PP2A Functions during Mitosis and Cytokinesis in Yeasts
Abstract
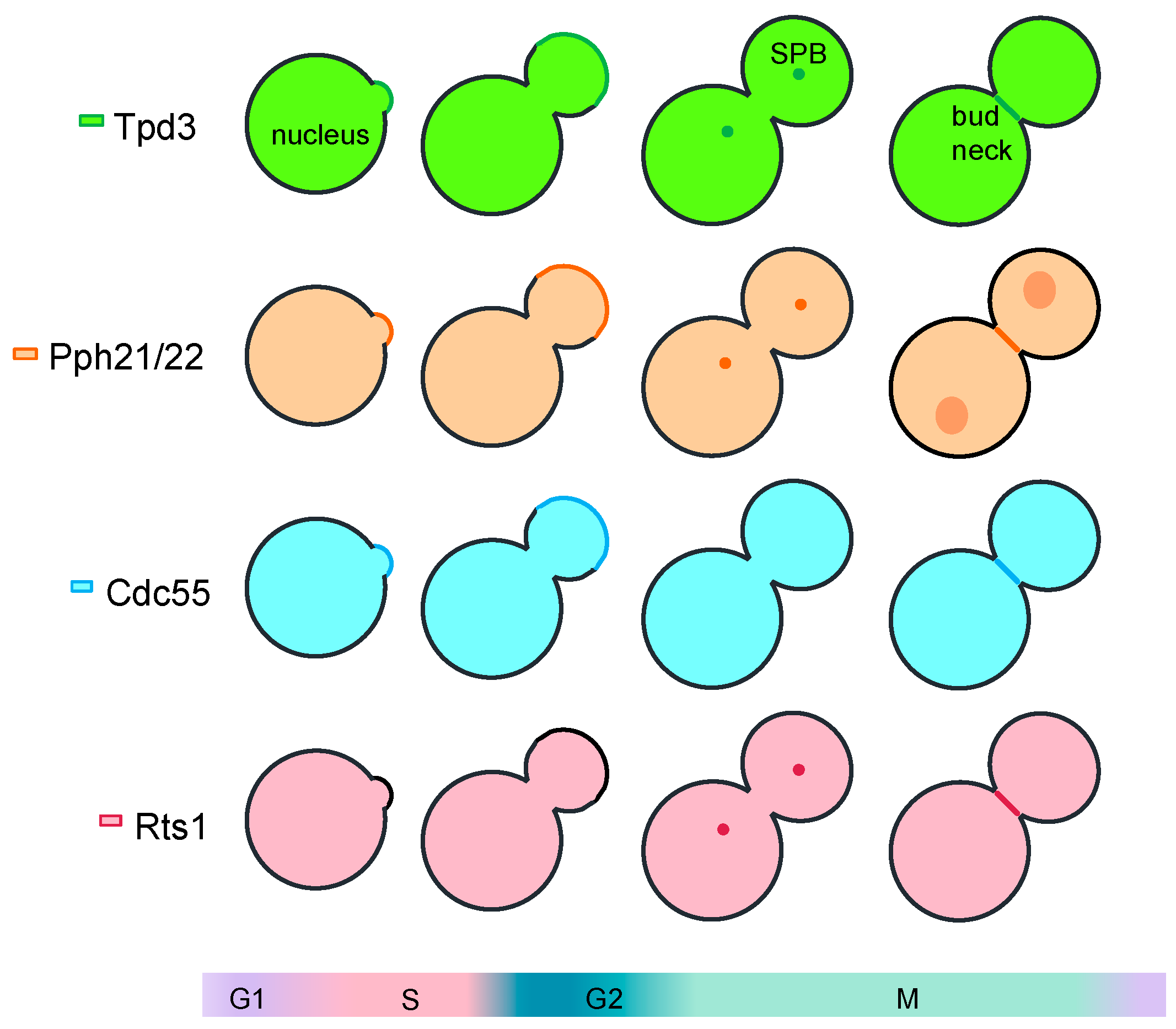
1. Introduction
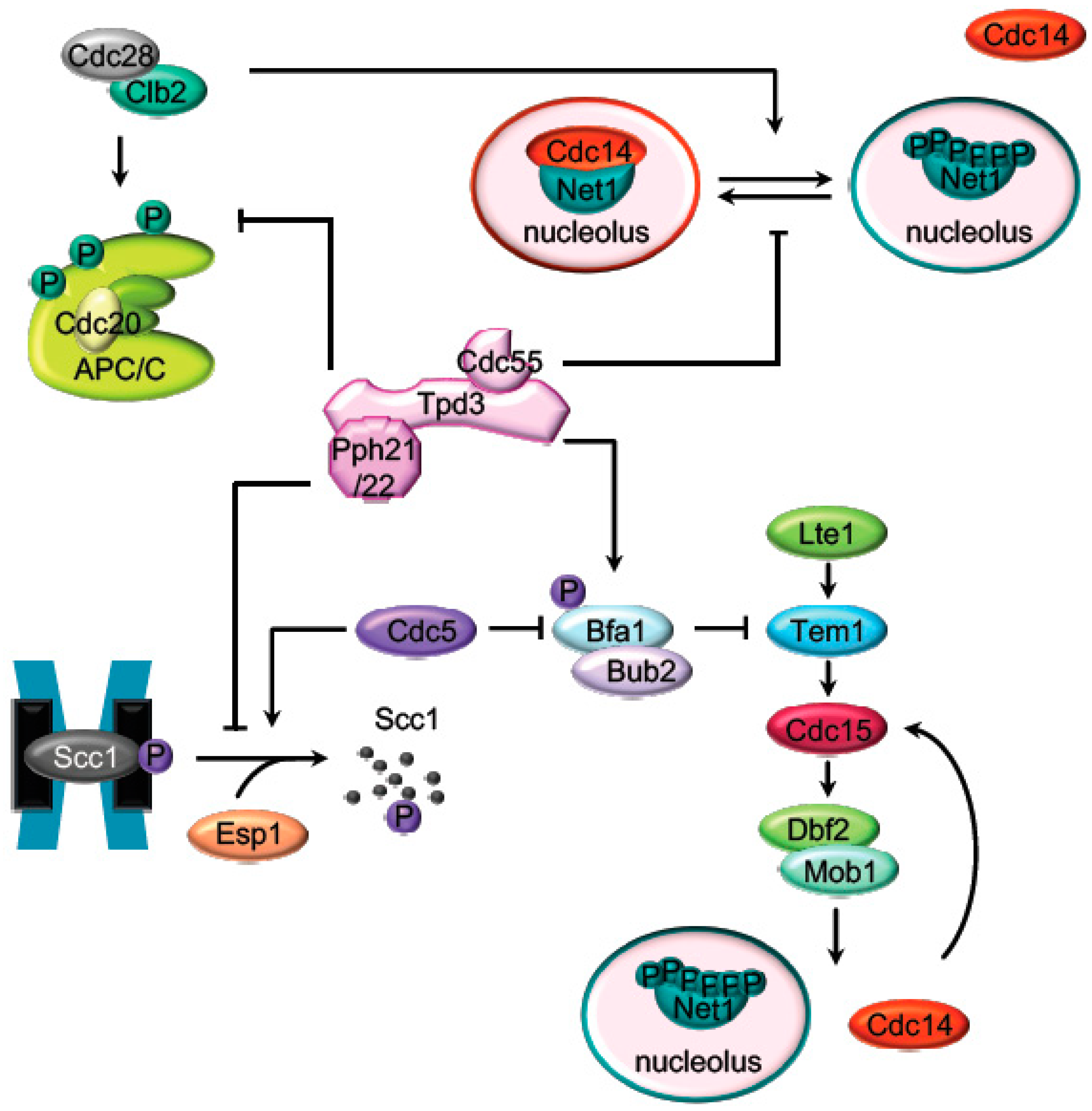
2. Mitosis Exit Regulation by PP2A
2.1. The APC Dephosphorylation by PP2ACdc55
2.2. The Regulation of the Cohesin Cleavage by PP2ACdc55
2.3. The FEAR-Cdc14 Release by PP2ACdc55
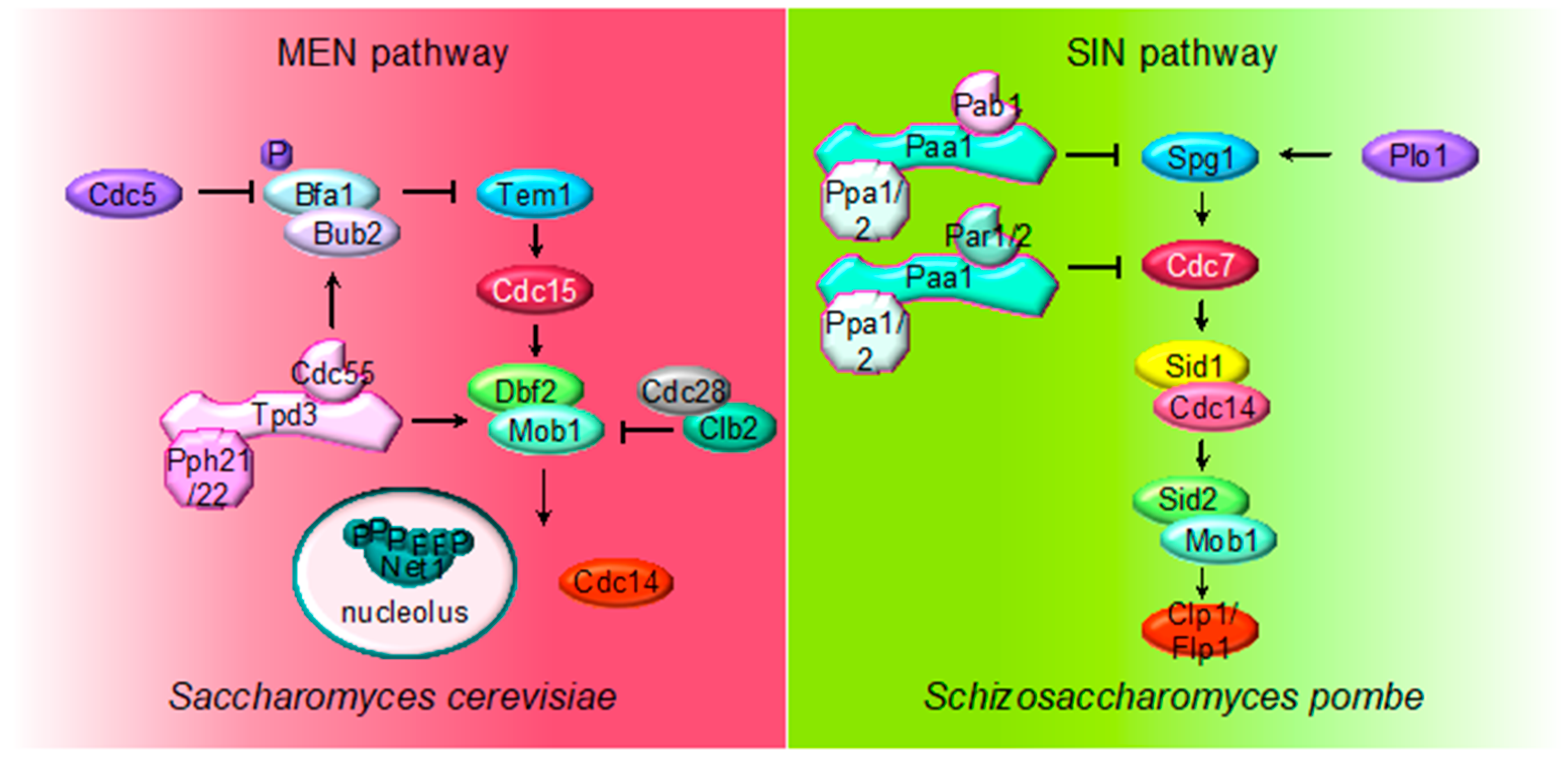
2.4. MEN (SIN) Regulation by PP2A
3. The Role of PP2A in Cytokinesis
4. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Ariño, J.; Velázquez, D.; Casamayor, A. Ser/Thr protein phosphatases in fungi: Structure, regulation and function. Microb. Cell (Graz, Austria) 2019, 6, 217–256. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K.; Nemoto, T.; Nabeshima, K.; Kondoh, H.; Niwa, H.; Yanagida, M. The regulatory subunits of fission yeast protein phosphatase 2A (PP2A) affect cell morphogenesis, cell wall synthesis and cytokinesis. Genes Cells 1996, 1, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Zolnierowicz, S.; Stapleton, A.E.; Goebl, M.; DePaoli-Roach, A.A.; Pringle, J.R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: Identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 1991, 11, 5767–5780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Mui, M.Z.; Chan, F.; Roopchand, D.E.; Marcellus, R.C.; Blanchette, P.; Li, S.; Berghuis, A.M.; Branton, P.E. Genetic Analysis of B55 /Cdc55 Protein Phosphatase 2A Subunits: Association with the Adenovirus E4orf4 Protein. J. Virol. 2011, 85, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Boguslawski, G.; Zitomer, R.S.; DePaoli-Roach, A.A. Saccharomyces cerevisiae Homologs of Mammalian B and B’ Subunits of Protein Phosphatase 2A Direct the Enzyme to Distinct Cellular Functions. J. Biol. Chem. 1997, 272, 8256–8262. [Google Scholar] [CrossRef]
- Shu, Y.; Yang, H.; Hallberg, E.; Hallberg, R. Molecular genetic analysis of Rts1p, a B’ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol. Cell. Biol. 1997, 17, 3242–3253. [Google Scholar] [CrossRef]
- Gentry, M.S.; Hallberg, R.L. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell 2002, 13, 3477–3492. [Google Scholar] [CrossRef]
- Lahoz, A.; Alcaide-Gavilan, M.; Daga, R.R.; Jimenez, J. Antagonistic roles of PP2A-Pab1 and Etd1 in the control of cytokinesis in fission yeast. Genetics 2010, 186, 1261–1270. [Google Scholar] [CrossRef]
- Le Goff, X.; Buvelot, S.; Salimova, E.; Guerry, F.; Schmidt, S.; Cueille, N.; Cano, E.; Simanis, V. The protein phosphatase 2A B′-regulatory subunit par1p is implicated in regulation of the S. Pombe septation initiation network. FEBS Lett. 2001, 508, 136–142. [Google Scholar] [CrossRef]
- Jiang, W.; Hallberg, R.L. Isolation and characterization of par1+ and par2+: Two schizosaccharomyces pombe genes encoding B’ subunits of protein phosphatase 2A. Genetics 2000, 154, 1025–1038. [Google Scholar] [PubMed]
