Autophagy-Like Cell Death Regulates Hydrogen Peroxide and Calcium Ion Distribution in Xa3/Xa26-Mediated Resistance to Xanthomonas oryzae pv. oryzae
Abstract
1. Introduction
2. Results
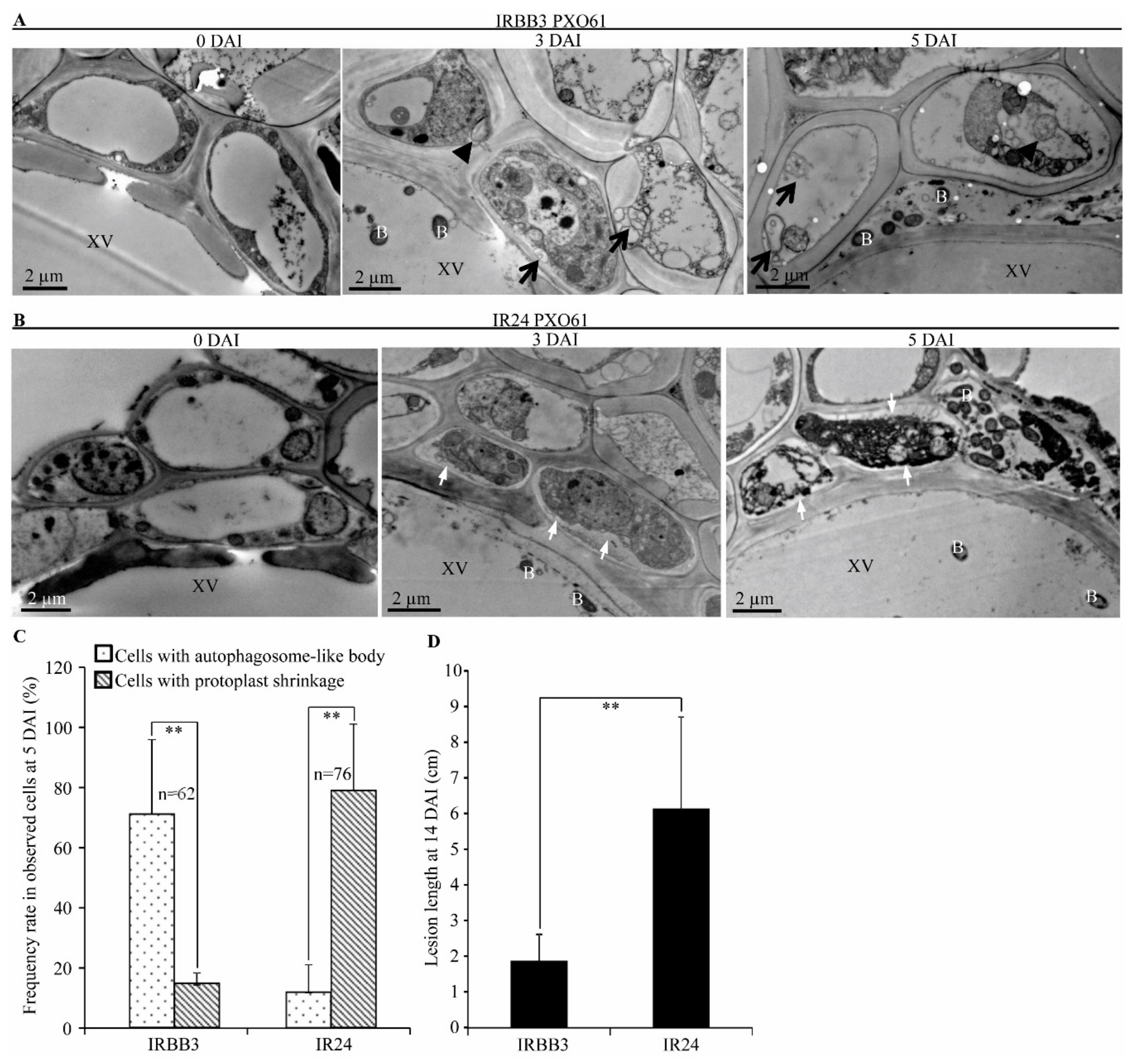
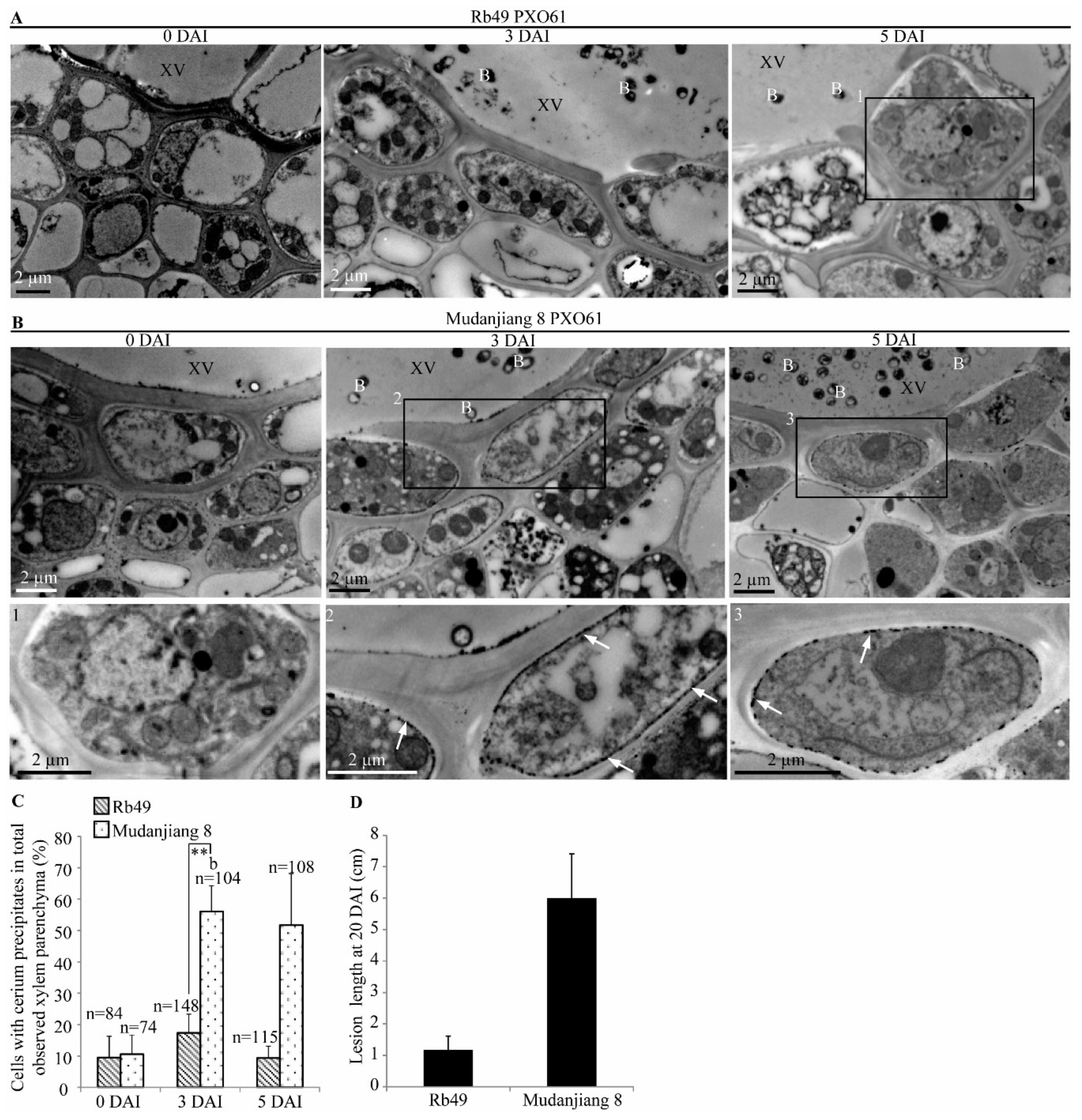
2.1. Autophagy-Like Ultrastructure in Xylem Parenchyma and Mesophyll Cells during Xa3/Xa26-Mediated Resistance Reaction
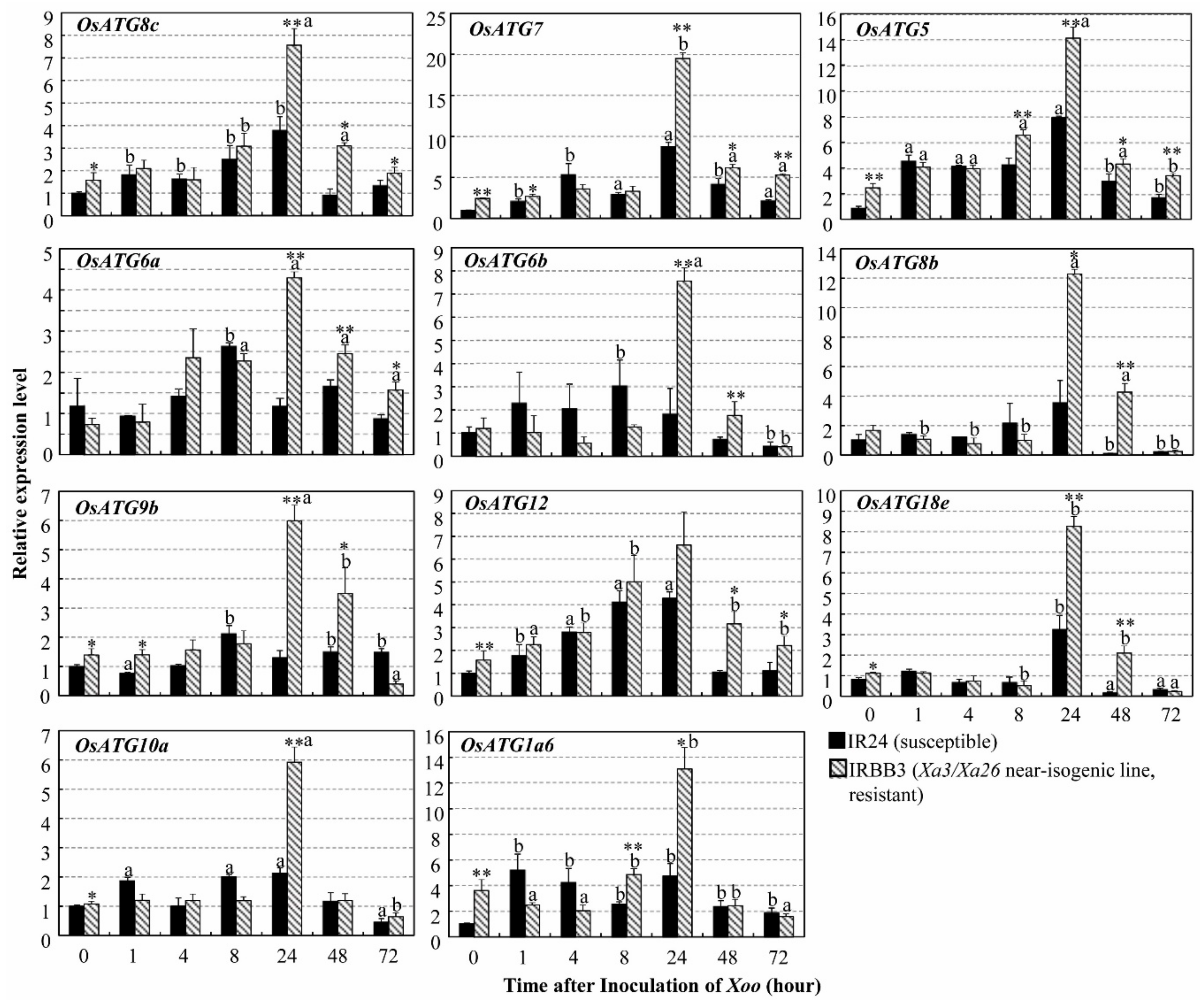
