Rapid Fabrication of Anatomically-Shaped Bone Scaffolds Using Indirect 3D Printing and Perfusion Techniques
Abstract
1. Introduction
2. Results
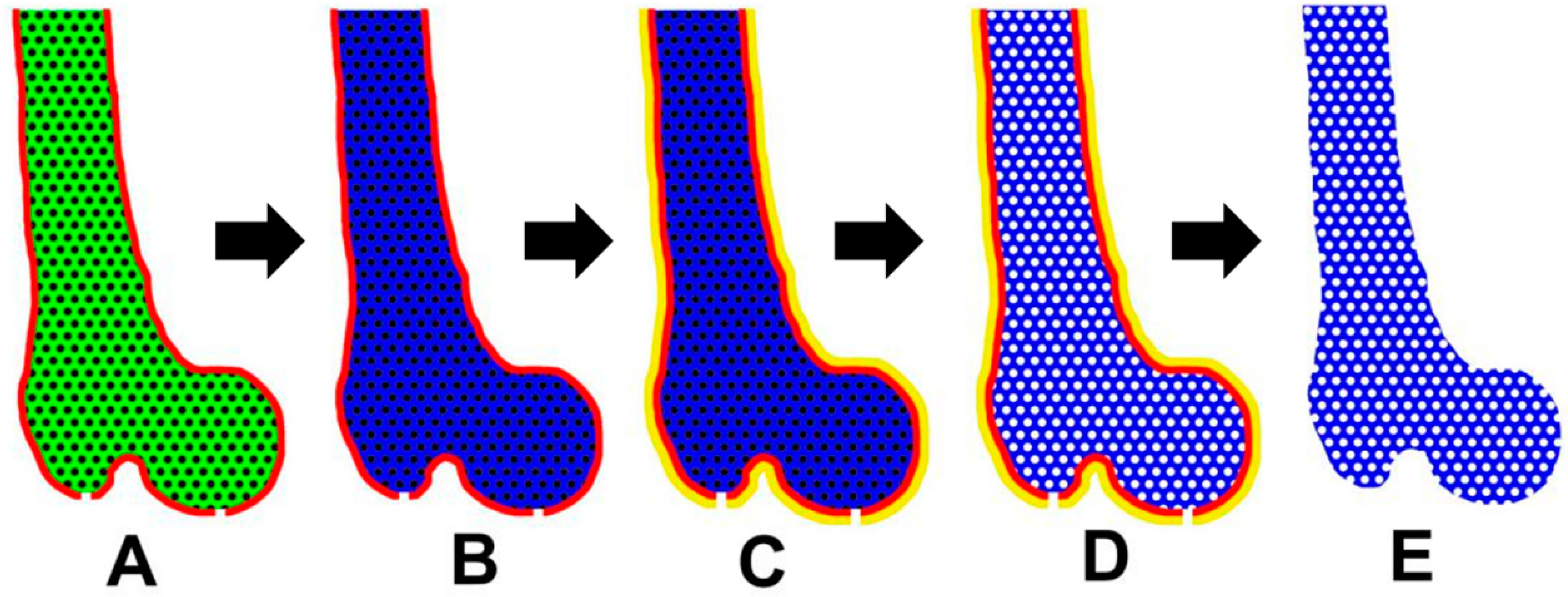
2.1. Anatomically-Shaped Digital Model Generation and Casting Mold Fabrication
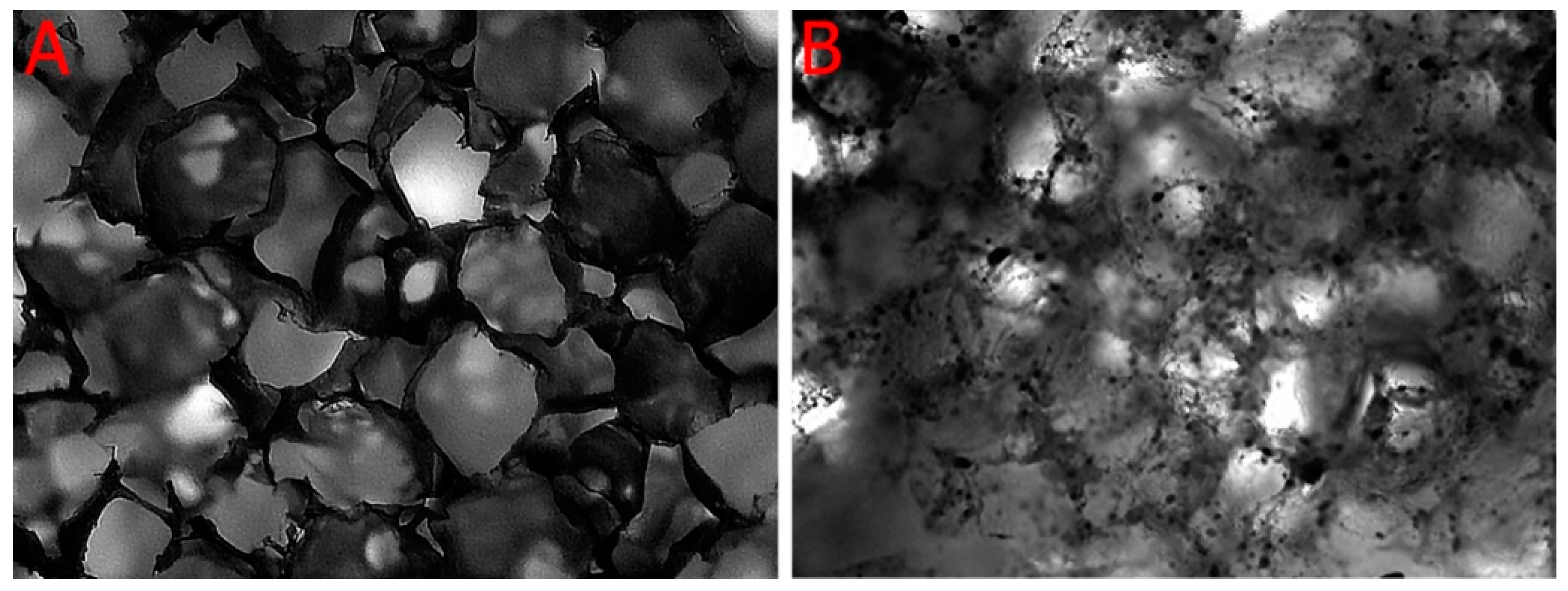
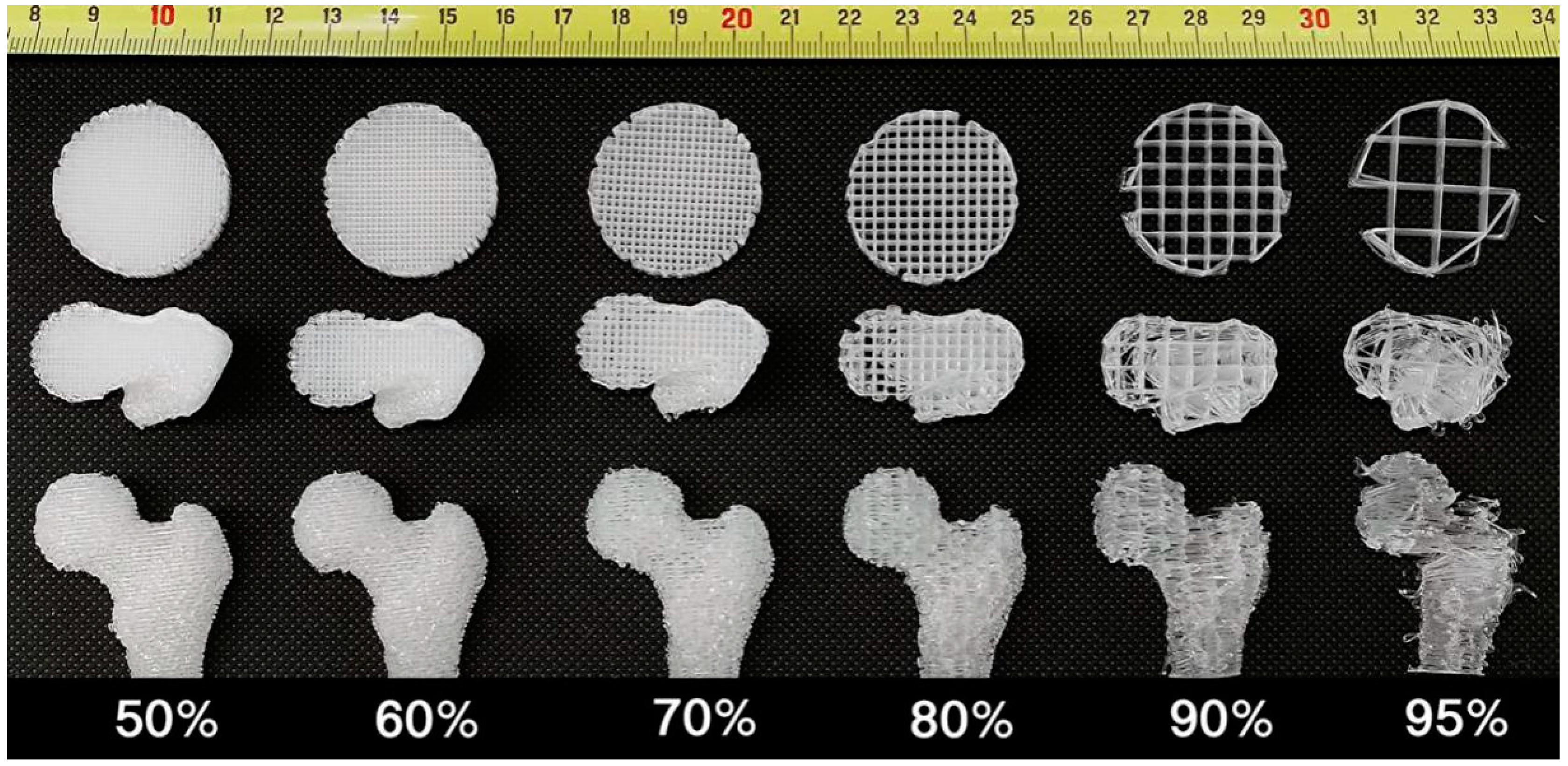
2.2. Porosity and Micro-Architecture of the Scaffold
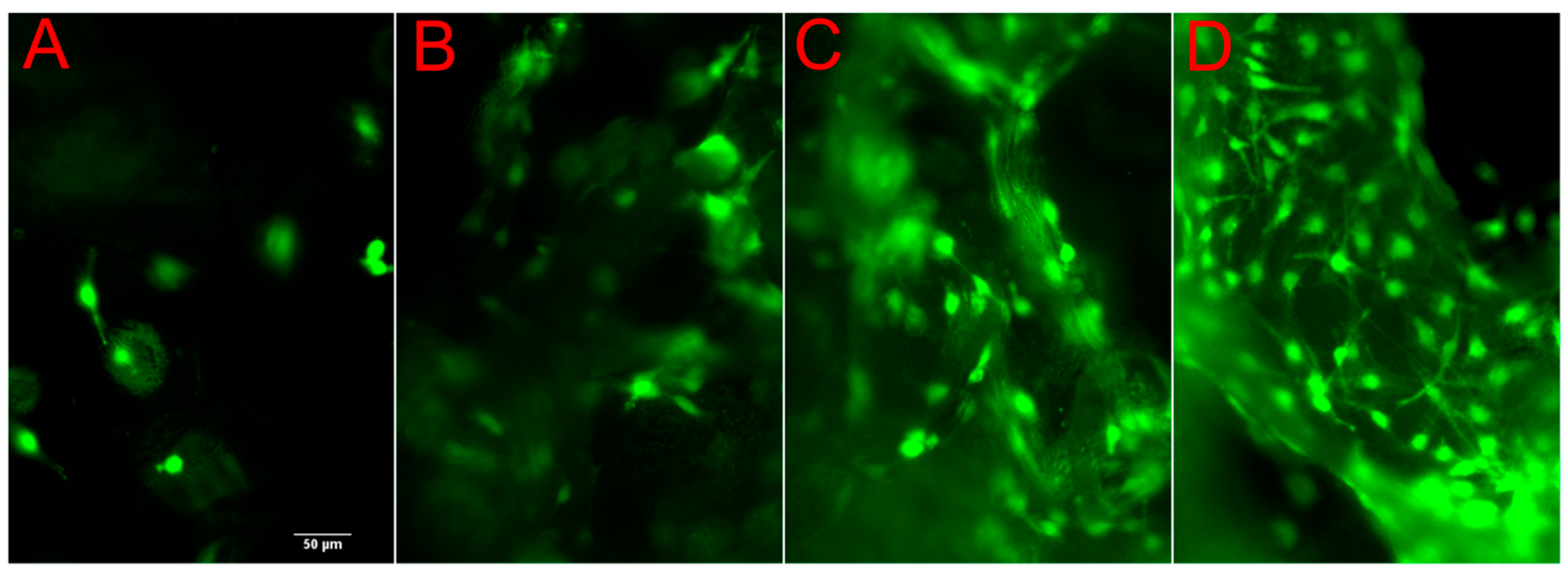
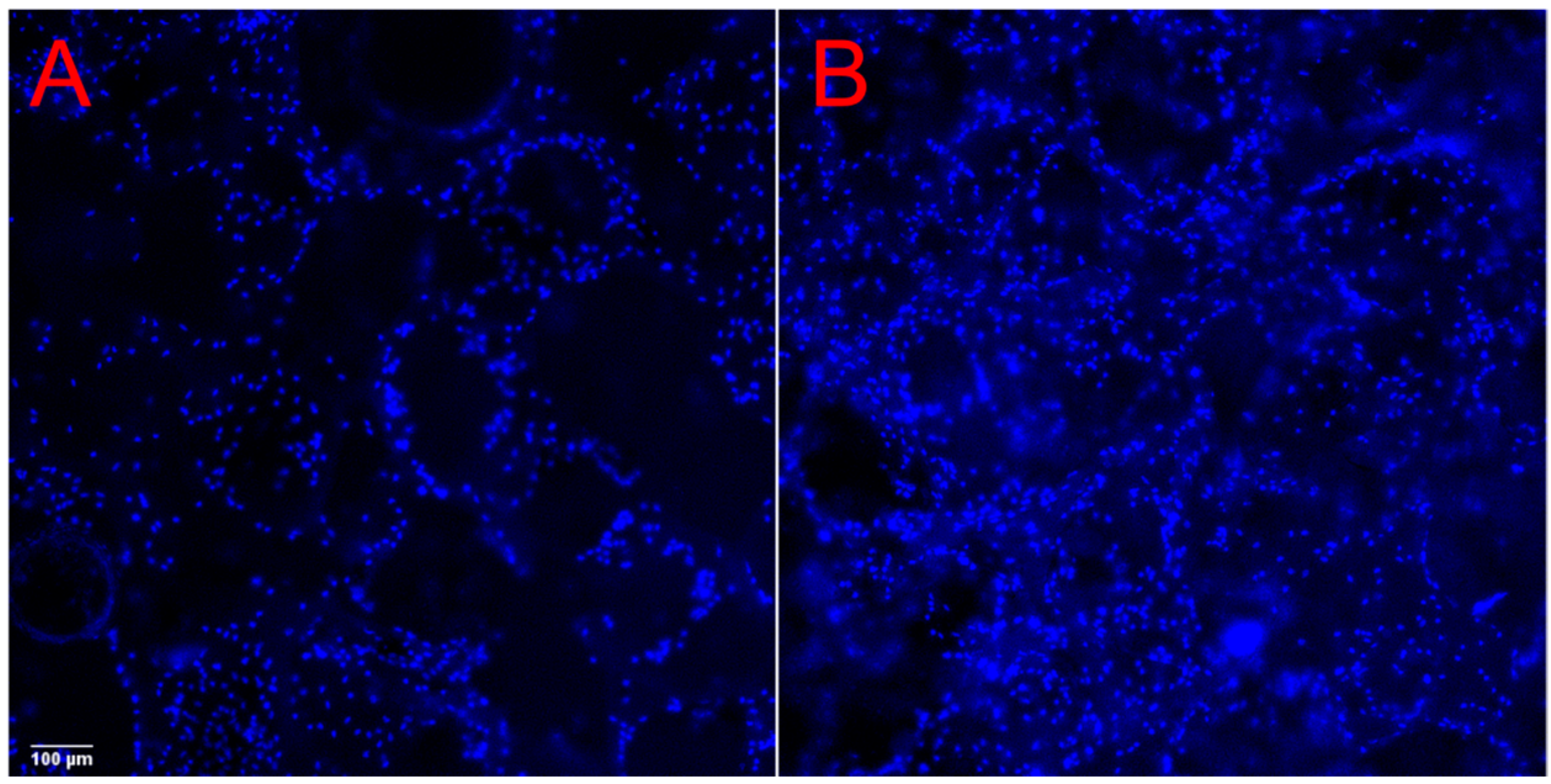
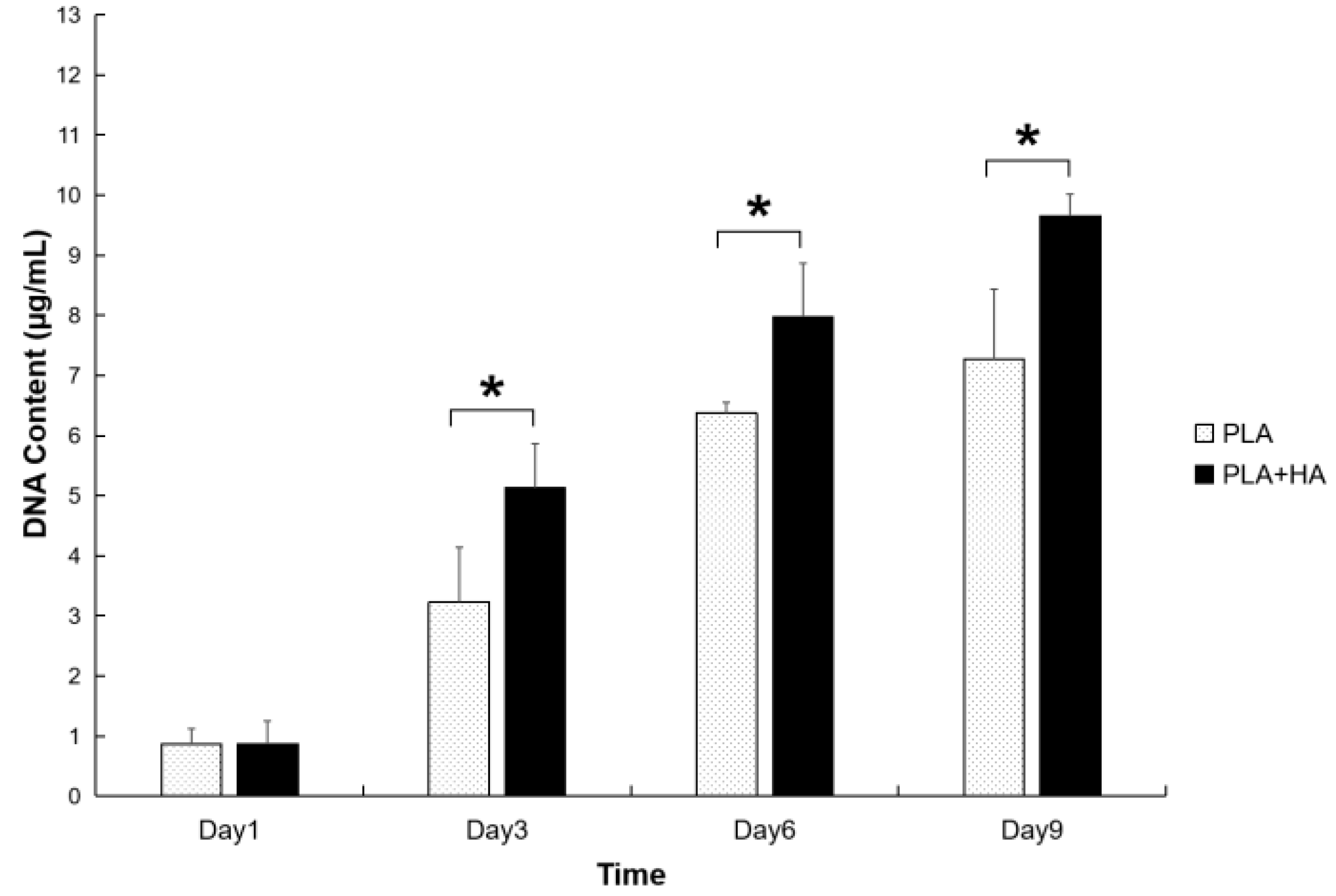
2.3. Cell Viability and Proliferation
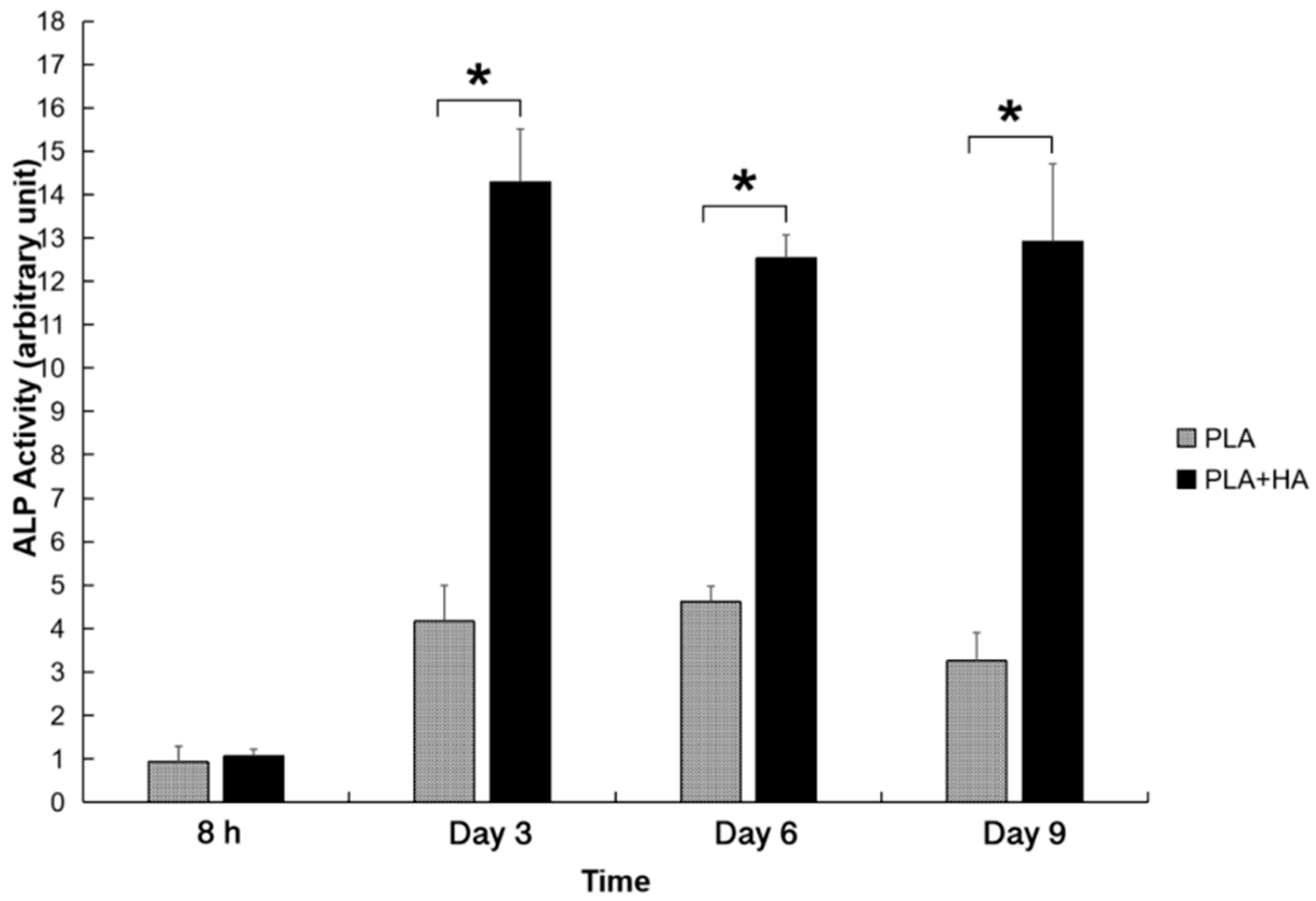
2.4. ALP Activities
2.5. Calcium Deposition
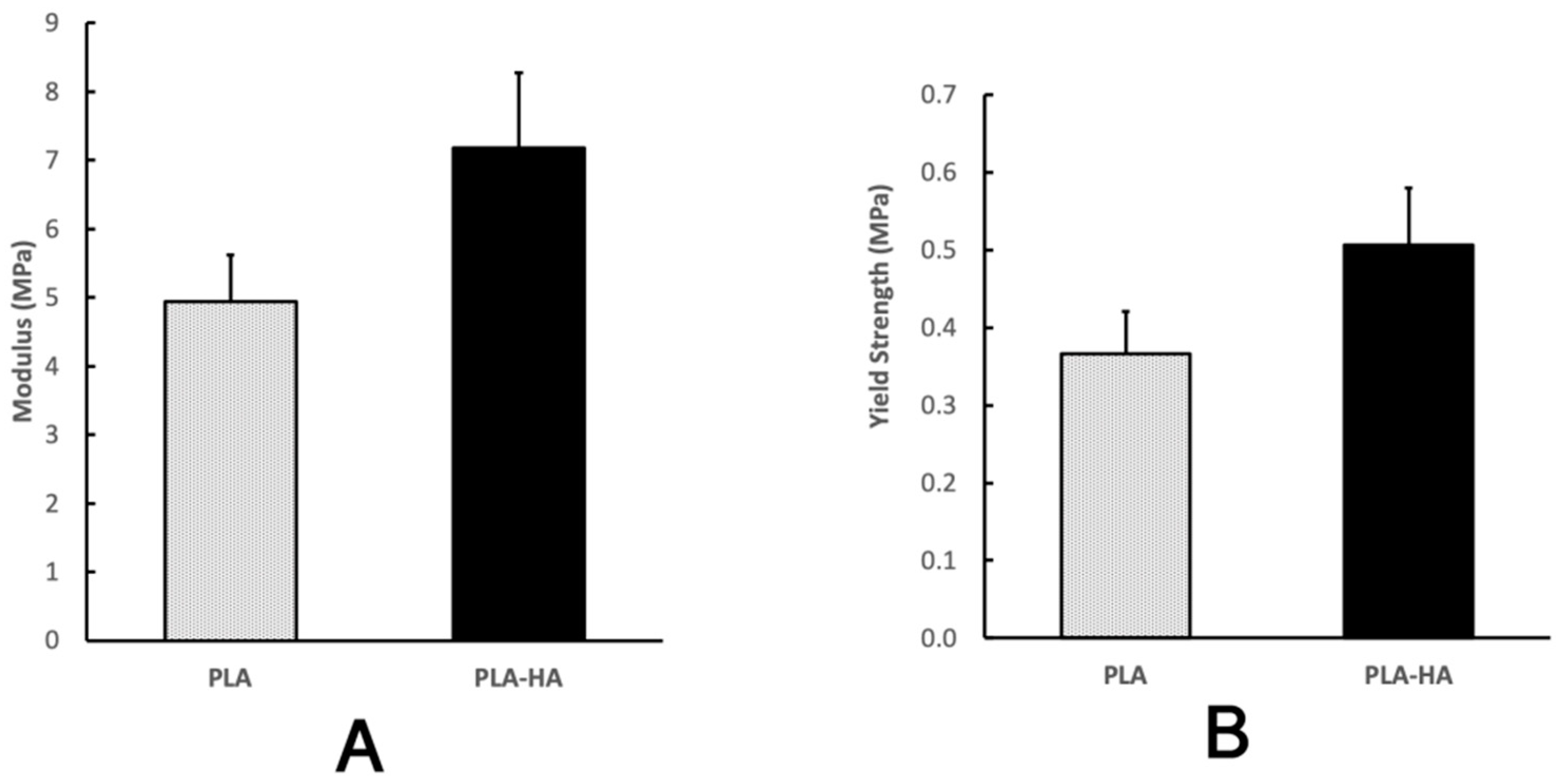
2.6. Mechanical Analysis
2.7. Comparison Assay with FDM Technology
3. Discussion
4. Materials and Methods
4.1. Generating Anatomically-Shaped 3D Model
4.2. Mold Generation by 3D Printing
4.3. Scaffold Manufacture
4.4. Micro-Architecture and Porosity Analysis
4.5. Cell Culture and Seeding into the Scaffolds
4.6. Cell Viability Analysis
4.7. Cell Proliferation Analysis
4.8. Alkaline-Phosphatase (ALP) Activity Assay
4.9. Calcium Content Measurement
4.10. Mechanical Property Analysis
4.11. Comparison with FDM Technology
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PLA | polylactic acid polymer |
