda, C.J.; Rutledge, M.A.; Jackson, R.L.; Fuchs, P.N. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp. Neurol. 2004, 188, 139–148. [Google Scholar] [CrossRef]
- LaBuda, C.J.; Fuchs, P.N. Attenuation of negative pain affect produced by unilateral spinal nerve injury in the rat following anterior cingulate cortex activation. Neuroscience 2005, 136, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; King, T.; Okun, A.; Lai, J.; Fields, H.L.; Porreca, F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain 2011, 152, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Barthas, F.; Sellmeijer, J.; Hugel, S.; Waltisperger, E.; Barrot, M.; Yalcin, I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol. Psychiatry 2015, 77, 236–245. [Google Scholar] [CrossRef]
- Juarez-Salinas, D.L.; Braz, J.M.; Etlin, A.; Gee, S.; Sohal, V.; Basbaum, A.I. GABAergic cell transplants in the anterior cingulate cortex reduce neuropathic pain aversiveness. Brain 2019, 142, 2655–2669. [Google Scholar] [CrossRef]
- LaGraize, S.C.; Borzan, J.; Peng, Y.B.; Fuchs, P.N. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp. Neurol. 2006, 197, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.Y.; Chen, Z.Y.; Zhong, W.; Ma, L.Q.; Chen, C.; Yang, Z.J.; Xie, W.L.; Wang, Y.W. Alleviation of neuropathic pain by regulating T-type calcium channels in rat anterior cingulate cortex. Mol. Pain 2015, 11, 7. [Google Scholar] [CrossRef]
- Bannister, K.; Qu, C.; Navratilova, E.; Oyarzo, J.; Xie, J.Y.; King, T.; Dickenson, A.H.; Porreca, F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017, 158, 2386–2395. [Google Scholar] [CrossRef]
- Gomtsian, L.; Bannister, K.; Eyde, N.; Robles, D.; Dickenson, A.H.; Porreca, F.; Navratilova, E. Morphine effects within the rodent anterior cingulate cortex and rostral ventromedial medulla reveal separable modulation of affective and sensory qualities of acute or chronic pain. Pain 2018, 159, 2512–2521. [Google Scholar] [CrossRef]
- Ren, L.Y.; Lu, Z.M.; Liu, M.G.; Yu, Y.Q.; Li, Z.; Shang, G.W.; Chen, J. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience 2008, 153, 268–278. [Google Scholar] [CrossRef]
- Gu, L.; Uhelski, M.L.; Anand, S.; Romero-Ortega, M.; Kim, Y.T.; Fuchs, P.N.; Mohanty, S.K. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS ONE 2015, 10, e0117746. [Google Scholar] [CrossRef]
- Kang, S.J.; Kwak, C.; Lee, J.; Sim, S.E.; Shim, J.; Choi, T.; Collingridge, G.L.; Zhuo, M.; Kaang, B.K. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol. Brain 2015, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Sellmeijer, J.; Mathis, V.; Hugel, S.; Li, X.H.; Song, Q.; Chen, Q.Y.; Barthas, F.; Lutz, P.E.; Karatas, M.; Luthi, A.; et al. Hyperactivity of Anterior Cingulate Cortex Areas 24a/24b Drives Chronic Pain-Induced Anxiodepressive-like Consequences. J. Neurosci. 2018, 38, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Kim, S.; Lee, J.; Kwak, C.; Lee, K.; Zhuo, M.; Kaang, B.K. Inhibition of anterior cingulate cortex excitatory neuronal activity induces conditioned place preference in a mouse model of chronic inflammatory pain. Korean J. Physiol. Pharmacol. 2017, 21, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Paxinos, G. Cytoarchitecture of mouse and rat cingulate cortex with human homologies. Brain Struct. Funct. 2014, 219, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Koga, K.; Descalzi, G.; Qiu, S.; Wang, J.; Zhang, L.S.; Zhang, Z.J.; He, X.B.; Qin, X.; Xu, F.Q.; et al. Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol. Pain 2014, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.S.; Chen, C.C.; Tsai, T.C.; Huang, C.C.; Chou, D.; Hsu, K.S. Alleviating Bone Cancer-induced Mechanical Hypersensitivity by Inhibiting Neuronal Activity in the Anterior Cingulate Cortex. Anesthesiology 2016, 125, 779–792. [Google Scholar] [CrossRef]
- Moon, H.C.; Heo, W.I.; Kim, Y.J.; Lee, D.; Won, S.Y.; Kim, H.R.; Ha, S.M.; Lee, Y.J.; Park, Y.S. Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve. J. Pain Res. 2017, 10, 2355–2364. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Chen, T.; Zhou, L.J.; Liu, X.G.; Zhuo, M. Heterosynaptic long-term potentiation from the anterior cingulate cortex to spinal cord in adult rats. Mol. Pain 2018, 14, 1744806918798406. [Google Scholar] [CrossRef]