2.2. High Expression of Autophagy-Related Genes in Xa3/Xa26-Mediated Resistance Reaction
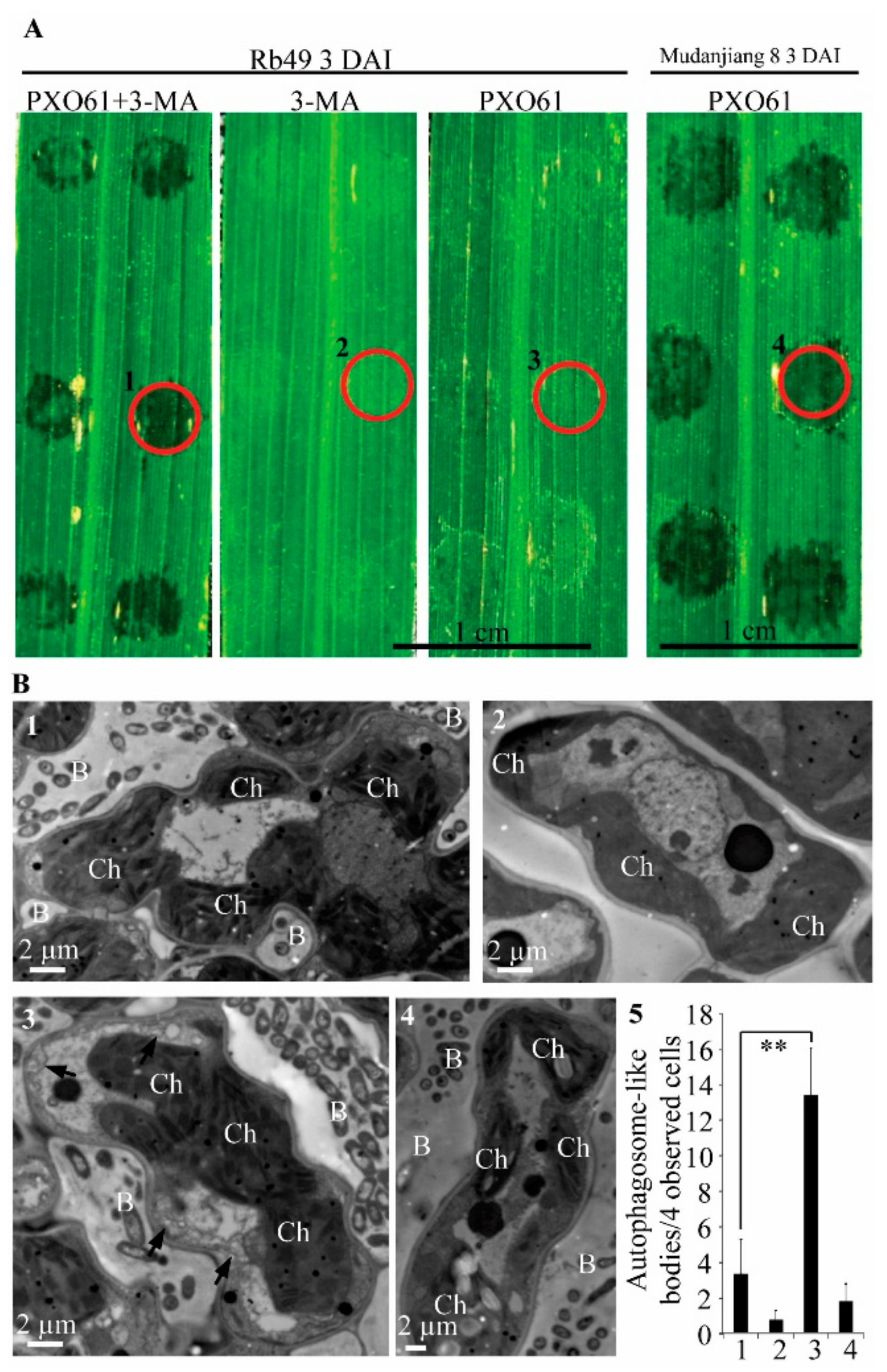
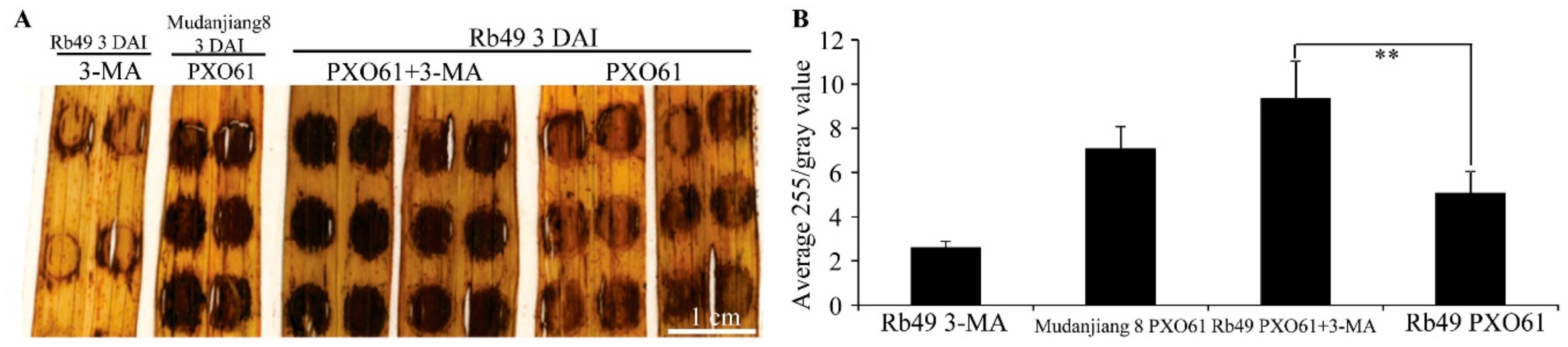
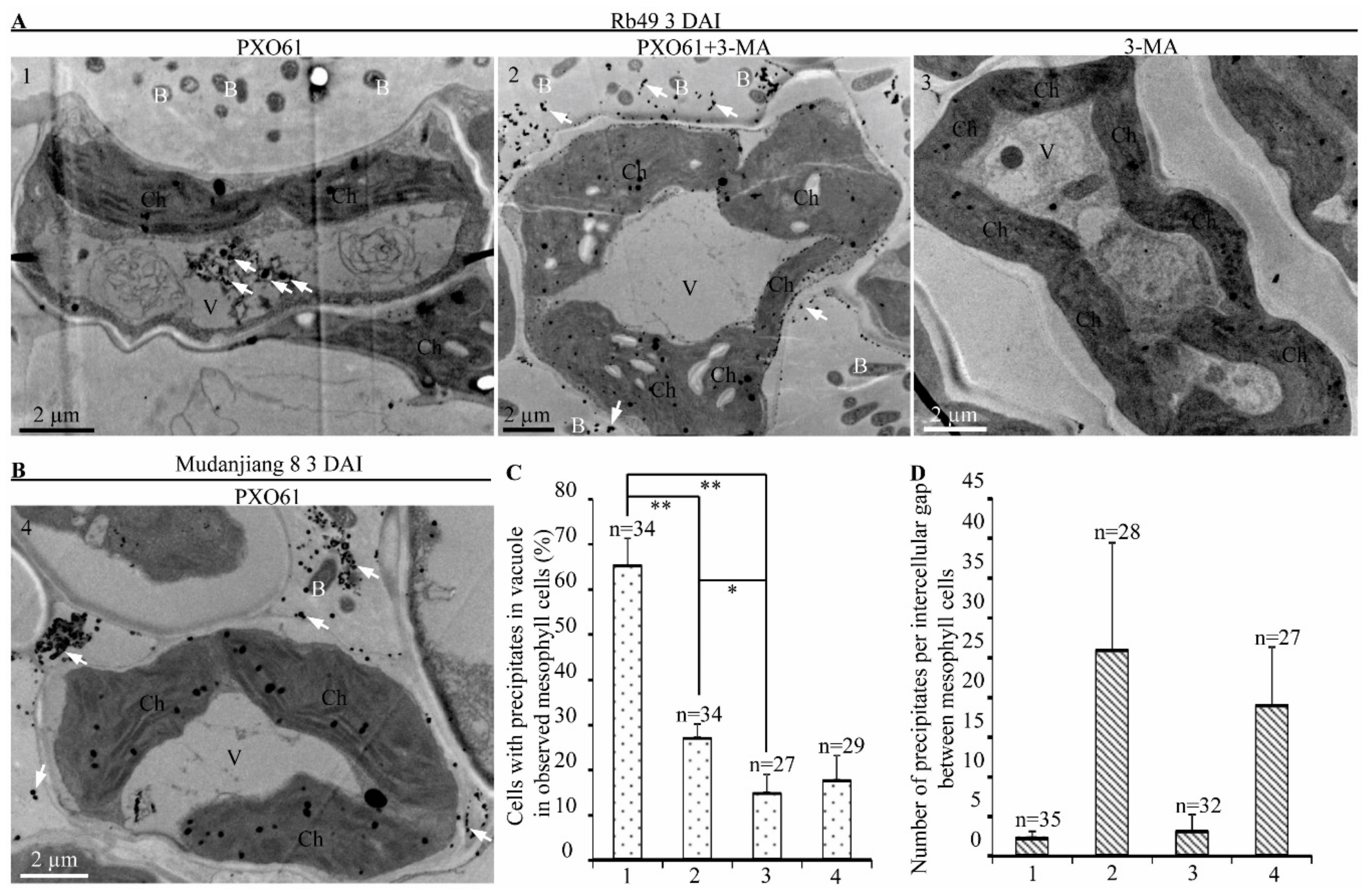
2.3. Autophagy Inhibitor 3-Methyladenine Partially Impaired Xa3/Xa26-Mediated Resistance by Reducing Autophagosome-Like Body Formation in Mesophyll Cells
2.4. Autophagy Inhibitor 3-Methyladenine Promoted H2O2 Accumulation in Impaired Xa3/Xa26-Mediated Resistance Reaction
