The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer
Abstract
1. Introduction
2. History of Bacteria Identification
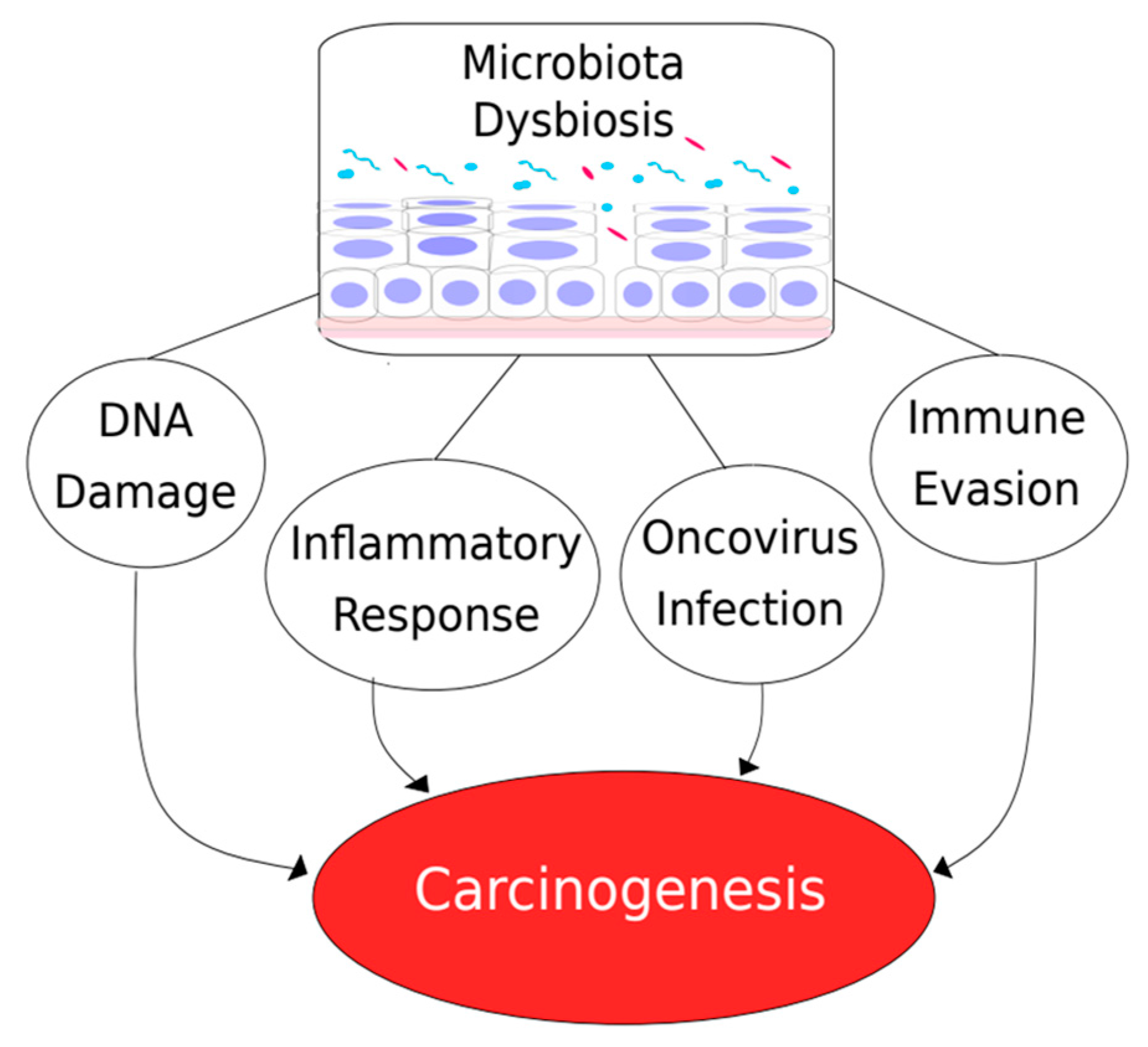
3. Microbiome and Cancer
4. Cervicovaginal Microbiome
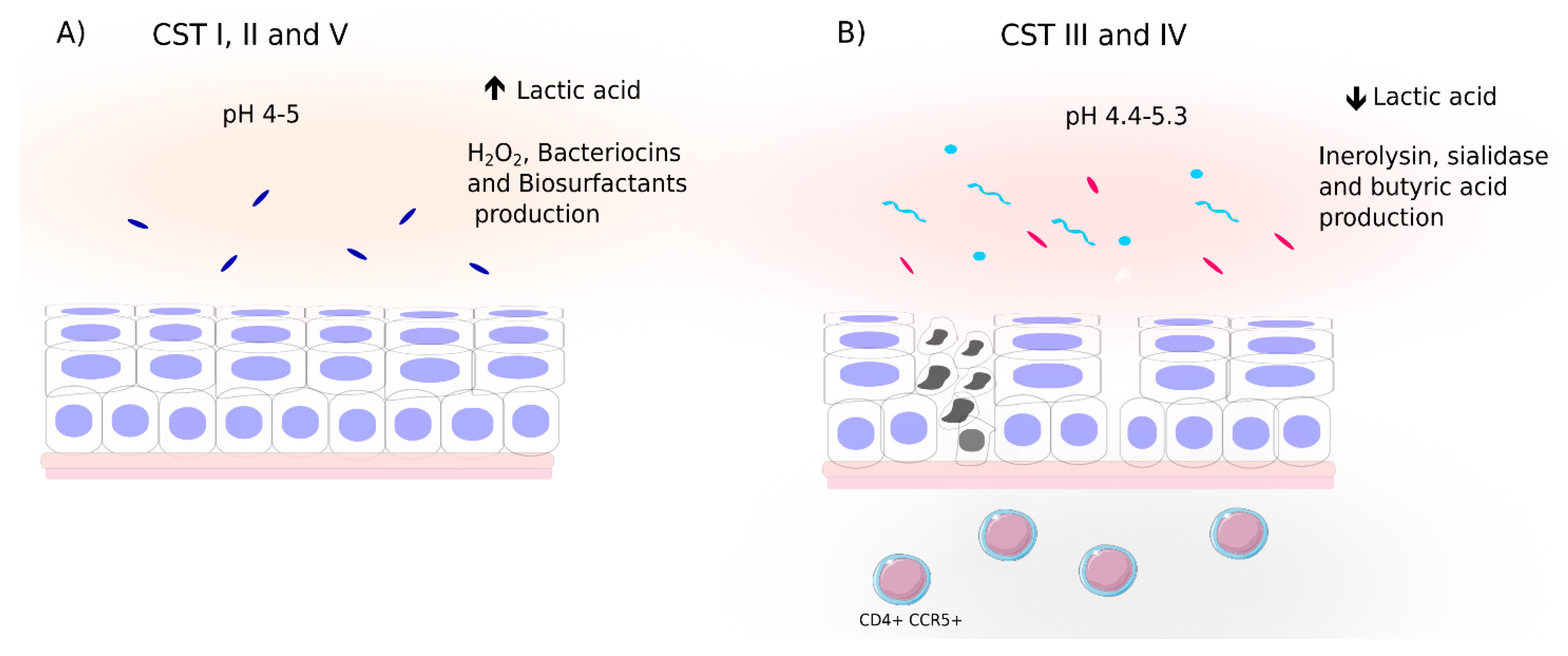
4.1. Cervicovaginal Bacterial Composition and Profiles
4.2. Cervicovaginal Microbiota and Relationship to Viral Infection
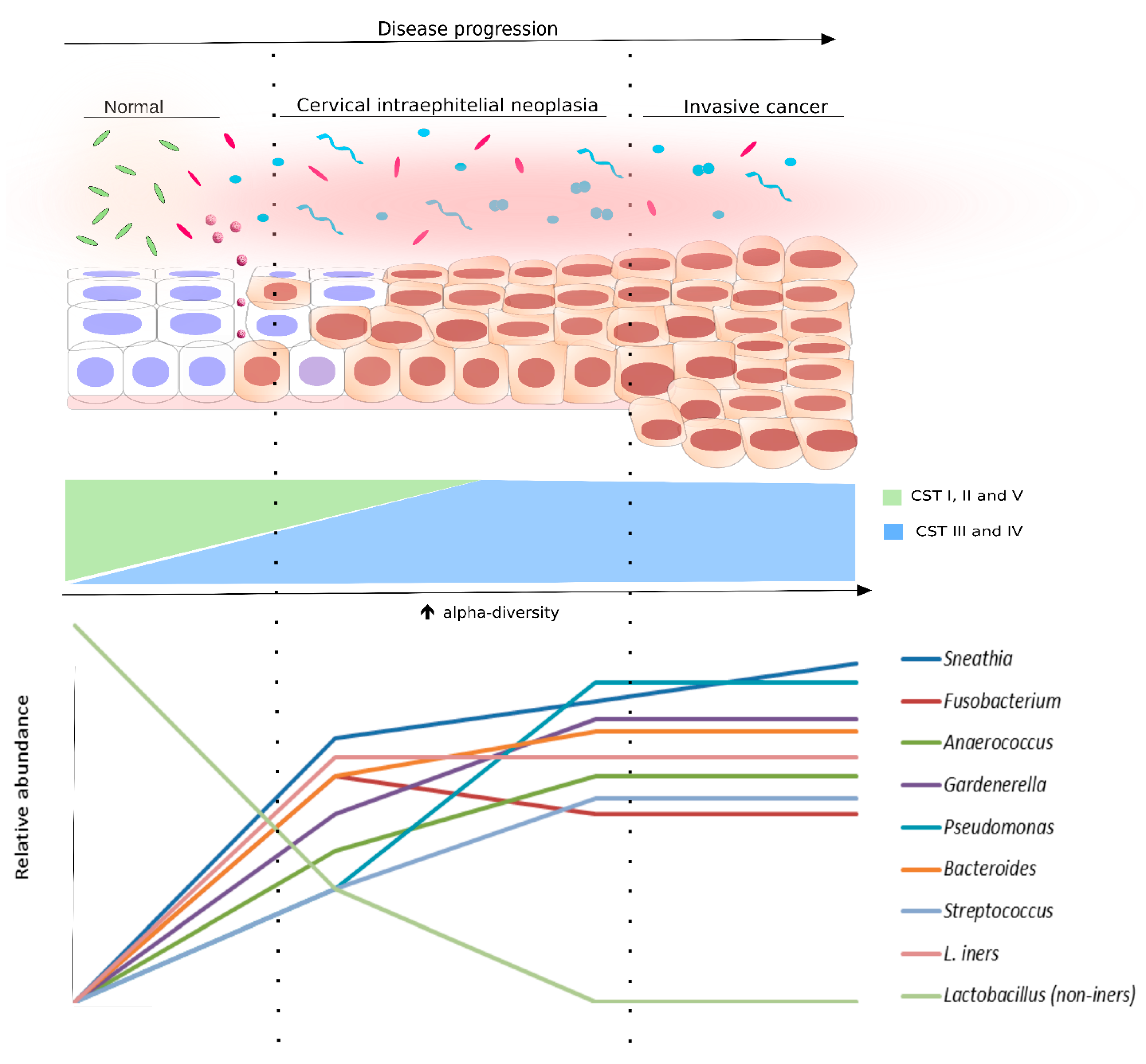
5. Microbiota as a Biomarker for HPV and Cervical Dysplasia
6. Clinical Molecular Diagnostics
7. Probiotics and Microbiota Manipulation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CIN | cervical intraepithelial neoplasia |
| HPV | human papillomavirus |
| CC | cervical cancer |
| lr-HPV | low-risk HPV |
| hr-HPV | high-risk HPV |
| HMC | Human Microbiome Project |
| MSM | men who have sex with men |
| DSB | DNA double-strand breaks |
| CDT | cytolethal distending toxin |
| CRC | colorectal cancer |
| TLR4 | toll-like receptor 4 |
| GGT | γ-glutamyl transpeptidase |
| Treg | regulatory T-cell |
| EBV | Epstein-Barr virus |
| KSHV | Kaposi’s sarcoma herpesvirus |
| HHV-8 | human herpesvirus humano type 8 |
| 16S-HTS | 16S rRNA high-throughput sequencing |
| CST | community state type |