- Wu, J.; Tolstykh, T.; Lee, J.; Boyd, K.; Stock, J.B.; Broach, J.R. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. Embo J. 2000, 19, 5672–5681. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ashby, D.G.; Moreno, C.S.; Ogris, E.; Yeong, F.M.; Corbett, A.H.; Pallas, D.C. Carboxymethylation of the PP2A Catalytic Subunit in Saccharomyces cerevisiae Is Required for Efficient Interaction with the B-type Subunits Cdc55p and Rts1p. J. Biol. Chem. 2001, 276, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Yabe, R.; Tsuji, S.; Mochida, S.; Ikehara, T.; Usui, T.; Ohama, T.; Sato, K. A stable association with PME-1 may be dispensable for PP2A demethylation–implications for the detection of PP2A methylation and immunoprecipitation. FEBS Open Bio 2018, 8, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Yabe, R.; Miura, A.; Usui, T.; Mudrak, I.; Ogris, E.; Ohama, T.; Sato, K. Protein phosphatase methyl-esterase PME-1 protects protein phosphatase 2A from ubiquitin/proteasome degradation. PLoS ONE 2015, 10, e0145226. [Google Scholar] [CrossRef] [PubMed]
- Játiva, S.; Calabria, I.; Moyano-Rodriguez, Y.; Garcia, P.; Queralt, E. Cdc14 activation requires coordinated Cdk1-dependent phosphorylation of Net1 and PP2A–Cdc55 at anaphase onset. Cell. Mol. Life Sci. 2019, 76, 3601–3620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Regulation of the Cell Cycle by Protein Phosphatase 2A in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 440–449. [Google Scholar] [CrossRef]
- Queralt, E.; Uhlmann, F. Cdk-counteracting phosphatases unlock mitotic exit. Curr. Opin. Cell Biol. 2008, 20, 661–668. [Google Scholar] [CrossRef]
- Stegmeier, F.; Amon, A. Closing mitosis: The functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 2004, 38, 203–232. [Google Scholar] [CrossRef]
- Cundell, M.J.; Hutter, L.H.; Bastos, R.N.; Poser, E.; Holder, J.; Mohammed, S.; Novak, B.; Barr, F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016, 214, 539–554. [Google Scholar] [CrossRef]
- Schmitz, M.H.A.; Held, M.; Janssens, V.; Hutchins, J.R.A.; Hudecz, O.; Ivanova, E.; Goris, J.; Trinkle-Mulcahy, L.; Lamond, A.I.; Poser, I.; et al. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 2010, 12, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Manchado, E.; Guillamot, M.; de Cárcer, G.; Eguren, M.; Trickey, M.; García-Higuera, I.; Moreno, S.; Yamano, H.; Cañamero, M.; Malumbres, M. Targeting Mitotic Exit Leads to Tumor Regression In Vivo: Modulation by Cdk1, Mastl, and the PP2A/B55α,δ Phosphatase. Cancer Cell 2010, 18, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Mochida, S.; Ikeo, S.; Gannon, J.; Hunt, T. Regulated activity of PP2A-B55 is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009, 28, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Touati, S.A.; Hofbauer, L.; Jones, A.W.; Snijders, A.P.; Kelly, G.; Uhlmann, F. Cdc14 and PP2A Phosphatases Cooperate to Shape Phosphoproteome Dynamics during Mitotic Exit. Cell Rep. 2019, 29, 2105–2119. [Google Scholar] [CrossRef]
- Queralt, E.; Lehane, C.; Novak, B.; Uhlmann, F. Downregulation of PP2ACdc55 Phosphatase by Separase Initiates Mitotic Exit in Budding Yeast. Cell 2006, 125, 719–732. [Google Scholar] [CrossRef]
- Lianga, N.; Williams, E.C.; Kennedy, E.K.; Doré, C.; Pilon, S.; Girard, S.L.; Deneault, J.S.; Rudner, A.D. A wee1 checkpoint inhibits anaphase onset. J. Cell Biol. 2013, 201, 843–862. [Google Scholar] [CrossRef]
- Zapata, J.; Dephoure, N.; Macdonough, T.; Yu, Y.; Parnell, E.J.; Mooring, M.; Gygi, S.P.; Stillman, D.J.; Kellogg, D.R. PP2ARts1 is a master regulator of pathways that control cell size. J. Cell Biol. 2014, 204, 359–376. [Google Scholar] [CrossRef]