- Calejesan, A.A.; Kim, S.J.; Zhuo, M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur. J. Pain 2000, 4, 83–96. [Google Scholar] [CrossRef]
- Traub, R.J.; Silva, E.; Gebhart, G.F.; Solodkin, A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci. Lett. 1996, 215, 165–168. [Google Scholar] [CrossRef]
- Gibney, S.M.; Gosselin, R.D.; Dinan, T.G.; Cryan, J.F. Colorectal distension-induced prefrontal cortex activation in the Wistar-Kyoto rat: Implications for irritable bowel syndrome. Neuroscience 2010, 165, 675–683. [Google Scholar] [CrossRef]
- Wang, Z.; Bradesi, S.; Charles, J.R.; Pang, R.D.; Maarek, J.M.; Mayer, E.A.; Holschneider, D.P. Functional brain activation during retrieval of visceral pain-conditioned passive avoidance in the rat. Pain 2011, 152, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Felice, V.D.; Gibney, S.M.; Gosselin, R.D.; Dinan, T.G.; O’Mahony, S.M.; Cryan, J.F. Differential activation of the prefrontal cortex and amygdala following psychological stress and colorectal distension in the maternally separated rat. Neuroscience 2014, 267, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tao, X.; Huang, S.T.; Wu, L.; Tang, H.L.; Song, Y.; Zhang, G.; Zhang, Y.M. Chronic Stress Is Associated with Pain Precipitation and Elevation in DeltaFosb Expression. Front. Pharmacol. 2016, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Kiritoshi, T.; Neugebauer, V. Group II mGluRs modulate baseline and arthritis pain-related synaptic transmission in the rat medial prefrontal cortex. Neuropharmacology 2015, 95, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; de Novellis, V.; Gatta, L.; Palazzo, E.; Vita, D.; Guida, F.; Giordano, C.; Siniscalco, D.; Marabese, I.; De Chiaro, M.; et al. Role of metabotropic glutamate receptor 1 in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology 2013, 66, 317–329. [Google Scholar] [CrossRef]
- Wang, G.Q.; Cen, C.; Li, C.; Cao, S.; Wang, N.; Zhou, Z.; Liu, X.M.; Xu, Y.; Tian, N.X.; Zhang, Y.; et al. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat. Commun. 2015, 6, 7660. [Google Scholar] [CrossRef]
- Li, A.L.; Yang, X.; Chiao, J.C.; Peng, Y.B. Reduced local field potential power in the medial prefrontal cortex by noxious stimuli. Brain Res. Bull. 2016, 127, 92–99. [Google Scholar] [CrossRef]
- Dale, J.; Zhou, H.; Zhang, Q.; Martinez, E.; Hu, S.; Liu, K.; Urien, L.; Chen, Z.; Wang, J. Scaling Up Cortical Control Inhibits Pain. Cell Rep. 2018, 23, 1301–1313. [Google Scholar] [CrossRef]
- Fu, B.; Wen, S.N.; Wang, B.; Wang, K.; Zhang, J.Y.; Liu, S.J. Acute and chronic pain affects local field potential of the medial prefrontal cortex in different band neural oscillations. Mol. Pain 2018, 14, 1744806918785686. [Google Scholar] [CrossRef]
- Ririe, D.G.; Boada, M.D.; MacGregor, M.K.; Martin, S.J.; Strassburg, T.J.; Kim, S.A.; Eisenach, J.C.; Martin, T.J. Incisional Nociceptive Input Impairs Attention-related Behavior and Is Associated with Reduced Neuronal Activity in the Prefrontal Cortex in Rats. Anesthesiology 2018, 129, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Cruz, H.; Paiva, P.; Monteiro, C.; Galhardo, V. Selective optogenetic inhibition of medial prefrontal glutamatergic neurons reverses working memory deficits induced by neuropathic pain. Pain 2019, 160, 805–823. [Google Scholar] [CrossRef] [PubMed]
- Sang, K.; Bao, C.; Xin, Y.; Hu, S.; Gao, X.; Wang, Y.; Bodner, M.; Zhou, Y.D.; Dong, X.W. Plastic change of prefrontal cortex mediates anxiety-like behaviors associated with chronic pain in neuropathic rats. Mol. Pain 2018, 14, 1744806918783931. [Google Scholar] [CrossRef]
- Palazzo, E.; Romano, R.; Luongo, L.; Boccella, S.; De Gregorio, D.; Giordano, M.E.; Rossi, F.; Marabese, I.; Scafuro, M.A.; de Novellis, V.; et al. MMPIP, an mGluR7-selective negative allosteric modulator, alleviates pain and normalizes affective and cognitive behavior in neuropathic mice. Pain 2015, 156, 1060–1073. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.; Kim, C.Y.; Yun, Y.C.; Yoon, S.H.; Kim, M.H.; Kim, Y.K.; Kim, S.J. Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci. Rep. 2017, 7, 9743. [Google Scholar] [CrossRef]