| LEEP | Loop Electrosurgical Excision Procedure |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.-M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; Zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Clifford, G.M.; Smith, J.S.; Aguado, T.; Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: A meta-analysis. Br. J. Cancer 2003, 89, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Braaten, K.P.; Laufer, M.R. Human Papillomavirus (HPV), HPV-Related Disease, and the HPV Vaccine. Rev. Obstet. Gynecol. 2008, 1, 2–10. [Google Scholar] [PubMed]
- Shulzhenko, N.; Lyng, H.; Sanson, G.F.; Morgun, A. Mé nage à trois: An evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 2014, 22, 345–353. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martínez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; Costa, R.L.; Siqueira, J.D.; Meyrelles, A.I.; Machado, E.S.; Soares, E.A.; Soares, M.A. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci. Rep. 2017, 7, 17364. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, B.-S.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Park, S.-Y.; Hong, K.-M.; Kim, H.-K.; Kim, M.K. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, e1-674. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wu, S.; Sukumaran, J.; Rodrigo, A. Models of microbiome evolution incorporating host and microbial selection. Microbiome 2017, 5, 127. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the ‘golden age’ of bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef]
- Hugenholtz, P.; Pace, N.R. Identifying microbial diversity in the natural environment: A molecular phylogenetic approach. Trends Biotechnol. 1996, 14, 190–197. [Google Scholar] [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [PubMed]
- Cho, I.; Blaser, M.J. Applications of Next-Generation Sequencing: The human microbiome: At the interface of health and disease. Nat. Publ. Gr. 2012, 13, 260. [Google Scholar]
- McGuire, A.L.; Colgrove, J.; Whitney, S.N.; Diaz, C.M.; Bustillos, D.; Versalovic, J. Ethical, legal, and social considerations in conducting the Human Microbiome Project. Genome Res. 2008, 18, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Hashem, A.; Allah, E.F.A. Exploring the human microbiome: The potential future role of next-generation sequencing in disease diagnosis and treatment. Front. Immunol. 2019, 10, 2868. [Google Scholar] [CrossRef]
- Di Bella, J.M.; Bao, Y.; Gloor, G.B.; Burton, J.P.; Reid, G. High throughput sequencing methods and analysis for microbiome research. J. Microbiol. Methods 2013, 95, 401–414. [Google Scholar] [CrossRef]
- Joshua Lederberg ’Ome Sweet ’Omics-A Genealogical Treasury of Words | The Scientist Magazine®. Available online: http: //www.the-scientist.com/?articles.view/articleNo/13313/title/-Ome-Sweet--Omics---A-Genealogical-Treasury-of-Words/ (accessed on 16 May 2019).