- Peplowska, K.; Wallek, A.U.; Storchova, Z. Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation. PLoS Genet. 2014, 10, e1004411. [Google Scholar] [CrossRef]
- Dobbelaere, J.; Gentry, M.S.; Hallberg, R.L.; Barral, Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev. Cell 2003, 4, 345–357. [Google Scholar] [CrossRef]
- Sherwin, D.; Wang, Y. The Opposing Functions of Protein Kinases and Phosphatases in Chromosome Bipolar Attachment. Int. J. Mol. Sci. 2019, 20, 6182. [Google Scholar] [CrossRef]
- Lu, D.; Hsiao, J.Y.; Davey, N.E.; van Voorhis, V.A.; Foster, S.A.; Tang, C.; Morgan, D.O. Multiple mechanisms determine the order of APC/C substrate degradation in mitosis. J. Cell Biol. 2014, 207, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Morgan, D.O. Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 2007, 8, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Fix, O.; Peters, J.M.; Kirschner, M.W.; Koshland, D. Anaphase initiation in saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996, 10, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Hilioti, Z.; Chung, Y.S.; Mochizuki, Y.; Hardy, C.F.J.; Cohen-Fix, O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 2001, 11, 1347–1352. [Google Scholar] [CrossRef]
- Lim, H.H.; Goh, P.Y.; Surana, U. Cdc20 is essential for the cyclosome-mediated proteolysis of both Pds1 and Clb2 during M phase in budding yeast. Curr. Biol. 1998, 8, 231–234. [Google Scholar] [CrossRef]
- Sullivan, M.; Uhlmann, F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat. Cell Biol. 2003, 5, 249–254. [Google Scholar] [CrossRef]
- Uhlmann, F.; Wernic, D.; Poupart, M.A.; Koonin, E.V.; Nasmyth, K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 2000, 103, 375–386. [Google Scholar] [CrossRef]
- Mirchenko, L.; Uhlmann, F. Sli15INCENP dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr. Biol. 2010, 20, 1396–1401. [Google Scholar] [CrossRef]
- Teichner, A.; Eytan, E.; Sitry-Shevah, D.; Miniowitz-Shemtov, S.; Dumin, E.; Gromis, J.; Hershko, A. p31comet Promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc. Natl. Acad. Sci. USA 2011, 108, 3187–3192. [Google Scholar] [CrossRef]
- Rossio, V.; Michimoto, T.; Sasaki, T.; Ohbayashi, I.; Kikuchi, Y.; Yoshida, S. Nuclear PP2A-Cdc55 prevents APC-Cdc20 activation during the spindle assembly checkpoint. J. Cell Sci. 2013, 126, 4396–4405. [Google Scholar] [CrossRef]
- Yellman, C.M.; Burke, D.J. The role of Cdc55 in the spindle checkpoint is through regulation of mitotic exit in Saccharomyces cerevisiae. Mol. Biol. Cell 2006, 17, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Rudner, A.D.; Murray, A.W. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 2000, 149, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Chiroli, E.; Francia, V.; Gross, F.; Ciliberto, A. Adaptation to the spindle checkpoint is regulated by the interplay between Cdc28/Clbs and PP2ACdc55. J. Cell Biol. 2013, 202, 765–778. [Google Scholar] [CrossRef]
- Wang, Y.; Burke, D.J. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol. Cell. Biol. 1997, 17, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ng, T.-Y.Y. Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol. Biol. Cell 2006, 17, 80–89. [Google Scholar] [CrossRef]