| HA | hydroxyapatite |
| TCP | β-tricalcium phosphate |
| CNC | computer-numerical-control |
| FDM | Fused deposition modeling |
| SCPL | Solvent casting and particulate leaching |
| PVA | Polyvinyl Alcohol |
| CT | computed tomography |
| STL | solid stereolithography |
| ABS | acrylonitrile butadiene styrene |
| PCL | polycaprolactone |
| S/V | surface area-to-volume ratio |
References
- Desai, B.M. Osteobiologics. Am. J. Orthop. (Belle Mead NJ) 2007, 36, 8–11. [Google Scholar] [PubMed]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2010, 16, BR24–BR31. [Google Scholar] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Calcei, J.G.; Rodeo, S.A. Orthobiologics for Bone Healing. Clin. Sports Med. 2019, 38, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Lane, J.M.; Sandhu, H.S. Current approaches to experimental bone grafting. Orthop. Clin. N. Am. 1987, 18, 213–225. [Google Scholar]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Reis, R.L. Materials in particulate form for tissue engineering. 2. Applications in bone. J. Tissue Eng. Regen. Med. 2007, 1, 97–109. [Google Scholar] [CrossRef]
- Li, J.; Baker, B.A.; Mou, X.; Ren, N.; Qiu, J.; Boughton, R.I.; Liu, H. Biopolymer/Calcium phosphate scaffolds for bone tissue engineering. Adv. Healthc. Mater. 2014, 3, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Frohlich, M.; Yeager, K.; Bhumiratana, S.; Chan, M.E.; Cannizzaro, C.; Wan, L.Q.; Liu, X.S.; Guo, X.E.; Vunjak-Novakovic, G. Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 2010, 107, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Giordano, R.A.; Wu, B.M.; Borland, S.W.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 1996, 8, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 64, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Tian, X.; Heng, B.C.; Fan, V.; Yeo, J.F.; Cao, T. Histological evaluation of osteogenesis of 3D-printed poly-lactic-co-glycolic acid (PLGA) scaffolds in a rabbit model. Biomed. Mater. 2009, 4, 021001. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. A 2014, 102, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Guo, R.; Merkel, A.R.; Sterling, J.A.; Davidson, J.M.; Guelcher, S.A. Substrate modulus of 3D-printed scaffolds regulates the regenerative response in subcutaneous implants through the macrophage phenotype and Wnt signaling. Biomaterials 2015, 73, 85–95. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-varepsilon-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Sa, M.W.; Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, Y.W.; Lee, A.K.