- Zhou, H.; Martinez, E.; Lin, H.H.; Yang, R.; Dale, J.A.; Liu, K.; Huang, D.; Wang, J. Inhibition of the Prefrontal Projection to the Nucleus Accumbens Enhances Pain Sensitivity and Affect. Front. Cell Neurosci. 2018, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Q.; Martinez, E.; Dale, J.; Robinson, E.; Huang, D.; Wang, J. A novel neuromodulation strategy to enhance the prefrontal control to treat pain. Mol. Pain 2019, 15, 1744806919845739. [Google Scholar] [CrossRef]
- David-Pereira, A.; Puga, S.; Goncalves, S.; Amorim, D.; Silva, C.; Pertovaara, A.; Almeida, A.; Pinto-Ribeiro, F. Metabotropic glutamate 5 receptor in the infralimbic cortex contributes to descending pain facilitation in healthy and arthritic animals. Neuroscience 2016, 312, 108–119. [Google Scholar] [CrossRef]
- David-Pereira, A.; Sagalajev, B.; Wei, H.; Almeida, A.; Pertovaara, A.; Pinto-Ribeiro, F. The medullary dorsal reticular nucleus as a relay for descending pronociception induced by the mGluR5 in the rat infralimbic cortex. Neuroscience 2017, 349, 341–354. [Google Scholar] [CrossRef]
- Yue, L.; Ma, L.Y.; Cui, S.; Liu, F.Y.; Yi, M.; Wan, Y. Brain-derived neurotrophic factor in the infralimbic cortex alleviates inflammatory pain. Neurosci. Lett. 2017, 655, 7–13. [Google Scholar] [CrossRef]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [PubMed]
- Baer, M.R.; Gao, X.Z.; Preisler, H.D. Sustained viability of acute myelogenous leukemia cells in liquid suspension culture correlates with occurrence of differentiation. Leuk. Res. 1988, 12, 647–650. [Google Scholar] [CrossRef]
- Millecamps, M.; Etienne, M.; Jourdan, D.; Eschalier, A.; Ardid, D. Decrease in non-selective, non-sustained attention induced by a chronic visceral inflammatory state as a new pain evaluation in rats. Pain 2004, 109, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, K.; Martinez, E.; Dale, J.; Huang, D.; Wang, J. AMPAkines and morphine provide complementary analgesia. Behav. Brain Res. 2017, 334, 1–5. [Google Scholar] [CrossRef]
- Giordano, C.; Cristino, L.; Luongo, L.; Siniscalco, D.; Petrosino, S.; Piscitelli, F.; Marabese, I.; Gatta, L.; Rossi, F.; Imperatore, R.; et al. TRPV1-dependent and -independent alterations in the limbic cortex of neuropathic mice: Impact on glial caspases and pain perception. Cereb. Cortex 2012, 22, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Qiu, C.S.; Kim, S.J.; Muglia, L.; Maas, J.W.; Pineda, V.V.; Xu, H.M.; Chen, Z.F.; Storm, D.R.; Muglia, L.J.; et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002, 36, 713–726. [Google Scholar] [CrossRef]
- Ding, M.; Shen, W.; Hu, Y. The Role of miR-539 in the Anterior Cingulate Cortex in Chronic Neuropathic Pain. Pain Med. 2017, 18, 2433–2442. [Google Scholar] [CrossRef]
- Li, X.Y.; Ko, H.G.; Chen, T.; Descalzi, G.; Koga, K.; Wang, H.; Kim, S.S.; Shang, Y.; Kwak, C.; Park, S.W.; et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 2010, 330, 1400–1404. [Google Scholar] [CrossRef]
- Ko, H.G.; Ye, S.; Han, D.H.; Park, P.; Lim, C.S.; Lee, K.; Zhuo, M.; Kaang, B.K. Transcription-independent expression of PKMzeta in the anterior cingulate cortex contributes to chronically maintained neuropathic pain. Mol. Pain 2018, 14, 1744806918783943. [Google Scholar] [CrossRef]
- Du, J.; Fang, J.; Wen, C.; Shao, X.; Liang, Y.; Fang, J. The Effect of Electroacupuncture on PKMzeta in the ACC in Regulating Anxiety-Like Behaviors in Rats Experiencing Chronic Inflammatory Pain. Neural. Plast. 2017, 2017, 3728752. [Google Scholar] [CrossRef]
- Millecamps, M.; Centeno, M.V.; Berra, H.H.; Rudick, C.N.; Lavarello, S.; Tkatch, T.; Apkarian, A.V. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain 2007, 132, 108–123. [Google Scholar] [CrossRef]
- De Freitas, R.L.; Salgado-Rohner, C.J.; Biagioni, A.F.; Medeiros, P.; Hallak, J.E.; Crippa, J.A.; Coimbra, N.C. NMDA and AMPA/kainate glutamatergic receptors in the prelimbic medial prefrontal cortex modulate the elaborated defensive behavior and innate fear-induced antinociception elicited by GABAA receptor blockade in the medial hypothalamus. Cereb. Cortex 2014, 24, 1518–1528. [Google Scholar] [CrossRef]