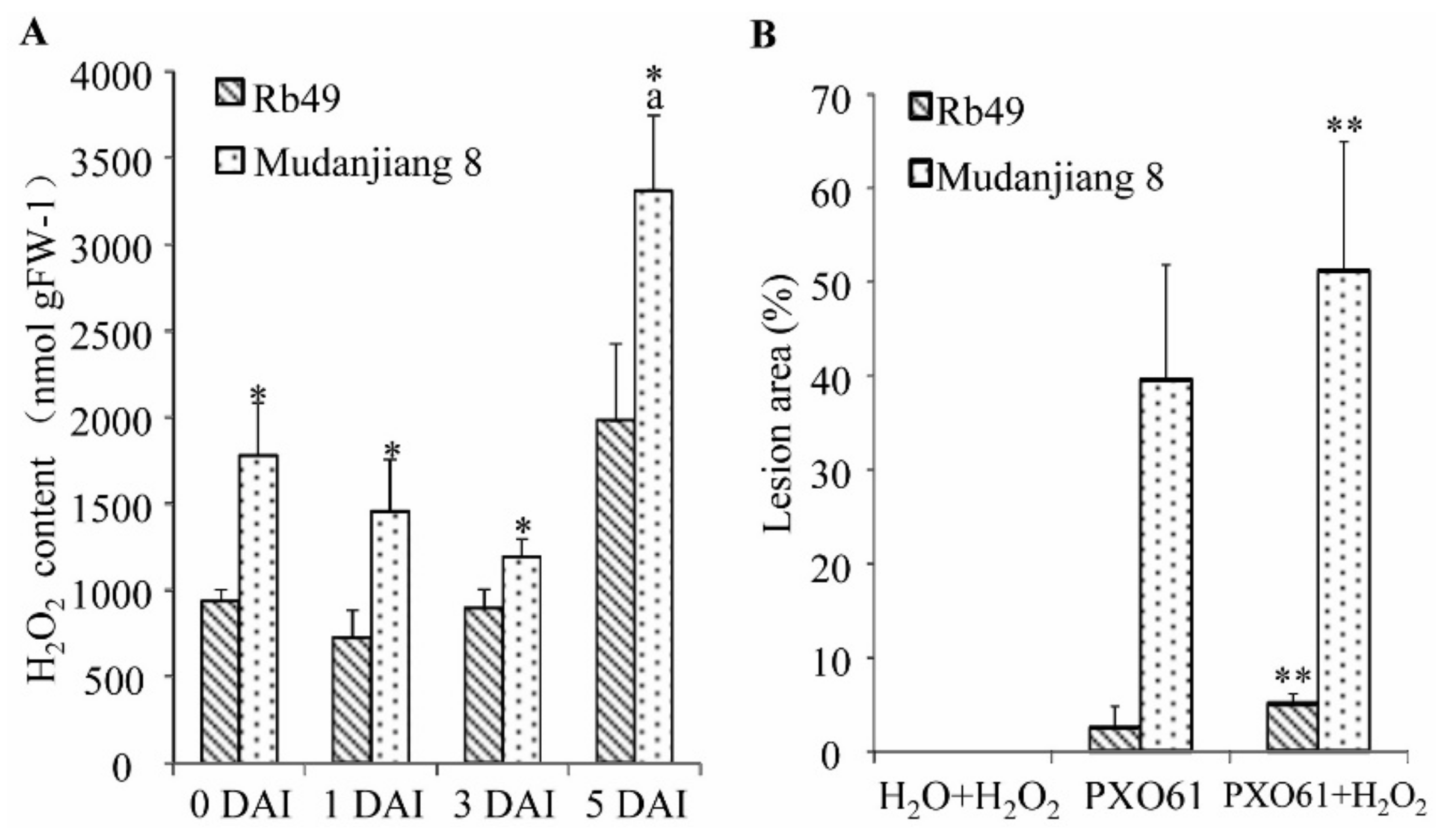
2.5. Low H2O2 Accumulation in Xa3/Xa26-Mediated Resistance and the Partially Impaired Resistance by in Vitro Spraying H2O2 Treatment
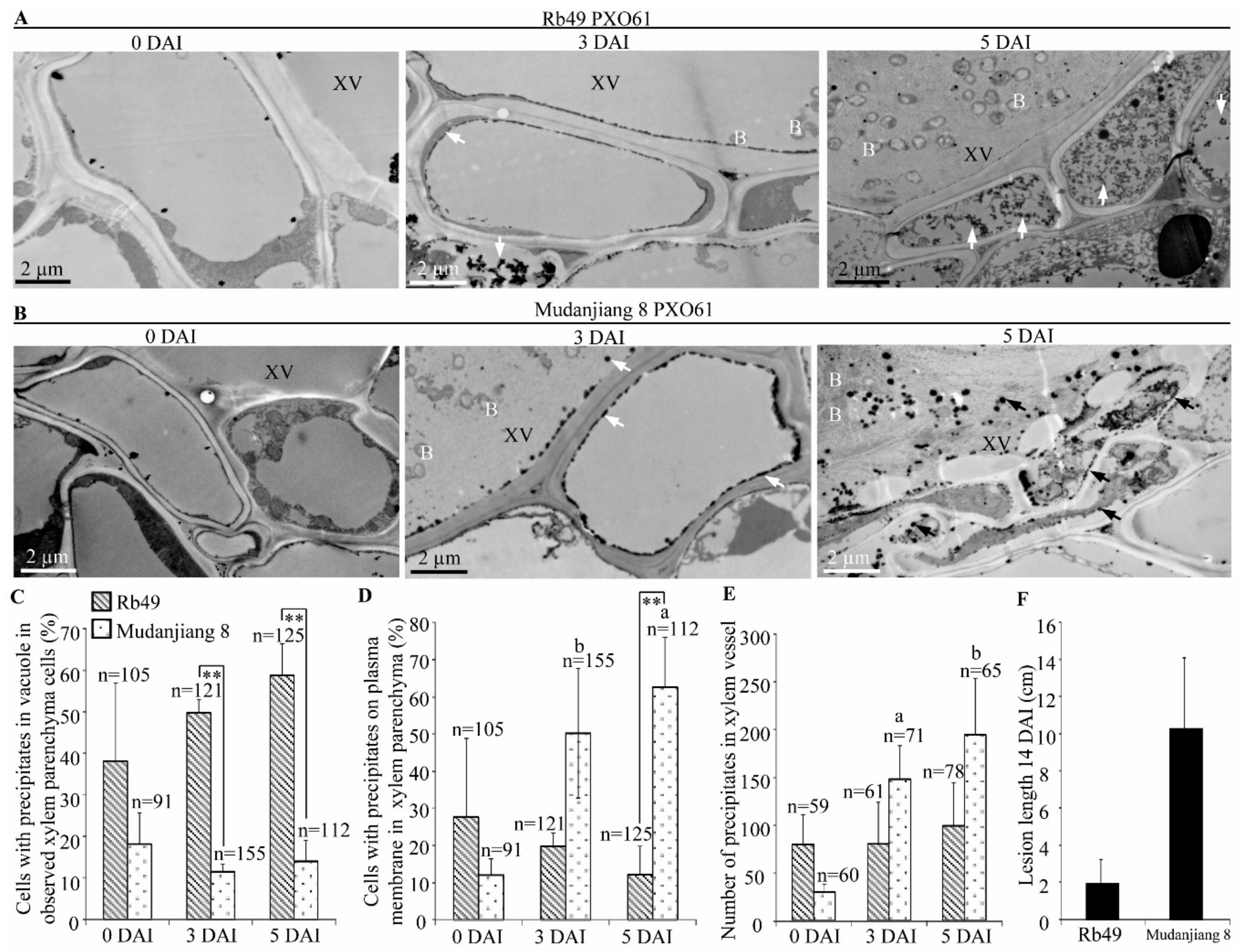
2.6. Lower H2O2 Accumulation on Plasma Membrane of Xylem Parenchyma Cells during Xa3/Xa26-Mediated Resistance
2.7. Autophagy Inhibitor 3-Methyladenine Reduced Calcium Ions Accumulation in Vacuole