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The Human Microbiome and Cancer. Cancer Prev. Res. 2017, 10, 226–234. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reida, G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Peck, K.N.; DeMichele, A.M.; Alwine, J.C.; Robertson, E.S. Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 2018, 9, 951. [Google Scholar] [CrossRef]
- Porter, C.M.; Shrestha, E.; Peiffer, L.B.; Sfanos, K.S. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; White, J.R.; Goros, M.; Gelfond, J.; Leach, R.; Johnson-Pais, T.; Lai, Z.; Rourke, E.; Basler, J.; Ankerst, D.; et al. Metabolic Biosynthesis Pathways Identified from Fecal Microbiome Associated with Prostate Cancer. Eur. Urol. 2018, 74, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.C.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.A.; Lhakhang, T.; Olsen, E.; et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Youssef, O.; Lahti, L.; Kokkola, A.; Karla, T.; Tikkanen, M.; Ehsan, H.; Carpelan-Holmström, M.; Koskensalo, S.; Böhling, T.; Rautelin, H.; et al. Stool Microbiota Composition Differs in Patients with Stomach, Colon, and Rectal Neoplasms. Dig. Dis. Sci. 2018, 63, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Corning, B.; Copland, A.P.; Frye, J.W. The Esophageal Microbiome in Health and Disease. Curr. Gastroenterol. Rep. 2018, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Noto, J.M.; Peek, R.M. The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer. PLOS Pathog. 2017, 13, e1006573. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Błasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 3456–3467. [Google Scholar] [CrossRef]
- Mert, I.; Walther-Antonio, M.; Mariani, A. Case for a role of the microbiome in gynecologic cancers: Clinician’s perspective. J. Obstet. Gynaecol. Res. 2018, 44, 1693–1704. [Google Scholar] [CrossRef]
- Walther-António, M.R.S.; Chen, J.; Multinu, F.; Hokenstad, A.; Distad, T.J.; Cheek, E.H.; Keeney, G.L.; Creedon, D.J.; Nelson, H.; Mariani, A.; et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Coukos, G.; Alwine, J.C.; Robertson, E.S. The ovarian cancer oncobiome. Oncotarget 2017, 8, 36225. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Macintyre, D.; Lee, Y.; Smith, A.; Marchesi, J.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.; et al. Characterisation of the vaginal microbiome in cervical intraepithelial neoplasia. The Lancet 2016, 387, S75. [Google Scholar] [CrossRef][Green Version]
- Goodman, B.; Gardner, H. The microbiome and cancer. J. Pathol. 2018, 244, 667–676. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Nougayrede, J.-P. Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Dalmasso, G.; Cougnoux, A.; Delmas, J.; Darfeuille-Michaud, A.; Bonnet, R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes 2015, 5, 675–680. [Google Scholar] [CrossRef]
- Nešić, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429–433. [Google Scholar] [CrossRef]
- Graillot, V.; Dormoy, I.; Dupuy, J.; Shay, J.W.; Huc, L.; Mirey, G.; Vignard, J. Genotoxicity of Cytolethal Distending Toxin (CDT) on Isogenic Human Colorectal Cell Lines: Potential Promoting Effects for Colorectal Carcinogenesis. Front. Cell. Infect. Microbiol. 2016, 6, 34. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Österreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Arthur, J.C.; Gharaibeh, R.Z.; Mühlbauer, M.; Perez-Chanona, E.; Uronis, J.M.; McCafferty, J.; Fodor, A.A.; Jobin, C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 2014, 5, 4727. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Shang, L.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; España, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G.; et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm. Bowel Dis. 2011, 17, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Hsu, D.; Xu, R.; Harpaz, N.; Dannenberg, A.J.; et al. Toll-Like Receptor-4 Promotes the Development of Colitis-Associated Colorectal Tumors. Gastroenterology 2007, 133, 1869. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Schmees, C.; Prinz, C.; Treptau, T.; Rad, R.; Hengst, L.; Voland, P.; Bauer, S.; Brenner, L.; Schmid, R.M.; Gerhard, M. Inhibition of T-Cell Proliferation by Helicobacter pylori γ-Glutamyl Transpeptidase. Gastroenterology 2007, 132, 1820–1833. [Google Scholar] [CrossRef]
- Mima, K.; Sukawa, Y.; Nishihara, R.; Qian, Z.R.; Yamauchi, M.; Inamura, K.; Kim, S.A.; Masuda, A.; Nowak, J.A.; Nosho, K.; et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015, 1, 653. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Beswick, E.J.; Pinchuk, I.V.; Das, S.; Powell, D.W.; Reyes, V.E. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect. Immun. 2007, 75, 4334–4341. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Asmar, N.; Ibrahim, T.; Rey, J.-F. Checkpoint Inhibitors: Conquering Cancer with a Little (T)-Help from Our Microbial Friends. Dig. Dis. Sci. 2018, 63, 2177–2179. [Google Scholar] [CrossRef]
- Adachi, K.; Tamada, K. Microbial biomarkers for immune checkpoint blockade therapy against cancer. J. Gastroenterol. 2018, 53, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F.; Jefferson, K.K.; Buck, G.A. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014, 160, 2272. [Google Scholar] [CrossRef] [PubMed]