- Ghoreishi-Haack, N.; Priebe, J.M.; Aguado, J.D.; Colechio, E.M.; Burgdorf, J.S.; Bowers, M.S.; Cearley, C.N.; Khan, M.A.; Moskal, J.R. NYX-2925 Is a Novel N-Methyl-d-Aspartate Receptor Modulator that Induces Rapid and Long-Lasting Analgesia in Rat Models of Neuropathic Pain. J. Pharmacol. Exp. Ther. 2018, 366, 485–497. [Google Scholar] [CrossRef]
- Wu, L.J.; Toyoda, H.; Zhao, M.G.; Lee, Y.S.; Tang, J.; Ko, S.W.; Jia, Y.H.; Shum, F.W.; Zerbinatti, C.V.; Bu, G.; et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J. Neurosci. 2005, 25, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Ko, S.W.; Wu, L.J.; Toyoda, H.; Xu, H.; Quan, J.; Li, J.; Jia, Y.; Ren, M.; Xu, Z.C.; et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J. Neurosci. 2006, 26, 8923–8930. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Steenland, H.W.; Kim, S.S.; Isiegas, C.; Abel, T.; Kaang, B.K.; Zhuo, M. Enhancement of presynaptic glutamate release and persistent inflammatory pain by increasing neuronal cAMP in the anterior cingulate cortex. Mol. Pain 2008, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Zhao, M.G.; Zhuo, M. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol. Pain 2009, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Bie, B.; Brown, D.L.; Naguib, M. Increased synaptic GluR1 subunits in the anterior cingulate cortex of rats with peripheral inflammation. Eur. J. Pharmacol. 2011, 653, 26–31. [Google Scholar] [CrossRef]
- Wang, H.; Xu, H.; Wu, L.J.; Kim, S.S.; Chen, T.; Koga, K.; Descalzi, G.; Gong, B.; Vadakkan, K.I.; Zhang, X.; et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci. Transl. Med. 2011, 3, 65ra63. [Google Scholar] [CrossRef]
- Lei, L.G.; Sun, S.; Gao, Y.J.; Zhao, Z.Q.; Zhang, Y.Q. NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp. Neurol. 2004, 189, 413–421. [Google Scholar] [CrossRef]
- Guo, S.G.; Lv, X.H.; Guan, S.H.; Li, H.L.; Qiao, Y.; Feng, H.; Cong, L.; Wang, G.M. Silencing the NR2B gene in rat ACC neurons by lentivirus-delivered shRNA alleviates pain-related aversion. Int. J. Clin. Exp. Med. 2015, 8, 6725–6734. [Google Scholar]
- Zhang, Y.; Ji, F.; Wang, G.; He, D.; Yang, L.; Zhang, M. BDNF Activates mTOR to Upregulate NR2B Expression in the Rostral Anterior Cingulate Cortex Required for Inflammatory Pain-Related Aversion in Rats. Neurochem. Res. 2018, 43, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Vadakkan, K.I.; Ao, H.; Gallitano-Mendel, A.; Wei, F.; Milbrandt, J.; Zhuo, M. Selective contribution of Egr1 (zif/268) to persistent inflammatory pain. J. Pain 2005, 6, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gao, Y.J.; Ren, W.H.; Li, T.T.; Duan, K.Z.; Cui, Y.H.; Cao, X.H.; Zhao, Z.Q.; Ji, R.R.; Zhang, Y.Q. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 2009, 29, 3307–3321. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zang, K.K.; Han, M.; Zhao, Z.Q.; Wu, G.C.; Zhang, Y.Q. Inhibition of p38 mitogen-activated protein kinase activation in the rostral anterior cingulate cortex attenuates pain-related negative emotion in rats. Brain Res. Bull. 2014, 107, 79–88. [Google Scholar] [CrossRef]
- Kang, W.B.; Yang, Q.; Guo, Y.Y.; Wang, L.; Wang, D.S.; Cheng, Q.; Li, X.M.; Tang, J.; Zhao, J.N.; Liu, G.; et al. Analgesic effects of adenylyl cyclase inhibitor NB001 on bone cancer pain in a mouse model. Mol. Pain 2016, 12. [Google Scholar] [CrossRef]
- Wei, F.; Zhuo, M. Activation of Erk in the anterior cingulate cortex during the induction and expression of chronic pain. Mol. Pain 2008, 4, 28. [Google Scholar] [CrossRef]
- Yang, J.X.; Hua, L.; Li, Y.Q.; Jiang, Y.Y.; Han, D.; Liu, H.; Tang, Q.Q.; Yang, X.N.; Yin, C.; Hao, L.Y.; et al. Caveolin-1 in the anterior cingulate cortex modulates chronic neuropathic pain via regulation of NMDA receptor 2B subunit. J. Neurosci. 2015, 35, 36–52. [Google Scholar] [CrossRef]
- Thibault, K.; Lin, W.K.; Rancillac, A.; Fan, M.; Snollaerts, T.; Sordoillet, V.; Hamon, M.; Smith, G.M.; Lenkei, Z.; Pezet, S. BDNF-dependent plasticity induced by peripheral inflammation in the primary sensory and the cingulate cortex triggers cold allodynia and reveals a major role for endogenous BDNF as a tuner of the affective aspect of pain. J. Neurosci. 2014, 34, 14739–14751. [Google Scholar] [CrossRef]