- Bokros, M.; Wang, Y. Spindle assembly checkpoint silencing and beyond. Cell Cycle 2016, 15, 1661–1662. [Google Scholar] [CrossRef]
- Yaakov, G.; Thorn, K.; Morgan, D.O. Separase Biosensor Reveals that Cohesin Cleavage Timing Depends on Phosphatase PP2ACdc55 Regulation. Dev. Cell 2012, 23, 124–136. [Google Scholar] [CrossRef]
- Uhlmann, F.; Lottspeich, F.; Nasmyth, K.; Lottspelch, F.; Nasmyth, K.; Lottspeich, F.; Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 1999, 400, 37–42. [Google Scholar] [CrossRef]
- Pakchuen, S.; Ishibashi, M.; Takakusagi, E.; Shirahige, K.; Sutani, T. Physical association of saccharomyces cerevisiae polo-like kinase cdc5 with chromosomal cohesin facilitates DNA damage response. J. Biol. Chem. 2016, 291, 17228–17246. [Google Scholar] [CrossRef]
- Alexandru, G.; Uhlmann, F.; Mechtler, K.; Poupart, M.-A.A.; Nasmyth, K. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 2001, 105, 459–472. [Google Scholar] [CrossRef]
- Hornig, N.C.; Uhlmann, F. Preferential cleavage of chromatin-bound cohesin after targeted phosphorylation by Polo-like kinase. EMBO J. 2004, 23, 3144–3153. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Y. Pds1/Esp1-dependent and -independent sister chromatid separation in mutants defective for protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 2006, 103, 16290–16295. [Google Scholar] [CrossRef]
- Azzam, R.; Chen, S.L.; Shou, W.; Mah, A.S.; Alexandru, G.; Nasmyth, K.; Annan, R.S.; Carr, S.A.; Deshaies, R.J. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science 2004, 305, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Shou, W.; Azzam, R.; Chen, S.L.; Huddleton, M.J.; Baskerville, C.; Charbonneau, H.; Annan, R.S.; Carr, S.A.; Deshaies, R.J.; Huddleston, M.J.; et al. Cdc5 influences phosphorylation of Net1 and disassembly of the RENT complex. BMC Mol. Biol. 2002, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, J.A.; Moyano, Y.; Játiva, S.; Queralt, E. Mitotic Exit Function of Polo-like Kinase Cdc5 Is Dependent on Sequential Activation by Cdk1. Cell Rep. 2016, 15, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Calabria, I.; Baro, B.; Rodriguez-Rodriguez, J.-A.; Russiñol, N.; Queralt, E. Zds1 regulates PP2ACdc55 activity and Cdc14 activation during mitotic exit through its Zds_C motif. J. Cell Sci. 2012, 125, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- De los Santos-Velázquez, A.I.; de Oya, I.G.; Manzano-López, J.; Monje-Casas, F. Late rDNA Condensation Ensures Timely Cdc14 Release and Coordination of Mitotic Exit Signaling with Nucleolar Segregation. Curr. Biol. 2017, 27, 3248–3263. [Google Scholar] [CrossRef]
- Queralt, E.; Uhlmann, F. Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase. J. Cell Biol. 2008, 182, 873–883. [Google Scholar] [CrossRef]
- Stegmeier, F.; Huang, J.; Rahal, R.; Zmolik, J.; Moazed, D.; Amon, A. The replication fork block protein Fob1 functions as a negative regulator of the FEAR network. Curr. Biol. 2004, 14, 467–480. [Google Scholar] [CrossRef]
- Stegmeier, F.; Visintin, R.; Amon, A. Separase, Polo Kinase, the Kinetochore Protein Slk19, and Spo12 Function in a Network that Controls Cdc14 Localization during Early Anaphase. Cell 2002, 108, 207–220. [Google Scholar] [CrossRef]
