How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites
Abstract
1. Introduction
2. Results and Discussion
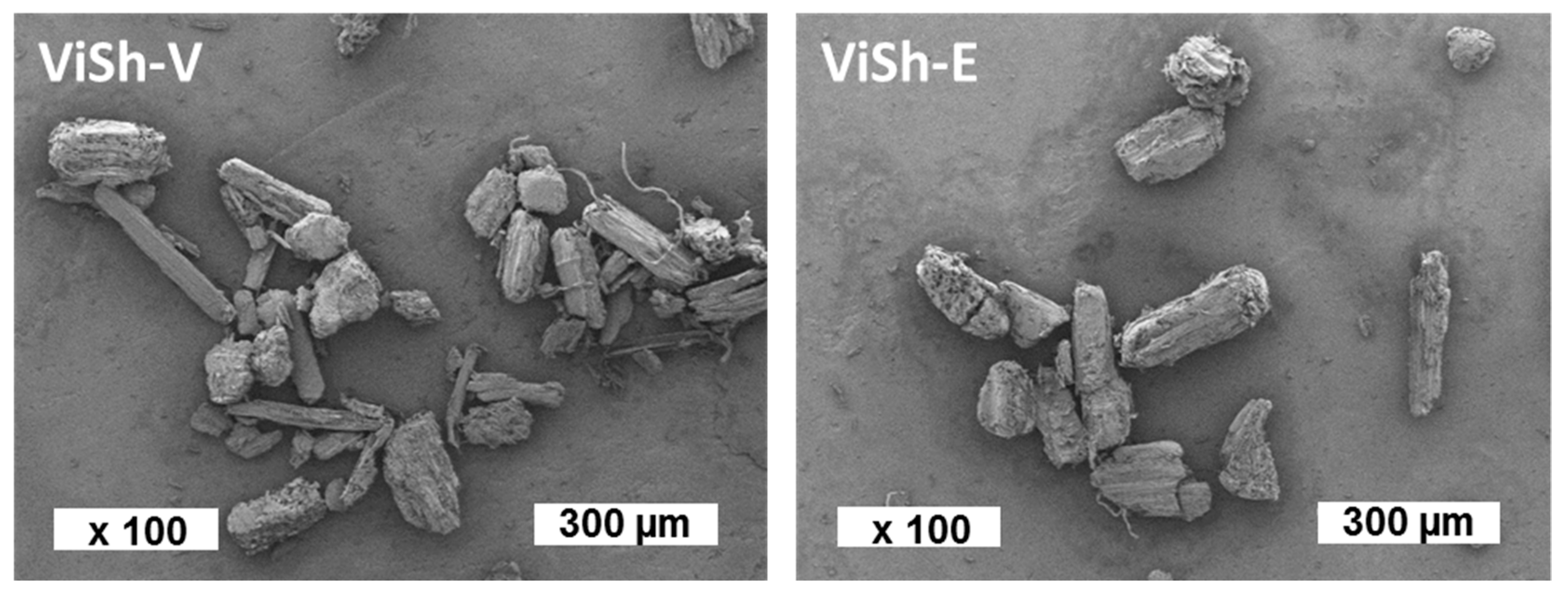
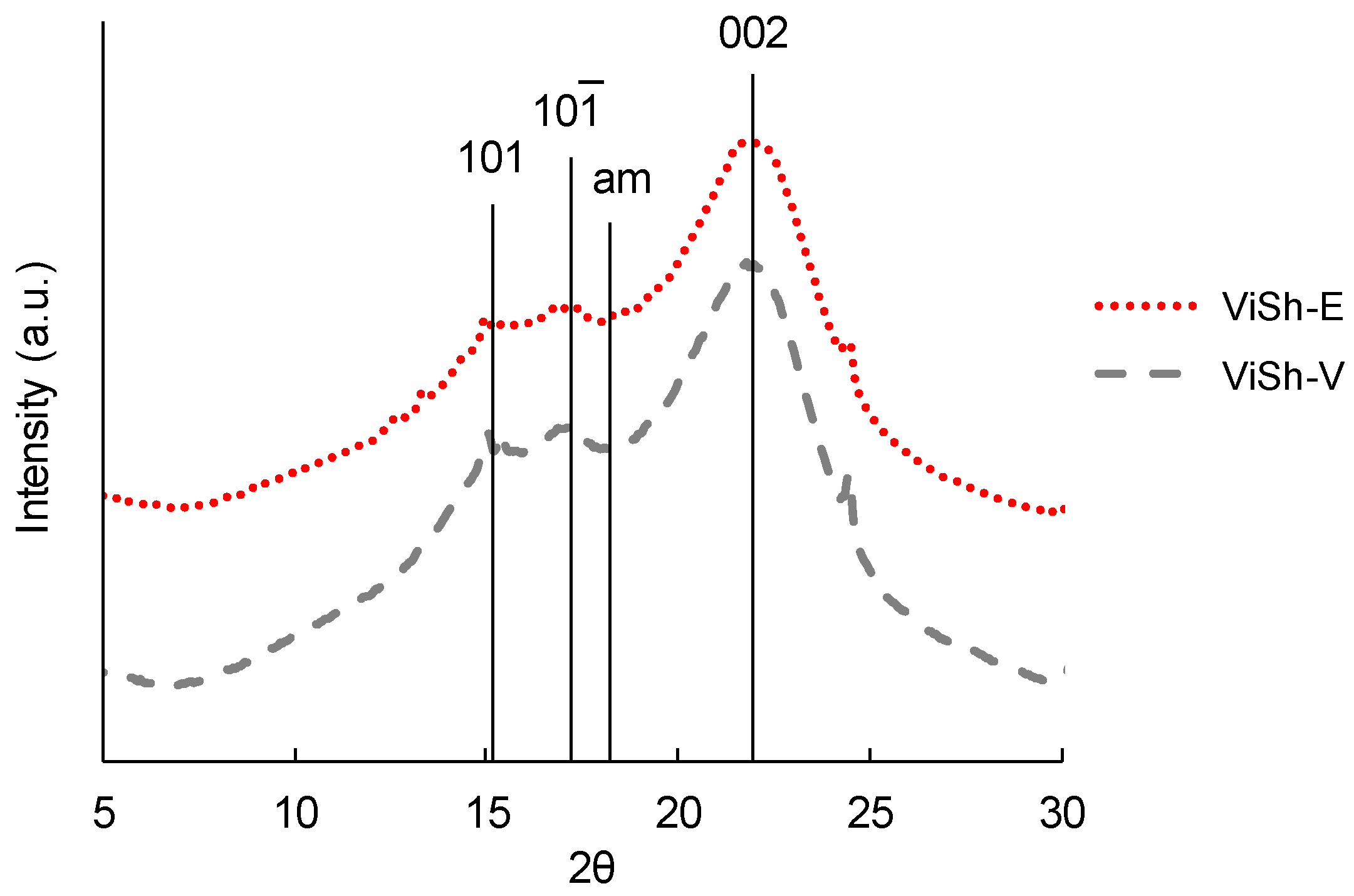
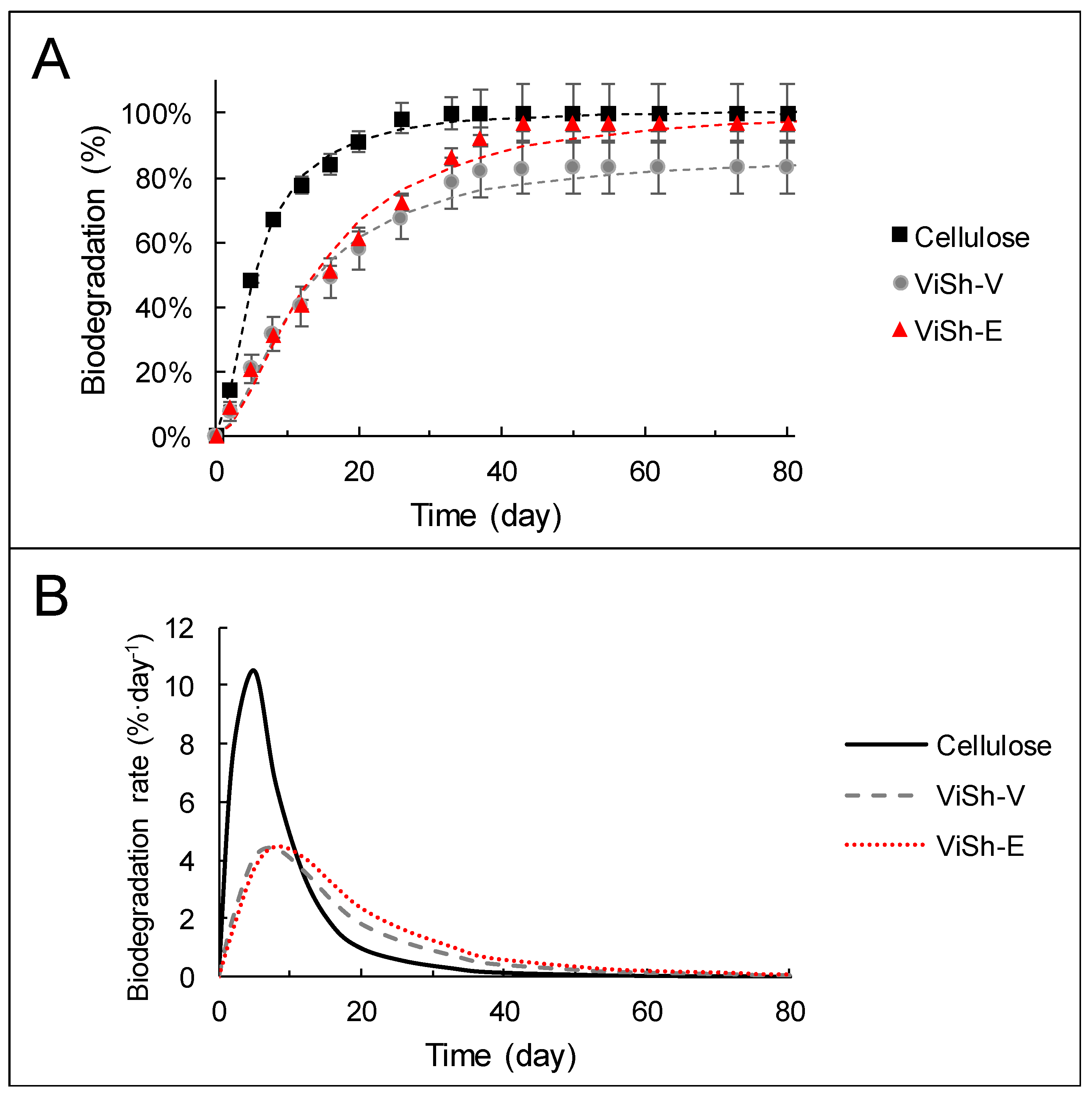
2.1. Biodegradability of Vine Shoots (ViSh) Fillers
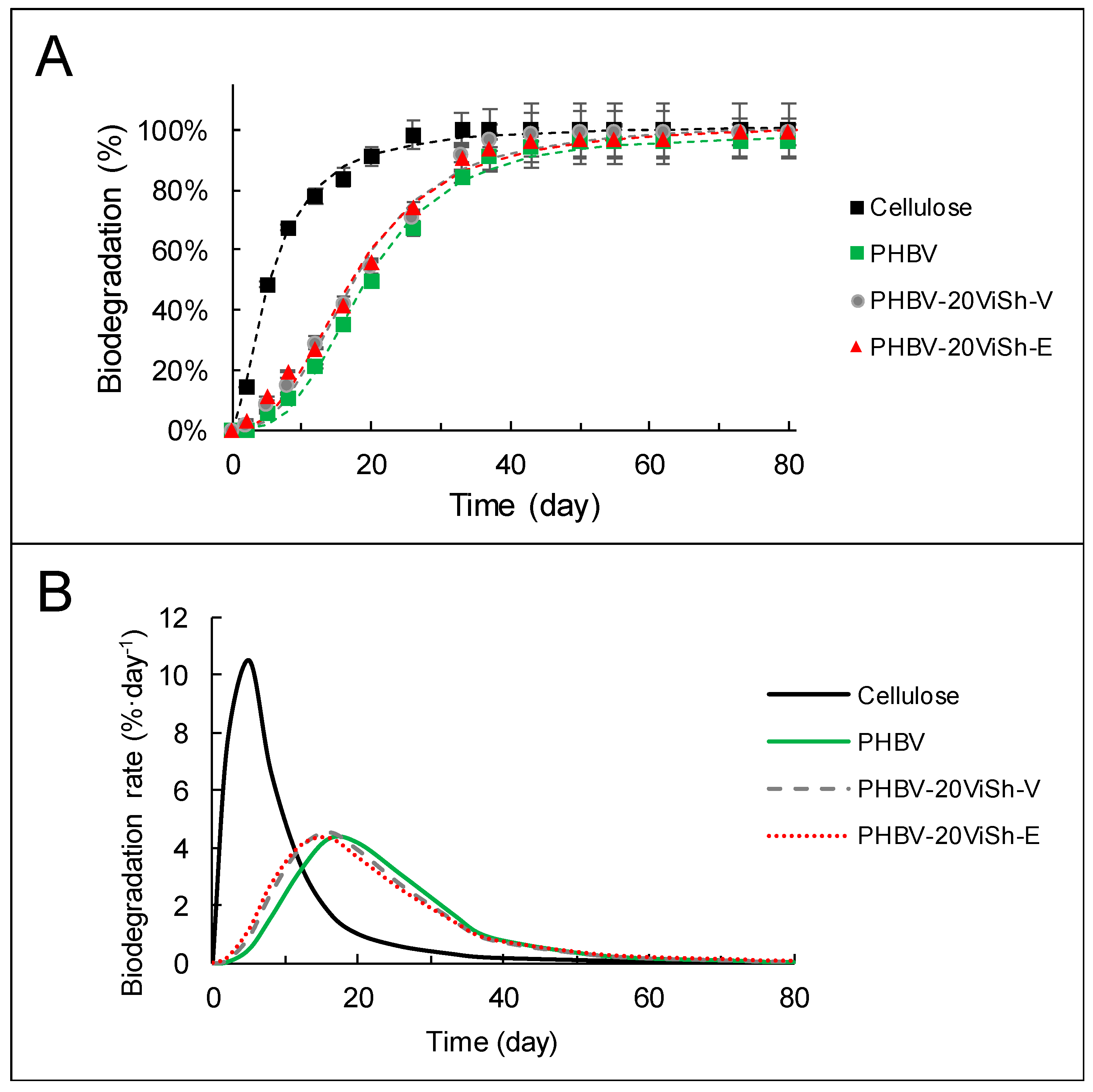
2.2. Biodegradability of PHBV-Based Composites
3. Materials and Methods
3.1. Materials
3.2. Preparation of Vine Shoot Fillers
3.3. Wide Angle X-Ray Diffraction (XRD)
3.4. Preparation of PHBV/ViSh Biocomposites
3.5. Scanning Electron Microscopy (SEM)
3.6. Biodegradation Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PHBV | Poly(3-HydroxyButyrate-3-hydroxyValerate) |
| ViSh | Vine shoots |
| ViSh-V | Vine shoots that are virgin, not exhausted |
| ViSh-E | Vine shoots that are exhausted |
References
- PlasticsEurope. Plastics—the Facts 2018; PlasticsEurope: Brussels, Belgium, 2018. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef] [PubMed]
- European Commission’s Directorate-General for Research and Innovation. A Circular Economy for Plastics, Insights from Research and Innovation to Inform Policy and Funding Decisions; EU Publications: Luxembourg, 2019. [Google Scholar]
- Ellen MacArthur Foundation. The New Plastics Economy: Rethinking the Future of Plastics; Ellen MacArthur Foundation: Cowes, UK, 2016. [Google Scholar]
- European Bioplastics. Nova-Institute Global Production of Bioplastics. 2018. Available online: https://www.european-bioplastics.org/market/ (accessed on 12 December 2019).