- Lu, B.; Jiang, J.; Sun, J.; Xiao, C.; Meng, B.; Zheng, J.; Li, X.; Wang, R.; Wu, G.; Chen, J. Inhibition of mammalian target of rapamycin activation in the rostral anterior cingulate cortex attenuates pain-related aversion in rats. Behav. Brain Res. 2016, 310, 51–58. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Ma, J.; Liu, C.; Liu, X.; Zhan, Y.; Zhang, M. Brain-derived neurotrophic factor (BDNF) in the rostral anterior cingulate cortex (rACC) contributes to neuropathic spontaneous pain-related aversion via NR2B receptors. Brain Res. Bull. 2016, 127, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Um, S.W.; Kim, M.J.; Leem, J.W.; Bai, S.J.; Lee, B.H. Pain-Relieving Effects of mTOR Inhibitor in the Anterior Cingulate Cortex of Neuropathic Rats. Mol. Neurobiol. 2019, 56, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, G.; Zou, X.; Yang, Z.; Wang, Q.; Feng, H.; Zhang, M. siRNA-mediated downregulation of GluN2B in the rostral anterior cingulate cortex attenuates mechanical allodynia and thermal hyperalgesia in a rat model of pain associated with bone cancer. Exp. Ther. Med. 2016, 11, 221–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.S.; Su, X.Y.; Song, X.L.; Qi, X.; Li, Y.; Wang, R.Q.; Maximyuk, O.; Krishtal, O.; Wang, T.; Fang, H.; et al. Protein Kinase C Lambda Mediates Acid-Sensing Ion Channel 1a-Dependent Cortical Synaptic Plasticity and Pain Hypersensitivity. J. Neurosci. 2019, 39, 5773–5793. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Wang, J.; Yao, H.; Ren, K.; Chen, J.; Yang, J.; Cai, G.; Liu, H.; Fan, Y.; Wang, W.; et al. Chronic Inflammatory Pain Impairs mGluR5-Mediated Depolarization-Induced Suppression of Excitation in the Anterior Cingulate Cortex. Cereb. Cortex 2018, 28, 2118–2130. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Li, X.; Li, Y.J.; Feng, D.; Shi, W.L.; Zhao, M.G.; Wang, J.B.; Wu, Y.M. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int. Immunopharmacol. 2016, 41, 66–73. [Google Scholar] [CrossRef]
- Li, S.; Han, J.; Wang, D.S.; Yang, Q.; Feng, B.; Kang, W.B.; Yang, L.; Liu, G.; Zhao, M.G. Sinomenine attenuates chronic inflammatory pain in mice. Metab. Brain Dis. 2017, 32, 211–219. [Google Scholar] [CrossRef]
- Fan, Y.F.; Guan, S.Y.; Luo, L.; Li, Y.J.; Yang, L.; Zhou, X.X.; Guo, G.D.; Zhao, M.G.; Yang, Q.; Liu, G. Tetrahydroxystilbene glucoside relieves the chronic inflammatory pain by inhibiting neuronal apoptosis, microglia activation, and GluN2B overexpression in anterior cingulate cortex. Mol. Pain 2018, 14, 1744806918814367. [Google Scholar] [CrossRef]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Masocha, W. Comprehensive analysis of the GABAergic system gene expression profile in the anterior cingulate cortex of mice with Paclitaxel-induced neuropathic pain. Gene. Expr. 2015, 16, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Nashawi, H.; Masocha, W.; Edafiogho, I.O.; Kombian, S.B. Paclitaxel Causes Electrophysiological Changes in the Anterior Cingulate Cortex via Modulation of the gamma-Aminobutyric Acid-ergic System. Med. Princ. Pract. 2016, 25, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Matsuzaki, Y.; Koga, K.; Matsumoto, T.; Mishima, K.; Hara, S.; Honda, K. Involvement of GABAB receptor in the antihypersensitive effect in anterior cingulate cortex of partial sciatic nerve ligation model. J. Pharmacol. Sci. 2018, 137, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.W.; He, T.; Wang, R.R.; Li, C.L.; Luo, W.J.; Wu, F.F.; Wang, Y.; Li, Z.; Lu, Y.F.; Guan, S.M.; et al. Ethanol Increases Mechanical Pain Sensitivity in Rats via Activation of GABAA Receptors in Medial Prefrontal Cortex. Neurosci. Bull. 2016, 32, 433–444. [Google Scholar] [CrossRef]
- Wang, H.; Ren, W.H.; Zhang, Y.Q.; Zhao, Z.Q. GABAergic disinhibition facilitates polysynaptic excitatory transmission in rat anterior cingulate cortex. Biochem. Biophys. Res. Commun. 2005, 338, 1634–1639. [Google Scholar] [CrossRef]
- LaGraize, S.C.; Fuchs, P.N. GABAA but not GABAB receptors in the rostral anterior cingulate cortex selectively modulate pain-induced escape/avoidance behavior. Exp. Neurol. 2007, 204, 182–194. [Google Scholar] [CrossRef][Green Version]