- Tomson, B.N.; Rahal, R.; Reiser, V.; Monje-Casas, F.; Mekhail, K.; Moazed, D.; Amon, A. Regulation of Spo12 phosphorylation and its essential role in the FEAR network. Curr. Biol. 2009, 19, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Nolt, J.K.; Rice, L.M.; Gallo-Ebert, C.; Bisher, M.E.; Nickels, J.T. PP2ACdc55 is required for multiple events during meiosis I. Cell Cycle 2011, 10, 1420–1434. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.W.; Sarkar, S.; Tibbles, K.L.; Petronczki, M.; Millar, J.B.A.; Arumugam, P. Meiotic nuclear divisions in budding yeast require PP2A Cdc55-mediated antagonism of Net1 phosphorylation by Cdk. J. Cell Biol. 2011, 193, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Bizzari, F.; Marston, A.L. Cdc55 coordinates spindle assembly and chromosome disjunction during meiosis. J. Cell Biol. 2011, 193, 1213–1228. [Google Scholar] [CrossRef]
- Kerr, G.W.; Wong, J.H.; Arumugam, P. PP2ACdc55′s role in reductional chromosome segregation during achiasmate meiosis in budding yeast is independent of its FEAR function. Sci. Rep. 2016, 6, 30397. [Google Scholar] [CrossRef]
- Baro, B.; Queralt, E.; Monje-Casas, F. Regulation of mitotic exit in saccharomyces cerevisiae. Methods Mol. Biol. 2017, 1505, 3–17. [Google Scholar]
- Mah, A.S.; Jang, J.; Deshaies, R.J. Protein kinase Cdc15 activates the Dbf2-Mob1 kinase complex. Proc. Natl. Acad. Sci. USA 2001, 98, 7325–7330. [Google Scholar] [CrossRef]
- Visintin, R.; Amon, A. Regulation of the mitotic exit protein kinases Cdc15 and Dbf2. Mol. Biol. Cell 2001, 12, 2961–2974. [Google Scholar] [CrossRef]
- Yoshida, S.; Toh-e, A. Regulation of the localization of Dbf2 and mob1 during cell division of saccharomyces cerevisiae. Genes Genet. Syst. 2001, 76, 141–147. [Google Scholar] [CrossRef][Green Version]
- Baro, B.; Rodriguez-Rodriguez, J.-A.; Calabria, I.; Hernáez, M.L.; Gil, C.; Queralt, E. Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase. PLoS Genet. 2013, 9, e1003966. [Google Scholar] [CrossRef]
- Geymonat, M.; Spanos, A.; Walker, P.A.; Johnston, L.H.; Sedgwick, S.G. In vitro regulation of budding yeast Bfa1/Bub2 GAP activity by Cdc5. J. Biol. Chem. 2003, 278, 14591–14594. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wang, Y.; Liu, D.; Li, Y.; Qin, J.; Elledge, S.J. Regulation of the Bub2/Bfa1 GAP complex by Cdc5 and cell cycle checkpoints. Cell 2001, 107, 655–665. [Google Scholar] [CrossRef]
- Asakawa, K.; Yoshida, S.; Otake, F.; Toh-e, A. A novel functional domain of Cdc15 kinase is required for its interaction with Tem1 GTPase in Saccharomyces cerevisiae. Genetics 2001, 157, 1437–1450. [Google Scholar] [PubMed]
- Visintin, R.; Hwang, E.S.; Amon, A. Cfi 1 prevents premature exit from mitosis by anchoring Cdc14 phosphatase in the nucleolus. Nature 1999, 398, 818–823. [Google Scholar] [CrossRef]
- Mohl, D.A.; Huddleston, M.J.; Collingwood, T.S.; Annan, R.S.; Deshaies, R.J. Dbf2-Mob1 drives relocalization of protein phosphatase Cdc14 to the cytoplasm during exit from mitosis. J. Cell Biol. 2009, 184, 527–539. [Google Scholar] [CrossRef]
- König, C.; Maekawa, H.; Schiebel, E.; Konig, C.; Maekawa, H.; Schiebel, E. Mutual regulation of cyclin-dependent kinase and the mitotic exit network. J. Cell Biol. 2010, 188, 351–368. [Google Scholar] [CrossRef][Green Version]