-X.; Yao, C.H.; Shie, M.Y. Development of mussel-inspired 3D-printed poly (lactic acid) scaffold grafted with bone morphogenetic protein-2 for stimulating osteogenesis. J. Mater. Sci. Mater. Med. 2019, 30, 78. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mikos, A.G. The importance of new processing techniques in tissue engineering. Bulletin 1996, 21, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Chen, C.F.; Chen, J.H.; Chiang, S.F.; Lin, Y.J.; Chang, K.Y. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Ma, P.X. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials 2009, 30, 6426–6434. [Google Scholar] [CrossRef]
- Suh, S.W.; Shin, J.Y.; Kim, J.; Kim, J.; Beak, C.H.; Kim, D.I.; Kim, H.; Jeon, S.S.; Choo, I.W. Effect of different particles on cell proliferation in polymer scaffolds using a solvent-casting and particulate leaching technique. ASAIO J. 2002, 48, 460–464. [Google Scholar] [CrossRef]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly (L-lactic acid) foams. Polymer 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacant, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Okada, K.; Hasegawa, F.; Kameshima, Y.; Nakajima, A. Bioactivity of CaSiO 3/poly-lactic acid (PLA) composites prepared by various surface loading methods of CaSiO 3 powder. J. Mater. Sci. Mater. Med. 2007, 18, 899–907. [Google Scholar] [CrossRef]