- Jiang, Z.C.; Pan, Q.; Zheng, C.; Deng, X.F.; Wang, J.Y.; Luo, F. Inactivation of the prelimbic rather than infralimbic cortex impairs acquisition and expression of formalin-induced conditioned place avoidance. Neurosci. Lett. 2014, 569, 89–93. [Google Scholar] [CrossRef][Green Version]
- Seminowicz, D.A.; Laferriere, A.L.; Millecamps, M.; Yu, J.S.; Coderre, T.J.; Bushnell, M.C. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage 2009, 47, 1007–1014. [Google Scholar] [CrossRef]
- Maarrawi, J.; Peyron, R.; Mertens, P.; Costes, N.; Magnin, M.; Sindou, M.; Laurent, B.; Garcia-Larrea, L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain 2007, 127, 183–194. [Google Scholar] [CrossRef]
- Sprenger, T.; Berthele, A.; Platzer, S.; Boecker, H.; Tolle, T.R. What to learn from in vivo opioidergic brain imaging? Eur. J. Pain 2005, 9, 117–121. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.Y.; Jia, H.; Tang, J.S. Roles of different subtypes of opioid receptors in mediating the ventrolateral orbital cortex opioid-induced inhibition of mirror-neuropathic pain in the rat. Neuroscience 2007, 144, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, M.; Caputi, F.F.; Mercatelli, D.; Romualdi, P.; Candeletti, S. Dynorphinergic system alterations in the corticostriatal circuitry of neuropathic mice support its role in the negative affective component of pain. Genes Brain Behav. 2018, e12467. [Google Scholar] [CrossRef] [PubMed]
- Condes-Lara, M. Different direct pathways of locus coeruleus to medial prefrontal cortex and centrolateral thalamic nucleus: Electrical stimulation effects on the evoked responses to nociceptive peripheral stimulation. Eur. J. Pain 1998, 2, 15–23. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessel, T.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Chu, K.L.; Xu, J.; Frost, J.; Li, L.; Gomez, E.; Dart, M.J.; Jarvis, M.F.; Meyer, M.D.; McGaraughty, S. A selective alpha2 B adrenoceptor agonist (A-1262543) and duloxetine modulate nociceptive neurones in the medial prefrontal cortex, but not in the spinal cord of neuropathic rats. Eur. J. Pain 2015, 19, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Alba-Delgado, C.; Llorca-Torralba, M.; Horrillo, I.; Ortega, J.E.; Mico, J.A.; Sanchez-Blazquez, P.; Meana, J.J.; Berrocoso, E. Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol. Psychiatry 2013, 73, 54–62. [Google Scholar] [CrossRef]
- Kaushal, R.; Taylor, B.K.; Jamal, A.B.; Zhang, L.; Ma, F.; Donahue, R.; Westlund, K.N. GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAalpha1 receptors in the medial prefrontal cortex. Neuroscience 2016, 334, 148–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Cordeiro Matos, S.; Jego, S.; Adamantidis, A.; Seguela, P. Norepinephrine drives persistent activity in prefrontal cortex via synergistic alpha1 and alpha2 adrenoceptors. PLoS ONE 2013, 8, e66122. [Google Scholar] [CrossRef]
- Jarcho, J.M.; Feier, N.A.; Labus, J.S.; Naliboff, B.; Smith, S.R.; Hong, J.Y.; Colloca, L.; Tillisch, K.; Mandelkern, M.A.; Mayer, E.A.; et al. Placebo analgesia: Self-report measures and preliminary evidence of cortical dopamine release associated with placebo response. Neuroimage Clin. 2016, 10, 107–114. [Google Scholar] [CrossRef]
- Dent, M.F.; Neill, D.B. Dose-dependent effects of prefrontal dopamine on behavioral state in rats. Behav. Neurosci. 2012, 126, 620–639. [Google Scholar] [CrossRef]
- Sogabe, S.; Yagasaki, Y.; Onozawa, K.; Kawakami, Y. Mesocortical dopamine system modulates mechanical nociceptive responses recorded in the rat prefrontal cortex. BMC Neurosci. 2013, 14, 65. [Google Scholar] [CrossRef]
- Santana, N.; Mengod, G.; Artigas, F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb. Cortex 2009, 19, 849–860. [Google Scholar] [CrossRef]
- Ford, G.K.; Moriarty, O.; McGuire, B.E.; Finn, D.P. Investigating the effects of distracting stimuli on nociceptive behaviour and associated alterations in brain monoamines in rats. Eur. J. Pain 2008, 12, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Llado-Pelfort, L.; Assie, M.B.; Newman-Tancredi, A.; Artigas, F.; Celada, P. In vivo electrophysiological and neurochemical effects of the selective 5-HT1A receptor agonist, F13640, at pre- and postsynaptic 5-HT1A receptors in the rat. Psychopharmacology 2012, 221, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Bisco, A.; Nevian, N.E.; Lacivita, E.; Leopoldo, M.; Nevian, T. The brain-penetrant 5-HT7 receptor agonist LP-211 reduces the sensory and affective components of neuropathic pain. Neurobiol. Dis. 2017, 106, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V.; Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat. Rev. Neurosci. 2009, 10, 383–390. [Google Scholar] [CrossRef]