- Gruneberg, U.; Campbell, K.; Simpson, C.; Grindlay, J.; Schiebel, E. Nud1p links astral microtubule organization and the control of exit from mitosis. EMBO J. 2000, 19, 6475–6488. [Google Scholar] [CrossRef]
- Cenamor, R.; Jiménez, J.; Cid, V.J.; Nombela, C.; Sanchez, M.; Jimenez, J.; Cid, V.J.; Nombela, C.; Sanchez, M.; Jiménez, J.; et al. The budding yeast Cdc 15 localizes to the spindle pole body in a cell-cycle-dependent manner. Mol. Cell. Biol. Res. Commun. 1999, 2, 178–184. [Google Scholar] [CrossRef]
- Jaspersen, S.L.; Morgan, D.O. Cdc14 activates cdc15 to promote mitotic exit in budding yeast. Curr. Biol. 2000, 10, 615–618. [Google Scholar] [CrossRef]
- Xu, S.; Huang, H.K.; Kaiser, P.; Latterich, M.; Hunter, T. Phosphorylation and spindle pole body localization of the Cdc15p mitotic regulatory protein kinase in budding yeast. Curr. Biol. 2000, 10, 329–332. [Google Scholar] [CrossRef]
- Menssen, R.; Neutzner, A.; Seufert, W. Asymmetric spindle pole localization of yeast Cdc15 kinase links mitotic exit and cytokinesis. Curr. Biol. 2001, 11, 345–350. [Google Scholar] [CrossRef]
- D’Aquino, K.E.; Monje-Casas, F.; Paulson, J.; Reiser, V.; Charles, G.M.; Lai, L.; Shokat, K.M.; Amon, A. The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol. Cell 2005, 19, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Bertazzi, D.T.; Kurtulmus, B.; Pereira, G. The cortical protein Lte1 promotes mitotic exit by inhibiting the spindle position checkpoint kinase Kin4. J. Cell Biol. 2011, 193, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.E.; Chan, L.Y.; Amon, A. Lte1 promotes mitotic exit by controlling the localization of the spindle position checkpoint kinase Kin4. Proc. Natl. Acad. Sci. USA 2011, 108, 12584–12590. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, H.; Priest, C.; Lechner, J.; Pereira, G.; Schiebel, E. The yeast centrosome translates the positional information of the anaphase spindle into a cell cycle signal. J. Cell Biol. 2007, 179, 423–436. [Google Scholar] [CrossRef]
- Pereira, G.; Schiebel, E. Kin4 kinase delays mitotic exit in response to spindle alignment defects. Mol. Cell 2005, 19, 209–221. [Google Scholar] [CrossRef]
- Chan, L.Y.; Amon, A. The protein phosphatase 2A functions in the spindle position checkpoint by regulating the checkpoint kinase Kin4. Genes Dev. 2009, 23, 1639–1649. [Google Scholar] [CrossRef][Green Version]
- Falk, J.E.; Campbell, I.W.; Joyce, K.; Whalen, J.; Seshan, A.; Amon, A. LTE1 promotes exit from mitosis by multiple mechanisms. Mol. Biol. Cell 2016, 27, 3991–4001. [Google Scholar] [CrossRef]
- Simanis, V. Pombe’s thirteen - control of fission yeast cell division by the septation initiation network. J. Cell Sci. 2015, 128, 1465–1474. [Google Scholar] [CrossRef]
- Jiang, W.; Hallberg, R.L. Correct regulation of the septation initiation network in Schizosaccharomyces pombe requires the activities of par1 and par2. Genetics 2001, 158, 1413–1429. [Google Scholar]