- Chen, J.-L.; Chiang, C.-H.; Yeh, M.-K. The mechanism of PLA microparticle formation by waterin-oil-in-water solvent evaporation method. J. Microencapsul. 2002, 19, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Larsen, L.B.; Trifol, J.; Szabo, P.; Burri, H.V.; Canali, C.; Dufva, M.; Emneus, J.; Wolff, A. Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.J.; Johnson, E.M.; Weisgerber, D.W.; Wheeler, M.B.; Harley, B.A.C. Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells. J. Mech. Behav. Biomed. Mater. 2019, 95, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kadri, O.E.; Sikavitsas, V.I.; Voronov, R.S. Scaffolds with a High Surface Area-to-Volume Ratio and Cultured Under Fast Flow Perfusion Result in Optimal O2 Delivery to the Cells in Artificial Bone Tissues. Appl. Sci. 2019, 9, 2381. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic characterization of 3D-printed PCL/beta-TCP scaffolds for biomedical devices and bone tissue engineering: Influence of composition and porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Kennedy, M.E.; Micallef, D.M. The effects of ultrasound irradiation on a biodegradable 50–50% copolymer of polylactic and polyglycolic acids. J. Biomed. Mater. Res. 1994, 28, 851–859. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef]
- Vozzi, G.; Corallo, C.; Carta, S.; Fortina, M.; Gattazzo, F.; Galletti, M.; Giordano, N. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: In vitro evidences. J. Biomed. Mater. Res. A 2014, 102, 1415–1421. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Poly (α--hydroxyl acids)/hydroxyapatite porous composites for bone--tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Kothapalli, C.R.; Shaw, M.T.; Wei, M. Biodegradable HA-PLA 3-D porous scaffolds: Effect of nano-sized filler content on scaffold properties. Acta Biomater. 2005, 1, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Dalela, M.; Sybil, D.; Patra, P.; Mohanty, S. Advances in three-dimensional bioprinting of bone: Progress and challenges. J. Tissue Eng. Regen. Med. 2019, 13, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical efficacy and effectiveness of 3D printing: A systematic review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yao, Y.; Grottkau, B. Rapid 3D Printing anatomically shaped bone scaffolds using novel molding and perfusion techniques. In Proceedings of the TERMIS, Washington, DC, USA, 13–16 December 2014. [Google Scholar]