- Parikh, V.; Kozak, R.; Martinez, V.; Sarter, M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron 2007, 56, 141–154. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Sarter, M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef]
- Alkondon, M.; Albuquerque, E.X. The nicotinic acetylcholine receptor subtypes and their function in the hippocampus and cerebral cortex. Prog. Brain Res. 2004, 145, 109–120. [Google Scholar] [CrossRef]
- Fucile, S.; Sucapane, A.; Eusebi, F. Ca2+ permeability through rat cloned alpha9-containing nicotinic acetylcholine receptors. Cell Calcium. 2006, 39, 349–355. [Google Scholar] [CrossRef]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: Some functions and mechanisms. J. Mol. Neurosci. 2010, 41, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Bubser, M.; Byun, N.; Wood, M.R.; Jones, C.K. Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb. Exp. Pharmacol. 2012, 121–166. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Hu, L.; Hung, Y.S.; Mouraux, A.; Iannetti, G.D. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J. Neurosci. 2012, 32, 7429–7438. [Google Scholar] [CrossRef]
- Schulz, E.; May, E.S.; Postorino, M.; Tiemann, L.; Nickel, M.M.; Witkovsky, V.; Schmidt, P.; Gross, J.; Ploner, M. Prefrontal Gamma Oscillations Encode Tonic Pain in Humans. Cereb. Cortex 2015, 25, 4407–4414. [Google Scholar] [CrossRef]
- Pafundo, D.E.; Miyamae, T.; Lewis, D.A.; Gonzalez-Burgos, G. Cholinergic modulation of neuronal excitability and recurrent excitation-inhibition in prefrontal cortex circuits: Implications for gamma oscillations. J. Physiol. 2013, 591, 4725–4748. [Google Scholar] [CrossRef]
- Koga, K.; Matsuzaki, Y.; Honda, K.; Eto, F.; Furukawa, T.; Migita, K.; Irie, K.; Mishima, K.; Ueno, S. Activations of muscarinic M1 receptors in the anterior cingulate cortex contribute to the antinociceptive effect via GABAergic transmission. Mol. Pain 2017, 13, 1744806917692330. [Google Scholar] [CrossRef]
- Koga, K.; Matsuzaki, Y.; Migita, K.; Shimoyama, S.; Eto, F.; Nakagawa, T.; Matsumoto, T.; Terada, K.; Mishima, K.; Furue, H.; et al. Stimulating muscarinic M1 receptors in the anterior cingulate cortex reduces mechanical hypersensitivity via GABAergic transmission in nerve injury rats. Brain Res. 2019, 1704, 187–195. [Google Scholar] [CrossRef]
- Navarria, A.; Wohleb, E.S.; Voleti, B.; Ota, K.T.; Dutheil, S.; Lepack, A.E.; Dwyer, J.M.; Fuchikami, M.; Becker, A.; Drago, F.; et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol. Dis. 2015, 82, 254–261. [Google Scholar] [CrossRef]
- Masocha, W. Gene expression profile of sodium channel subunits in the anterior cingulate cortex during experimental paclitaxel-induced neuropathic pain in mice. PeerJ 2016, 4, e2702. [Google Scholar] [CrossRef]
- Li, T.T.; Ren, W.H.; Xiao, X.; Nan, J.; Cheng, L.Z.; Zhang, X.H.; Zhao, Z.Q.; Zhang, Y.Q. NMDA NR2A and NR2B receptors in the rostral anterior cingulate cortex contribute to pain-related aversion in male rats. Pain 2009, 146, 183–193. [Google Scholar] [CrossRef] [PubMed]
- La Porta, C.; Bura, S.A.; Llorente-Onaindia, J.; Pastor, A.; Navarrete, F.; Garcia-Gutierrez, M.S.; De la Torre, R.; Manzanares, J.; Monfort, J.; Maldonado, R. Role of the endocannabinoid system in the emotional manifestations of osteoarthritis pain. Pain 2015, 156, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Madasu, M.K.; Roche, M.; Finn, D.P. Supraspinal Transient Receptor Potential Subfamily V Member 1 (TRPV1) in Pain and Psychiatric Disorders. Mod. Trends Pharm. 2015, 30, 80–93. [Google Scholar] [CrossRef]
- Vertes, R.P. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 2004, 51, 32–58. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Neugebauer, V. Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics. Mol. Brain 2012, 5, 36. [Google Scholar] [CrossRef]