- Singh, N.S.; Shao, N.; McLean, J.R.; Sevugan, M.; Ren, L.; Chew, T.G.; Bimbo, A.; Sharma, R.; Tang, X.; Gould, K.L.; et al. SIN-inhibitory phosphatase complex promotes Cdc11p dephosphorylation and propagates SIN asymmetry in fission yeast. Curr. Biol. 2011, 21, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Grallert, A.; Boke, E.; Hagting, A.; Hodgson, B.; Connolly, Y.; Griffiths, J.R.; Smith, D.L.; Pines, J.; Hagan, I.M. A PP1-PP2A phosphatase relay controls mitotic progression. Nature 2014, 517, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bardin, A.J.; Amon, A. Men and sin: What’s the difference? Nat. Rev. Mol. Cell Biol. 2001, 2, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Meitinger, F.; Palani, S.; Pereira, G. The power of MEN in cytokinesis. Cell Cycle 2012, 11, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.C.; Mody, M.; Kurischko, C.; Roof, D.M.; Giddings, T.H.; Winey, M. Saccharomyces cerevisiae Mob1p Is Required for Cytokinesis and Mitotic Exit. Mol. Cell. Biol. 2001, 21, 6972–6983. [Google Scholar] [CrossRef] [PubMed]
- Frenz, L.M.; Lee, S.E.; Fesquet, D.; Johnston, L.H. The budding yeast Dbf2 protein kinase localises to the centrosome and moves to the bud neck in late mitosis. J. Cell Sci. 2000, 113 Pt 19, 3399–3408. [Google Scholar]
- Song, S.; Grenfell, T.Z.; Garfield, S.; Erikson, R.L.; Lee, K.S. Essential Function of the Polo Box of Cdc5 in Subcellular Localization and Induction of Cytokinetic Structures. Mol. Cell. Biol. 2000, 20, 286–298. [Google Scholar] [CrossRef]
- Jin, Q.-W.; Zhou, M.; Bimbo, A.; Balasubramanian, M.K.; McCollum, D. A role for the septation initiation network in septum assembly revealed by genetic analysis of sid2-250 suppressors. Genetics 2006, 172, 2101–2112. [Google Scholar] [CrossRef]
- Hachet, O.; Simanis, V. Mid1p/anillin and the septation initiation network orchestrate contractile ring assembly for cytokinesis. Genes Dev. 2008, 22, 3205–3216. [Google Scholar] [CrossRef]
- Yang, X.; Yu, K.; Hao, Y.; Li, D.M.; Stewart, R.; Insogna, K.L.; Xu, T. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat. Cell Biol. 2004, 6, 609–617. [Google Scholar] [CrossRef]
- Palani, S.; Meitinger, F.; Boehm, M.E.; Lehmann, W.D.; Pereira, G. Cdc14-dependent dephosphorylation of Inn1 contributes to Inn1-Cyk3 complex formation. J. Cell Sci. 2012, 125, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.P.; Hall, H.; Chaparian, R.; Mara, M.; Mueller, A.; Hall, M.C.; Shannon, K.B. Dephosphorylation of Iqg1 by Cdc14 regulates cytokinesis in budding yeast. Mol. Biol. Cell 2015, 26, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Kuilman, T.; Maiolica, A.; Godfrey, M.; Scheidel, N.; Aebersold, R.; Uhlmann, F. Identification of Cdk targets that control cytokinesis. EMBO J. 2015, 34, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Devrekanli, A.; Foltman, M.; Roncero, C.; Sanchez-Diaz, A.; Labib, K. Inn1 and Cyk3 regulate chitin synthase during cytokinesis in budding yeasts. J. Cell Sci. 2012, 125, 5453–5466. [Google Scholar] [CrossRef]
- Baro, B.; Játiva, S.; Calabria, I.; Vinaixa, J.; Bech-Serra, J.J.; De LaTorre, C.; Rodrigues, J.; Hernáez, M.L.; Gil, C.; Barceló-Batllori, S.; et al. SILAC-based phosphoproteomics reveals new PP2A-Cdc55-regulated processes in budding yeast. Gigascience 2018, 7, giy047. [Google Scholar] [CrossRef]