- Grottkau, B.H.; Zhi, X.; Pang, Y. Rapid 3D printing of anatomically shaped bone scaffolds using novel molding and perfusion techniques. In Proceedings of the AAOS, Las Vegas, NV, USA, 12–16 March 2019. [Google Scholar]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, J.; Hui, Z.; Grottkau, B.E. Robotic Patterning a Superhydrophobic Surface for Collective Cell Migration Screening. Tissue Eng. Part C Methods 2018, 24, 205–213. [Google Scholar] [CrossRef]
- Connerty, H.V.; Briggs, A.R. Determination of serum calcium by means of orthocresolphthalein complexone. Am. J. Clin. Pathol. 1966, 45, 290–296. [Google Scholar] [CrossRef]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.; Eames, B.F.; Chen, D.X. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef]



| Processing Time | Perfusion (perf.) | Conventional Methods (conv.) | Time Ratio (Tperf./Tconv.) |
|---|---|---|---|
| Solvent Removal | 15 min | 48 h | 1/192 |
| Salt particle Removal | 4 h | 48 h | 1/12 |
| Residual water removal | 10 min | 10 h | 1/60 |
| Porosity Analysis | Femoral Head | Femoral Neck | Proximal Femoral Shaft | Distal Femoral Shaft |
|---|---|---|---|---|
| PLA | 97.31 ± 0.85 | 96.80 ± 1.79 | 96.36 ± 0.86 | 97.38 ± 0.95 |
| PLA-HA | 92.02 ± 2.24 | 92.62 ± 0.43 | 90.78 ± 1.71 | 92.75 ± 2.08 |
| FDM Technology | 3D P&P | ||||||
|---|---|---|---|---|---|---|---|