- Van de Wijgert, J.H.H.M.; Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017, 168, 859–864. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Creedon, D.J.; Nelson, K.E.; Wilson, B.A. The vaginal microbiome in health and disease. Trends Endocrinol. Metab. 2011, 22, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.H. The Microbiota of the Vagina and Its Influence on Women’s Health and Disease. Am. J. Med. Sci. 2012, 343, 2–9. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 2011, 193, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Macklaim, J.M.; Gloor, G.B.; Anukam, K.C.; Cribby, S.; Reid, G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. USA 2011, 108, 4688–4695. [Google Scholar] [CrossRef]
- Vyshenska, D.; Lam, K.C.; Shulzhenko, N.; Morgun, A. Interplay between viruses and bacterial microbiota in cancer development. Semin. Immunol. 2017, 32, 14–24. [Google Scholar] [CrossRef]
- McClelland, R.S.; Lingappa, J.R.; Srinivasan, S.; Kinuthia, J.; John-Stewart, G.C.; Jaoko, W.; Richardson, B.A.; Yuhas, K.; Fiedler, T.L.; Mandaliya, K.N.; et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: A nested case-control study. Lancet Infect. Dis. 2018, 18, 554–564. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.-M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the Vaginal Microbiota with Human Papillomavirus Infection in a Korean Twin Cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Heal. 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; van de Wijgert, J.H. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef]
- Von Nicolai, H.; Hammann, R.; Salehnia, S.; Zilliken, F. A newly discovered sialidase from Gardnerella vaginalis. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1984, 258, 20–26. [Google Scholar] [CrossRef]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ochiai, K. Effect of microbial coinfection with HIV-1 and butyric acid-producing anaerobic bacteria on AIDS progression. J. Oral Biosci. 2013, 55, 55–60. [Google Scholar] [CrossRef]
- Fernandes, J.; Fernandes, T.; de Azevedo, J.; Cobucci, R.; de Carvalho, M.; Andrade, V.; de Ara�jo, J. Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol. Lett. 2015, 9, 1015–1026. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Afiuni-Zadeh, S.; Boylan, K.L.M.; Jagtap, P.D.; Griffin, T.J.; Rudney, J.D.; Peterson, M.L.; Skubitz, A.P.N. Evaluating the potential of residual Pap test fluid as a resource for the metaproteomic analysis of the cervical-vaginal microbiome. Sci. Rep. 2018, 8, 10868. [Google Scholar] [CrossRef]
- Zegels, G.; Van Raemdonck, G.A.A.; Coen, E.P.; Tjalma, W.A.A.; Van Ostade, X.W.M. Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome Sci. 2009, 7, 17. [Google Scholar] [CrossRef]
- Van Ostade, X.; Dom, M.; Tjalma, W.; Van Raemdonck, G. Candidate biomarkers in the cervical vaginal fluid for the (self-) diagnosis of cervical precancer. Arch. Gynecol. Obstet. 2018, 297, 295–311. [Google Scholar] [CrossRef]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; van de Wijgert, J.H.H.M. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef]
- Yim, E.-K.; Park, J.-S. Biomarkers in cervical cancer. Biomark. Insights 2007, 1, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabuddhe, V.V.; Luhn, P.; Wentzensen, N. Human papillomavirus and cervical cancer: Biomarkers for improved prevention efforts. Future Microbiol. 2011, 6, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, M.L.; Buonaguro, L.; Giorgi-Rossi, P.; Buonaguro, F.M. Viral and cellular biomarkers in the diagnosis of cervical intraepithelial neoplasia and cancer. Biomed. Res. Int. 2013, 2013, 519619. [Google Scholar] [CrossRef] [PubMed]
- Dillner, J.; Rebolj, M.; Birembaut, P.; Petry, K.-U.; Szarewski, A.; Munk, C.; de Sanjose, S.; Naucler, P.; Lloveras, B.; Kjaer, S.; et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European cohort study. BMJ 2008, 337, a1754. [Google Scholar] [CrossRef] [PubMed]
- The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. Available online: https://www.cancer.org/cancer/cervical-cancer/prevention-and-early-detection/cervical-cancer-screening-guidelines.html (accessed on 29 May 2019).
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 2013, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Shannon, B.; Yi, T.J.; Perusini, S.; Gajer, P.; Ma, B.; Humphrys, M.S.; Thomas-Pavanel, J.; Chieza, L.; Janakiram, P.; Saunders, M.; et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal. Immunol. 2017, 10, 1310–1319. [Google Scholar] [CrossRef]
- Campisciano, G.; Gheit, T.; De Seta, F.; Cason, C.; Zanotta, N.; Delbue, S.; Ricci, G.; Ferrante, P.; Tommasino, M.; Comar, M. Oncogenic virome benefits from the different vaginal microbiome-immune axes. Microorganisms 2019, 7, 414. [Google Scholar] [CrossRef]
- Das Purkayastha, S.; Bhattacharya, M.K.; Prasad, H.K.; Upadhyaya, H.; Das Lala, S.; Pal, K.; Das, M.; Sharma, G.D.; Bhattacharjee, M.J. Contrasting diversity of vaginal lactobacilli among the females of Northeast India. BMC Microbiol. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Łaniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 7593. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Gao, W.; Pan, Y.; Gao, Y.; Shen, J.; Xiong, H. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med. 2018, 7, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, J.; Lu, Y.; Cai, Q.; Liu, H.; Xu, C. Cervical microbiome is altered in cervical intraepithelial neoplasia after loop electrosurgical excision procedure in china. Sci. Rep. 2018, 8, 4923. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Adler, D.; Wallace, M.; Bennie, T.; Abar, B.; Sadeghi, R.; Meiring, T.; Williamson, A.-L.; Bekker, L.-G. High risk human papillomavirus persistence among HIV-infected young women in South Africa. Int. J. Infect. Dis. 2015, 33, 219–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Vuyst, H.; Lillo, F.; Broutet, N.; Smith, J.S. HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. Eur. J. Cancer Prev. 2008, 17, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Serraino, D.; Carrieri, P.; Pradier, C.; Bidoli, E.; Dorrucci, M.; Ghetti, E.; Schiesari, A.; Zucconi, R.; Pezzotti, P.; Dellamonica, P.; et al. Risk of invasive cervical cancer among women with, or at risk for, HIV infection. Int. J. Cancer 1999, 82, 334–337. [Google Scholar] [CrossRef]