- Jost, V. Packaging related properties of commercially available biopolymers - An overview of the status quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, Y.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHBV films in a pilot-scale composting condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Salomez, M.; George, M.; Fabre, P.; Touchaleaume, F.; Cesar, G.; Lajarrige, A.; Gastaldi, E. A comparative study of degradation mechanisms of PHBV and PBSA under laboratory-scale composting conditions. Polym. Degrad. Stab. 2019, 167, 102–113. [Google Scholar] [CrossRef]
- Mergaert, J.; Webb, A.; Anderson, C.; Wouters, A.; Swings, J. Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in soils. Appl. Environ. Microbiol. 1993, 59, 3233–3238. [Google Scholar]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J.; Kersters, K. Biodegradation of polyhydroxyalkanoates. FEMS Microbiol. Lett. 1992, 103, 317–321. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Galanakis, C.M. Handbook of Grape Processing by-products; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-809870-7. [Google Scholar]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crop. Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Karacabey, E.; Mazza, G.; Bayindirli, L.; Artik, N. Extraction of Bioactive Compounds from Milled Grape Canes (Vitis vinifera) Using a Pressurized Low-Polarity Water Extractor. Food Bioprocess Technol. 2012, 5, 359–371. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-shoot waste aqueous extracts for re-use in agriculture obtained by different extraction techniques: Phenolic, volatile, and mineral compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Gontard, N.; Angellier-Coussy, H. Assessing the potential of vine shoots particles as fillers in biopolyester based biocomposites. In Proceedings of the Polymer Blends Conference 2019, Palermo, Italy, 24 April 2019. [Google Scholar]
- David, G.; Heux, L.; Pradeau, S.; Gontard, N.; Angellier-Coussy, H. Upcycling vine shoots for biocomposites applications: About the interest of filler surface esterification to improve their performance. Unpublished work. 2019. [Google Scholar]
- Casarin, S.A.; Rodrigues, C.P.; Souza Júnior, O.F.d.; Rosário, F.; Agnelli, J.A.M. Biodegradation in Soil of the PHB/Wood Flour (80/20) and PHB/Sisal Fiber (80/20) Tubes. Mater. Res. 2017, 20, 47–50. [Google Scholar] [CrossRef][Green Version]
- Lammi, S.; Gastaldi, E.; Gaubiac, F.; Angellier-Coussy, H. How olive pomace can be valorized as fillers to tune the biodegradation of PHBV based composites. Polym. Degrad. Stab. 2019, 166, 325–333. [Google Scholar] [CrossRef]
- Cinelli, P.; Seggiani, M.; Mallegni, N.; Gigante, V.; Lazzeri, A. Processability and degradability of PHA-based composites in terrestrial environments. Int. J. Mol. Sci. 2019, 20, 284. [Google Scholar] [CrossRef]
- Chan, C.M.; Vandi, L.J.; Pratt, S.; Halley, P.; Richardson, D.; Werker, A.; Laycock, B. Insights into the biodegradation of PHA/wood composites: Micro- and macroscopic changes. Sustain. Mater. Technol. 2019, 21, e00099. [Google Scholar] [CrossRef]
- Avella, M.; La Rota, G.; Martuscelli, E.; Raimo, M.; Sadocco, P.; Elegir, G.; Riva, R. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: Thermal, mechanical properties and biodegradation behaviour. J. Mater. Sci. 2000, 35, 829–836. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J.; Tsunoda, K. Effects of heartwood extractive fractions of Thuja plicata and Chamaecyparis nootkatensis on wood degradation by termites or fungi. J. Wood Sci. 2006, 52, 147–153. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Santana, A.L.B.D.; Maranhão, C.A.; de Miranda, R.D.C.M.; Galvão de Lima, V.L.A.; da Silva, S.I.; Nascimento, M.S.; Bieber, L. Natural resistance of five woods to Phanerochaete chrysosporium degradation. Int. Biodeterior. Biodegrad. 2010, 64, 711–715. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Santana, A.L.B.D.; Maranhão, C.A.; Oliveira, L.S.; Bieber, L. Phenolic Extractives and Natural Resistance of Wood. In Biodegradation—Life of Science; Intechopen: London, UK, 2013; pp. 349–370. [Google Scholar]