| Porosity | 50% | 60% | 70% | 80% | 90% | 95% | 97% |
| Integrity | H | H | H | M | L | EL | H |
| Surface Detail | H | H | M | L | EL | EL | H |
| S/V | 5.0 | 4.0 | 3.0 | 2.0 | 1.0 | 0.5 | 15.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grottkau, B.E.; Hui, Z.; Yao, Y.; Pang, Y. Rapid Fabrication of Anatomically-Shaped Bone Scaffolds Using Indirect 3D Printing and Perfusion Techniques. Int. J. Mol. Sci. 2020, 21, 315. https://doi.org/10.3390/ijms21010315
Grottkau BE, Hui Z, Yao Y, Pang Y. Rapid Fabrication of Anatomically-Shaped Bone Scaffolds Using Indirect 3D Printing and Perfusion Techniques. International Journal of Molecular Sciences. 2020; 21(1):315. https://doi.org/10.3390/ijms21010315
Chicago/Turabian StyleGrottkau, Brian E., Zhixin Hui, Yang Yao, and Yonggang Pang. 2020. "Rapid Fabrication of Anatomically-Shaped Bone Scaffolds Using Indirect 3D Printing and Perfusion Techniques" International Journal of Molecular Sciences 21, no. 1: 315. https://doi.org/10.3390/ijms21010315
APA StyleGrottkau, B. E., Hui, Z., Yao, Y., & Pang, Y. (2020). Rapid Fabrication of Anatomically-Shaped Bone Scaffolds Using Indirect 3D Printing and Perfusion Techniques. International Journal of Molecular Sciences, 21(1), 315. https://doi.org/10.3390/ijms21010315