- Price, J.T.; Vwalika, B.; Hobbs, M.; Nelson, J.A.E.; Stringer, E.M.; Zou, F.; Rittenhouse, K.J.; Azcarate-Peril, A.; Kasaro, M.P.; Stringer, J.S.A. Highly diverse anaerobe-predominant vaginal microbiota among HIV-infected pregnant women in Zambia. PLoS ONE 2019, 14, e0223128. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Kooistra-Smid, A.M.D.; Raangs, E.C.; Rosema, S.; Veloo, A.C.M.; et al. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.-J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef]
- Hildebrand, F.; Tadeo, R.; Voigt, A.Y.; Bork, P.; Raes, J. LotuS: An efficient and user-friendly OTU processing pipeline. Microbiome 2014, 2, 30. [Google Scholar] [CrossRef]
- Robertson, C.E.; Harris, J.K.; Wagner, B.D.; Granger, D.; Browne, K.; Tatem, B.; Feazel, L.M.; Park, K.; Pace, N.R.; Frank, D.N. Explicet: Graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics 2013, 29, 3100–3101. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis: Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar]
- Fredricks, D.N.; Fiedler, T.L.; Thomas, K.K.; Oakley, B.B.; Marrazzo, J.M. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J. Clin. Microbiol. 2007, 45, 3270–3276. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Smith, W.L.; Chadwick, S.G.; Toner, G.; Mordechai, E.; Adelson, M.E.; Aguin, T.J.; Sobel, J.D.; Gygax, S.E. Development and Validation of a Highly Accurate Quantitative Real-Time PCR Assay for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 2016, 54, 1017–1024. [Google Scholar] [CrossRef]
- Coleman, J.S.; Gaydos, C.A. Molecular Diagnosis of Bacterial Vaginosis: An Update. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Van der Veer, C.; van Houdt, R.; van Dam, A.; de Vries, H.; Bruisten, S. Accuracy of a commercial multiplex PCR for the diagnosis of bacterial vaginosis. J. Med. Microbiol. 2018, 67, 1265–1270. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Achilles, S.L.; Bradshaw, C.S.; Burgener, A.; Crucitti, T.; Fredricks, D.N.; Jaspan, H.B.; Kaul, R.; Kaushic, C.; Klatt, N.; et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS Res. Hum. Retroviruses 2019, 35, 219–228. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Womens. Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017, 30, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9251055130. [Google Scholar]
- Reid, G. The development of probiotics for women’s health. Can. J. Microbiol. 2016, 63, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefanski, G.; Mikolajczyk-Cichonska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebocontrolled trial. BMC Womens Health 2015, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Benedetti Panici, P. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016, 293, 101–107. [Google Scholar] [CrossRef]
- McMillan, A.; Rulisa, S.; Gloor, G.B.; Macklaim, J.M.; Sumarah, M.; Reid, G. Pilot assessment of probiotics for pregnant women in Rwanda. PLoS ONE 2018, 13, e0195081. [Google Scholar] [CrossRef]
- Verdenelli, M.C.; Cecchini, C.; Coman, M.M.; Silvi, S.; Orpianesi, C.; Coata, G.; Cresci, A.; Di Renzo, G.C. Impact of Probiotic SYNBIO®Administered by Vaginal Suppositories in Promoting Vaginal Health of Apparently Healthy Women. Curr. Microbiol. 2016, 73, 483–490. [Google Scholar] [CrossRef]
- Tomusiak, A.; Strus, M.; Heczko, P.B.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: A multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des. Devel. Ther. 2015, 9, 5345–5354. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Pires, M.C.V.; Leão, T.L.; Hernández, Z.P.; Rodriguez, M.L.; Martins, A.K.S.; Miranda, L.S.; Martins, F.S.; Nicoli, J.R. Selection of Lactobacillus strains as potential probiotics for vaginitis treatment. Microbiol 2016, 162, 1195–1207. [Google Scholar] [CrossRef]
- Ouarabi, L.; Chait, Y.A.; Seddik, H.A.; Drider, D.; Bendali, F. Newly Isolated Lactobacilli strains from Algerian Human Vaginal Microbiota: Lactobacillus fermentum Strains Relevant Probiotic’s Candidates. Probiotics Antimicrob. Proteins 2017, 11, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Domig, K.J.; Kiss, H.; Petricevic, L.; Viernstein, H.; Unger, F.; Kneifel, W. Strategies for the evaluation and selection of potential vaginal probiotics from human sources: An exemplary study. Benef. Microb. 2014, 5, 3920. [Google Scholar] [CrossRef] [PubMed]
- Laue, C.; Papazova, E.; Liesegang, A.; Pannenbeckers, A.; Arendarski, P.; Linnerth, B.; Domig, K.J.; Kneifel, W.; Petricevic, L.; Schrezenmeir, J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women-a double-blind, randomised, controlled clinical pilot trial. Benef. Microbes 2018, 9, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Shirzad, M.; Raoofian, R.; Hasheminasab, S.-M.; Hatami, M.; Dianatpour, M.; Modarressi, M.-H. Differences in Vaginal Lactobacilli Composition of Iranian Healthy and Bacterial Vaginosis Infected Women: A Comparative Analysis of Their Cytotoxic Effects with Commercial Vaginal Probiotics. Iran. Red Crescent Med. J. 2013, 15, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Motevaseli, E.; Shirzad, M.; Akrami, S.M.; Mousavi, A.S.; Mirsalehian, A.; Modarressi, M.H. Normal and Tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013, 62, 1065–1072. [Google Scholar] [CrossRef]