- De Bertoldi, M.; Vallini, G.; Pera, A. The Biology of Composting: A Review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. Empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bastioli, C. Handbook of Biodegradable Polymers; Smithers Rapra Press: Akron, OH, USA, 2005. [Google Scholar]
- Mais, U.; Esteghlalian, A.R.; Saddler, J.N.; Mansfield, S.D. Enhancing the Enzymatic Hydrolysis of Cellulosic Materials Using Simultaneous Ball Milling. In Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 2002; pp. 815–832. [Google Scholar]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar] [PubMed]
- Cooperband, L. The Art and Science of Composting: A Resource for Farmers and Compost Producers; University of Wisconsin: Madison, WI, USA, 2002. [Google Scholar]
- Scalbert, A. Tannins in Woods and Their Contribution to Microbial Decay Prevention. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 935–952. [Google Scholar]
- Saxena, R.K.; Sharmila, P.; Singh, V.P. Microbial degradation of tannins. Prog. Ind. Microbiol. 1995, 32, 259–270. [Google Scholar] [CrossRef]
- Riedewald, F. Bacterial adhesion to surfaces: The influence of surfaces roughness. PDA J. Pharm. Sci. Technol. 2006, 60, 164–171. [Google Scholar] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- David, G. Eco-Conversion of Agricultural Lignocellulosic Residues into Bioplyester-Based Composite Materials; University of Montpellier: Montpellier, France, 2019. [Google Scholar]
- Nicholson, D.J.; Leavitt, A.T.; Francis, R.C. A Three-Stage Klason Method for More Accurate Determinations of Hardwood Lignin Content. Cellul. Chem. Technol 2014, 48, 53–59. [Google Scholar]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein - Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Roumeas, L.; Aouf, C.; Dubreucq, E.; Fulcrand, H. Depolymerisation of condensed tannins in ethanol as a gateway to biosourced phenolic synthons. Green Chem. 2013, 15, 3268–3275. [Google Scholar] [CrossRef]
- Chevillard, A.; Angellier-Coussy, H.; Guillard, V.; Gontard, N.; Gastaldi, E. Investigating the biodegradation pattern of an ecofriendly pesticide delivery system based on wheat gluten and organically modified montmorillonites. Polym. Degrad. Stab. 2012, 97, 2060–2068. [Google Scholar] [CrossRef]

| Sample | Klason Lignin | Ashes | Tannins | Resveratrol | Proteins | C | N |
|---|---|---|---|---|---|---|---|
| ViSh-V | 19.4 ± 0.5 | 3.9 ± 0.2 | 1.25 ± 0.07 | 0.07 ± 0.01 | 3.3 | 46.05 | 0.53 |
| ViSh-E | 17.7 ± 0.5 | 4.7 ± 0.5 | 0.41 ± 0.07 | 0.01 ± 0.00 | 1.7 | 45.96 | 0.27 |
| WVP (×1013 mol·m/(m2·s·Pa) | Young’s Modulus (GPa) | PHBV Crystallinity (%) | |
|---|---|---|---|
| PHBV | 4.3 ± 1.3 | 2.16 ± 0.02 | 55.9 |
| PHBV-20ViSh-V | 14.0 ± 1.6 | 2.50 ± 0.03 | 52.0 |
| PHBV-20ViSh-E | 21.1 ± 2.0 | 2.43 ± 0.04 | 55.4 |
| Hill Parameters | Timerate max (day) | Degrate max (%∙day−1) | ||||
|---|---|---|---|---|---|---|
| Degmax (%) | k (day) | n | R2 | |||
| Cellulose | 101 (±1) | 5.5 (±0.1) | 1.7 (±0.1) | 0.99 | 5 | 10.5 |
| ViSh-V | 87 (±2) | 11.8 (±0.6) | 1.7 (±0.1) | 0.98 | 8 | 4.5 |
| ViSh-E | 102 (±2) | 13.9 (±0.8) | 1.8 (±0.2) | 0.98 | 8 | 4.3 |
| PHBV | 98 (±1) | 19.1 (±0.4) | 3.1 (±0.2) | 0.99 | 16 | 4.3 |
| PHBV-20ViSh-V | 102 (±1) | 17.6 (±0.5) | 2.8 (±0.1) | 0.99 | 16 | 4.4 |
| PHBV-20ViSh-E | 102 (±1) | 17.3 (±0.5) | 2.6 (±0.2) | 0.99 | 16 | 4.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, G.; Michel, J.; Gastaldi, E.; Gontard, N.; Angellier-Coussy, H. How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites. Int. J. Mol. Sci. 2020, 21, 228. https://doi.org/10.3390/ijms21010228
David G, Michel J, Gastaldi E, Gontard N, Angellier-Coussy H. How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites. International Journal of Molecular Sciences. 2020; 21(1):228. https://doi.org/10.3390/ijms21010228
Chicago/Turabian StyleDavid, Grégoire, Julie Michel, Emmanuelle Gastaldi, Nathalie Gontard, and Hélène Angellier-Coussy. 2020. "How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites" International Journal of Molecular Sciences 21, no. 1: 228. https://doi.org/10.3390/ijms21010228
APA StyleDavid, G., Michel, J., Gastaldi, E., Gontard, N., & Angellier-Coussy, H. (2020). How Vine Shoots as Fillers Impact the Biodegradation of PHBV-Based Composites. International Journal of Molecular Sciences, 21(1), 228. https://doi.org/10.3390/ijms21010228