- Parnell, E.J.; Yu, Y.; Lucena, R.; Yoon, Y.; Bai, L.; Kellogg, D.R.; Stillman, D.J. The rts1 regulatory subunit of PP2A phosphatase controls expression of the ho endonuclease via localization of the Ace2 transcription factor. J. Biol. Chem. 2014, 289, 35431–35437. [Google Scholar] [CrossRef]
- Kovacech, B.; Nasmyth, K.; Schuster, T. EGT2 gene transcription is induced predominantly by Swi5 in early G1. Mol. Cell. Biol. 1996, 16, 3264–3274. [Google Scholar] [CrossRef]
- O’Conalláin, C.; Doolin, M.T.; Taggart, C.; Thornton, F.; Butler, G. Regulated nuclear localisation of the yeast transcription factor Ace2p controls expression of chitinase (CTS1) in Saccharomyces cerevisiae. Mol. Gen. Genet. 1999, 262, 275–282. [Google Scholar] [CrossRef]
- Kuznetsov, E.; Váchová, L.; Palková, Z. Cellular localization of Sun4p and its interaction with proteins in the yeast birth scar. Cell Cycle 2016, 15, 1898–1907. [Google Scholar] [CrossRef][Green Version]
- Alcaide-Gavilán, M.; Lahoz, A.; Daga, R.R.; Jimenez, J. Feedback regulation of SIN by Etd1 and Rho1 in fission yeast. Genetics 2014, 196, 455–470. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, S.; Lu, L. Mitotic-spindle organizing protein MztA mediates septation signaling by suppressing the regulatory subunit of protein phosphatase 2A-ParA in Aspergillus nidulans. Front. Microbiol. 2018, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Pan, C.; Wang, Y.; Wang, N.; Wang, Y.; Sang, J. The PP2A regulatory subunits, Cdc55 and Rts1, play distinct roles in Candida albicans’ growth, morphogenesis, and virulence. Fungal Genet. Biol. 2019, 131, 103240. [Google Scholar] [CrossRef]
- Liu, Q.; Han, Q.; Wang, N.; Yao, G.; Zeng, G.; Wang, Y.; Huang, Z.; Sang, J.; Wang, Y. Tpd3-Pph21 phosphatase plays a direct role in Sep7 dephosphorylation in Candida albicans. Mol. Microbiol. 2016, 101, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Muñiz, J.M.; Renshaw, H.; Richards, A.D.; Waitt, G.; Soderblom, E.J.; Moseley, M.A.; Asfaw, Y.; Juvvadi, P.R.; Steinbach, W.J. Dephosphorylation of the Core Septin, AspB, in a Protein Phosphatase 2A-Dependent Manner Impacts Its Localization and Function in the Fungal Pathogen Aspergillus fumigatus. Front. Microbiol. 2016, 7, 997. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Jiang, P.; Qiao, W.; Zhang, Y.; Wei, W.; Lu, L. Protein phosphatase 2A (PP2A) regulatory subunits ParA and PabA orchestrate septation and conidiation and are essential for PP2A activity in Aspergillus nidulans. Eukaryot. Cell 2014, 13, 1494–1506. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano-Rodriguez, Y.; Queralt, E. PP2A Functions during Mitosis and Cytokinesis in Yeasts. Int. J. Mol. Sci. 2020, 21, 264. https://doi.org/10.3390/ijms21010264
Moyano-Rodriguez Y, Queralt E. PP2A Functions during Mitosis and Cytokinesis in Yeasts. International Journal of Molecular Sciences. 2020; 21(1):264. https://doi.org/10.3390/ijms21010264
Chicago/Turabian StyleMoyano-Rodriguez, Yolanda, and Ethel Queralt. 2020. "PP2A Functions during Mitosis and Cytokinesis in Yeasts" International Journal of Molecular Sciences 21, no. 1: 264. https://doi.org/10.3390/ijms21010264
APA StyleMoyano-Rodriguez, Y., & Queralt, E. (2020). PP2A Functions during Mitosis and Cytokinesis in Yeasts. International Journal of Molecular Sciences, 21(1), 264. https://doi.org/10.3390/ijms21010264