- Verhoeven, V.; Renard, N.; Makar, A.; Van Royen, P.; Bogers, J.-P.; Lardon, F.; Peeters, M.; Baay, M. Probiotics enhance the clearance of human papillomavirus-related cervical lesions. Eur. J. Cancer Prev. 2012, 22, 46–51. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef]
- Sánchez, M.T.; Ruiz, M.A.; Castán, H.; Morales, M.E. A novel double-layer mucoadhesive tablet containing probiotic strain for vaginal administration: Design, development and technological evaluation. Eur. J. Pharm. Sci. 2018, 112, 63–70. [Google Scholar] [CrossRef]
- Lagenaur, L.A.; Swedek, I.; Lee, P.P.; Parks, T.P. Robust vaginal colonization of macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS ONE 2015, 10, e0122730. [Google Scholar] [CrossRef]
- Reid, G. Has knowledge of the vaginal microbiome altered approaches to health and disease? F1000Research 2018, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, A.J.; Sim, K.; Deierl, A.; Kroll, J.S.; Brannigan, E.; Darby, J. “Vaginal seeding” of infants born by caesarean section. BMJ 2016, 352, i227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, I.; Corwin, E.J.; Brennan, P.A.; Jordan, S.; Murphy, J.R.; Dunlop, A. The Infant Microbiome: Implications for Infant Health and Neurocognitive Development. Nurs. Res. 2016, 65, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Castanys-Muñoz, E.; Martin, M.J.; Vazquez, E. Building a Beneficial Microbiome from Birth. Adv. Nutr. 2016, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. Lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Thavagnanam, S.; Fleming, J.; Bromley, A.; Shields, M.D.; Cardwell, C.R. A meta-analysis of the association between Caesarean section and childhood asthma. Clin. Exp. Allergy 2008, 38, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Pistiner, M.; Gold, D.R.; Abdulkerim, H.; Hoffman, E.; Celedón, J.C. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J. Allergy Clin. Immunol. 2008, 122, 274–279. [Google Scholar] [CrossRef]
- Huh, S.Y.; Rifas-Shiman, S.L.; Zera, C.A.; Edwards, J.W.R.; Oken, E.; Weiss, S.T.; Gillman, M.W. Delivery by caesarean section and risk of obesity in preschool age children: A prospective cohort study. Arch. Dis. Child. 2012, 97, 610–616. [Google Scholar] [CrossRef]
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; De Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Delong, K.; Bensouda, S.; Zulfiqar, F.; Zierden, H.C.; Hoang, T.M.; Abraham, A.G.; Coleman, J.S.; Cone, R.A.; Gravitt, P.E.; Hendrix, C.W.; et al. Conceptual design of a universal donor screening approach for vaginal microbiota transplant. Front. Cell. Infect. Microbiol. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Study | Treatment | Study Characteristics | Main Outcomes |
|---|---|---|---|
| Heczko et al., 2015 [129] | Oral metronidazole 500 mg twice daily for seven days together with an oral probiotic preparation (prOVag®) containing L. fermentum 57A, L. plantarum 57B and L. gasseri 57C twice daily for ten days. | 578 participants (118 receiving antibiotic together with prOVag and 241 treated with antibiotic together with placebo); Patients recruited from nine private gynaecological clinics in Poland; Women with history of recurrent BV/AV and with current symptoms were recruited and underwent five assessments. | Treatment with probiotics lengthened the time to a clinical relapse. The average time to a BV/AV relapse event was 71.4 days for women treated with prOVag and 47.3 days for the placebo group (p = 0.0125). Microbiologically confirmed BV/AV patients at visit V were significantly less frequent in the prOVag group (p = 0.04632). Nugent score achieved statistically significant differences between visits I and III, I and IV and IV and V for prOVag group. For placebo subjects, differences were found only between visits I and III, III and IV. |
| Recine et al., 2016 [130] | Oral metronidazole 500 mg twice a day for seven days together with vaginal tablets of L. rhamnosus BMX 54 (NORMOGIN®). Administration of the probiotic occured once a day for 10 days, twice a week for 15 days and once every 5 days for 7 months. | 250 participants (Group A: 125 women subjected to metronidazol alone and Group B: 125 patients receiving antibiotic together with probiotic). Women sexually active, non-pregnant and with BV diagnostic were recruited at University of Rome. Patients were assessed after 2, 6 and 9 months. | After 2 months of treatment, 90.4% of Group B patients showed BV clinical remission, compared to 79.4% in Group A subjects (p = 0.014). After 6 months, physiological vaginal microbiota was found in 74.6% of Group B participants, compared to 25.4% of Group A women (p < 0.0001). After 9 months, healthy microbiota were observed in 79.7% of Group B subjects, compared to 20.3% in Group A (p < 0.001). Vaginal pH was significantly higher in Group A compared to that of Group B at 6-month (p = 0.034) and at 9-month (p < 0.001) follow-ups. |