- Mukherjee, A.; Caroni, P. Infralimbic cortex is required for learning alternatives to prelimbic promoted associations through reciprocal connectivity. Nat. Commun. 2018, 9, 2727. [Google Scholar] [CrossRef]
- Gutman, D.A.; Keifer, O.P., Jr.; Magnuson, M.E.; Choi, D.C.; Majeed, W.; Keilholz, S.; Ressler, K.J. A DTI tractography analysis of infralimbic and prelimbic connectivity in the mouse using high-throughput MRI. Neuroimage 2012, 63, 800–811. [Google Scholar] [CrossRef]
- Berendse, H.W.; Galis-de Graaf, Y.; Groenewegen, H.J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992, 316, 314–347. [Google Scholar] [CrossRef]
- Moorman, D.E.; James, M.H.; McGlinchey, E.M.; Aston-Jones, G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015, 1628, 130–146. [Google Scholar] [CrossRef]

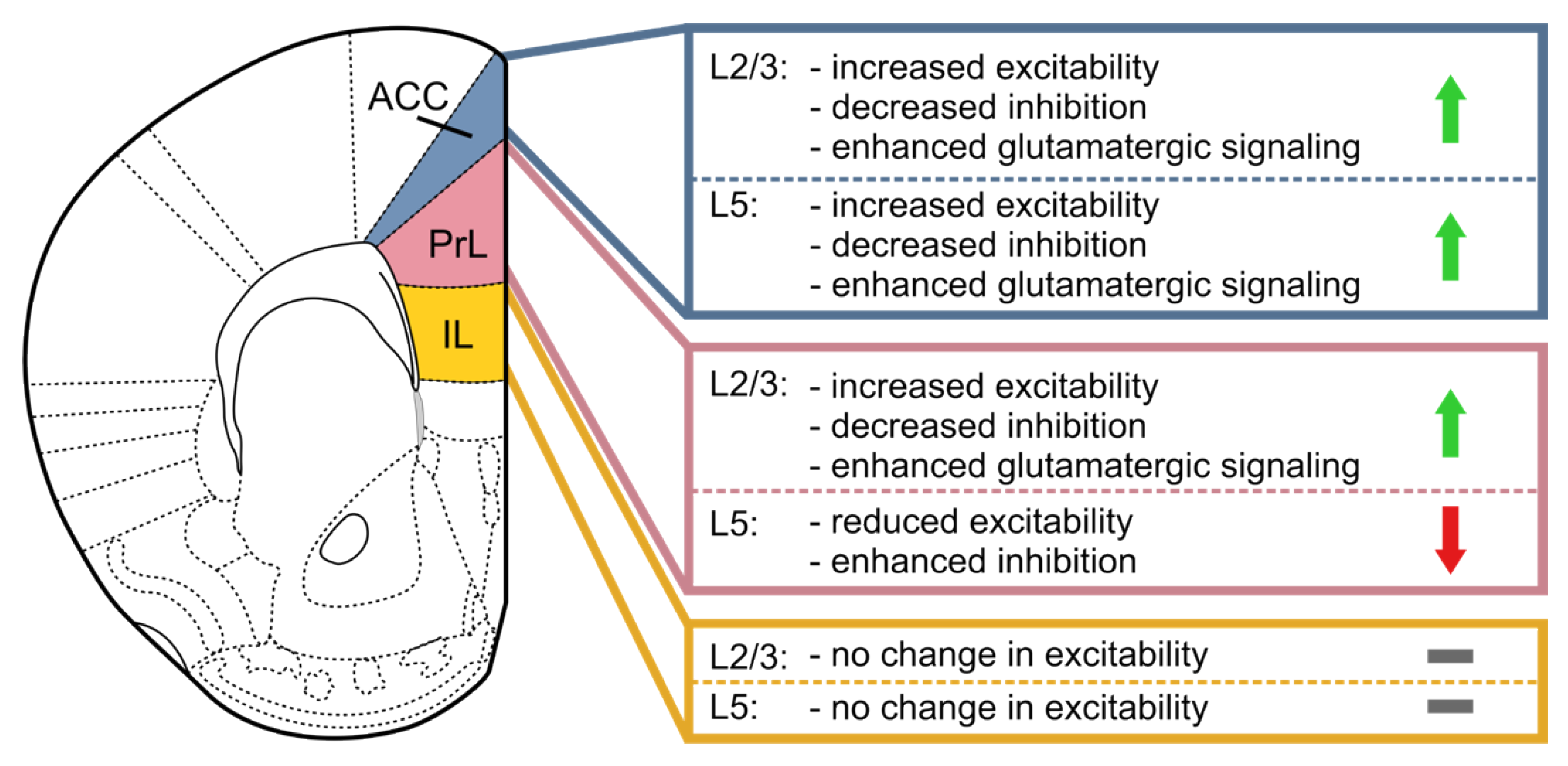
| Pala | Region (Layer) | Regulation | Pain Model | Reference |
|---|---|---|---|---|
| AChR M1 | PrL/IL (L5) | down | SNI | [155] |
| AMPA GluA1 | ACC | up | CFA-induced chronic inflammatory pain | [243] |
| AMPA GluA1 | ACC | up | CFA-induced chronic inflammatory pain | [261] |
| AMPA GluA1 | ACC | up | CFA-induced chronic inflammatory pain | [262] |
| AMPA GluA1 | ACC | increased membrane insertion | peripheral nerve ligation | [200] |
| AMPA GluA1 | ACC | increased membrane insertion | peripheral nerve ligation | [158] |
| GABAA β2 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [266] |
| GABAA β3 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [266] |
| GABAA δ | ACC | up | Paclitaxel-induced thermal hyperalgesia | [266] |
| GABAB | ACC | up | Paclitaxel-induced thermal hyperalgesia | [266] |
| HCN1 | ACC | down | CCI | [134] |
| HCN | ACC/PrL (L5) | functional reduction of Ih | SNI | [72] |
| HCN | ACC/PrL/IL (L2/3) | Ih modulation by noradrenergic alpha2 activation | SNI | [140] |
| HCN | ACC (L5) | functional reduction of Ih | CCI | [157] |
| mGluR1 | ACC | up | CCI | [134] |
| mGluR5 | ACC | down | CFA-induced chronic inflammatory pain | [260] |
| mGluR5 | PrL | up | SNL | [220] |
| mGluR7 | PrL | down | SNI | [219] |
| Nav1.1 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| Nav1.2 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| Nav1.6 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| Nax | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| Nav β1 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| Nav β3 | ACC | up | Paclitaxel-induced thermal hyperalgesia | [306] |
| NMDA NR2A | ACC | up | formalin-induced conditioned place avoidance | [307] |
| NMDA NR2A | ACC | up | CFA-induced chronic inflammatory pain | [261] |
| NMDA NR2A | ACC | down | CFA-induced chronic inflammatory pain | [263] |
| NMDA NR2B | mPFC | up | SNI | [173] |
| NMDA NR2B | ACC | up | CFA-induced chronic inflammatory pain | [239] |
| NMDA NR2B | ACC | up | formalin-induced conditioned place avoidance | [307] |
| NMDA NR2B | ACC | up | CFA-induced chronic inflammatory pain | [261] |
| NMDA NR2B | ACC | up | CFA-induced chronic inflammatory pain | [262] |
| NMDA NR2B | ACC | up | CFA-induced chronic inflammatory pain | [263] |
| NMDA NR2B | ACC | up | SNI | [247] |
| TRPV1 | PrL/IL (L2/3) | up | SNI | [174] |
| TRPV1 | PrL/IL (L2/3 and L5) | up | SNI | [230] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kummer, K.K.; Mitrić, M.; Kalpachidou, T.; Kress, M. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. Int. J. Mol. Sci. 2020, 21, 3440. https://doi.org/10.3390/ijms21103440
Kummer KK, Mitrić M, Kalpachidou T, Kress M. The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. International Journal of Molecular Sciences. 2020; 21(10):3440. https://doi.org/10.3390/ijms21103440
Chicago/Turabian StyleKummer, Kai K., Miodrag Mitrić, Theodora Kalpachidou, and Michaela Kress. 2020. "The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain" International Journal of Molecular Sciences 21, no. 10: 3440. https://doi.org/10.3390/ijms21103440
APA StyleKummer, K. K., Mitrić, M., Kalpachidou, T., & Kress, M. (2020). The Medial Prefrontal Cortex as a Central Hub for Mental Comorbidities Associated with Chronic Pain. International Journal of Molecular Sciences, 21(10), 3440. https://doi.org/10.3390/ijms21103440