| Laue et al., 2018 [137] | 500 mg of oral metronidazole twice a day for seven days together with 125g yoghurt drink twice daily for 4 weeks. The yoghurt drink (verum) contained L. crispatus LbV 88, L. gasseri LbV 150N, L. jensenii LbV 116 and L. rhamnosus LbV 96 | 36 participants were randomly assigned to a metronidazole plus probiotic arm (n = 18) or a metronidazole plus placebo arm (n = 18). Women newly diagnosed with BV were recruited from Schleswig-Holstein region in Germany. | Post-intervention, all women receiving antibiotic plus probiotics showed recovery from BV, while 35.3% of patients after antibiotic plus placebo remained with the condition according to Amsel criteria (p = 0.018). Amsel score decreased by 3.41 ± 0.71 for the probiotic group compared to 1.94 ± 1.95 for placebo subjects (p = 0.037). Nugent score decreased by 4.65 ± 2.85 for probiotic subjects compared to 2.82 ± 3.59 for the placebo group (p = 0.158). |
| Verdenelli et al., 2016 [132] | Vaginal suppository SYNBIO®gin containing L. rhamnosus IMC 501 and L. paracasei IMC 502 once daily for seven days. | 35 apparently healthy women from Italy were enrolled. Assessments were made three times: before treatment, immediately after treatment and 21 days after treatment. | After treatment, 50% of the women with an intermediate Nugent score reverted to the normal state. There were no significant differences in vaginal pH comparing the time points before and after the treatment. After SYNBIOgin, L. rhamnosus IMC 501 and L. paracasei IMC 502 exhibited increased abundance in the vaginal microbiota that slowly declined over the following 21 days. |
| Tomusiak et al., 2015 [133] | InVag® vaginal capsules containing L. fermentum 57A, L. plantarum 57B and L. gasseri 57C once a day for seven days. | 160 women of European descent and with dysbiotic vaginal microbiome were enrolled. Patients were randomly assigned either to a group receiving the InVag preparation or to a placebo group. Four visits were included in the trial. Assessments were made at visits I, III and IV. | For InVag subjects, there was a significant reduction in vaginal pH between visits I and III (p < 0.0016) and visits I and IV (p < 0.0001). For placebo patients, differences were not significant. For the InVag arm, Nugent score decreased significantly from visits I to III (p = 0.0001), I to IV (p < 0.0001) and III to IV (p = 0.0238). For the placebo arm, Nugent score also decreased significantly from visits I to III (p < 0.0001) and I to IV (p = 0.0002). However, visits III and IV were not significantly different from each other. InVag subjects significantly increased L. plantarum and L. fermentum in their vaginal microbiota by approximately 1,000 times at visit III and the levels slowly declined until visit IV. In placebo subjects, L. plantarum and L. fermentum increased much more slowly, by approximately 10 times at visit IV. L. fermentum 57A, L. plantarum 57B and L. gasseri 57C were confirmed to be present on the vaginal epithelium of 82% of InVag participants at visit III and on 47.5% at visit IV. |
| Palma et al., 2018 [141] | 500 mg of metronidazole twice a day for 7 days or daily fluconazole (150 mg) for two consecutive days together with vaginal tablets of L. rhamnosus BMX 54 for 3 months (short-term) or 6 months (long-term). | 117 subjects were randomly assigned to the short-term probiotic administration (group 1, n = 60) or to the long-term Lactobacilli implementation (group 2, n = 57) at University of Rome. Women diagnosed with yeast vaginitis / BV together with HPV infection / cytological abnormalities were enrolled. Assessments were made before treatment, and at 3, 6 and 9 months after intervention. | 3 months after treatment, statistically significant differences were not found between groups 1 and 2. After 9 months, 79.4% of patients subjected to the long-term probiotic administration solved the cytological abnormalities, against 37.5% in group 1 (p = 0.041). After 9 months, 11.6% women from group 1 cleared HPV infection, compared to 31.2% from group 2 (p = 0.044). |
| Verhoeven et al., 2012 [140] | Daily consumption of a commercially available probiotic drink (Yakult) containing L. casei Shirota during the study period (6 months). | 54 HPV+ women with LSIL were assigned to a group receiving probiotics or to a group without intervention (control). The study was developed at the University of Antwerp, Belgium. Assessments were made at study entry (t1), 3 months after (t2) and 6 months after (end of the study, t3). | 60% of probiotic-consuming patients solved the cytological abnormalities against 30.7% patients without intervention (p = 0.047). After 3 months, 16% of probiotic subjects cleared the HPV infection against 7.7% in control women (p = 0.13). After 6 months, 29.2% of probiotic intakers cleared the HPV infection compared to 19.2% of control subjects (p = 0.41). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curty, G.; de Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2020, 21, 222. https://doi.org/10.3390/ijms21010222
Curty G, de Carvalho PS, Soares MA. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. International Journal of Molecular Sciences. 2020; 21(1):222. https://doi.org/10.3390/ijms21010222
Chicago/Turabian StyleCurty, Gislaine, Pedro S. de Carvalho, and Marcelo A. Soares. 2020. "The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer" International Journal of Molecular Sciences 21, no. 1: 222. https://doi.org/10.3390/ijms21010222
APA StyleCurty, G., de Carvalho, P. S., & Soares, M. A. (2020). The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. International Journal of Molecular Sciences, 21(1), 222. https://doi.org/10.3390/ijms21010222