of Mesophyll Cells in Impaired Xa3/Xa26-Mediated Resistance Reaction
2.8. Accumulation of Calcium Ions in Vacuole of Xylem Parenchyma Cells in Xa3/Xa26-Mediated Resistance
3. Discussion
3.1. Xa3/Xa26 Induces Autophagy-Like Cell Death to Defend Xoo
3.2. Autophagy-Like Cell Death Negatively Regulates H2O2 Accumulation to Reduce Oxidative stress in Xa3/Xa26-Mediated Resistance
3.3. Autophagy-Like Cell Death Regulates Calcium Ion Distribution in Xa3/Xa26 Mediated Resistance
4. Materials and Methods
4.1. Rice Materials
4.2. Pathogen Inoculation
4.3. Cell Death Ultrastructure Analysis
4.4. Autophagy-Related Gene Expression Analysis
4.5. DAB Staining, H2O2 Treatment, and H2O2 Content Assay
4.6. H2O2 and Calcium Ion Localization Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ke, Y.G.; Deng, H.Q.; Wang, S.P. Advances in understanding broad-spectrum resistance to pathogens in rice. Plant J. 2017, 90, 738–748. [Google Scholar] [CrossRef]
- Zhang, H.T.; Wang, S.P. Rice versus Xanthomonas oryzae pv oryzae: A unique pathosystem. Curr. Opin. Plant Biol. 2013, 16, 188–195. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, L.Y.; Zhang, F.; Ali, J.; Cruz, C.V.; Zhuo, D.L.; Du, Z.L.; Li, Z.K.; Zhou, Y.L. Comparative transcriptome profiling of a rice line carrying Xa39 and its parents triggered by Xanthomonas oryzae pv. oryzae provides novel insights into the broad-spectrum hypersensitive response. BMC Genom. 2015, 16, 111. [Google Scholar]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Doorn, W.G.V.; Beers, E.P.; Dangl, J.L.; Franklin-Tong, V.E.; Gallois, P.; Hara-Nishimura, I.; Jones, A.M.; Kawai-Yamada, M.; Lam, E.; Mundy, J. Morphological classification of plant cell deaths. Cell Death Differ. 2011, 18, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.P.; Tsao, J.; Dinesh-Kumar, S.P. Autophagy and plant innate immunity: Defense through degradation. Semin. Cell Dev. Biol. 2009, 20, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, W.G.; Papini, A. Ultrastructure of autophagy in plant cells: A review. Autophagy 2013, 9, 1922–1936. [Google Scholar] [CrossRef]
- Cao, J.B.; Zhang, M.; Xiao, J.H.; Li, X.H.; Yuan, M.; Wang, S.P. Dominant and recessive major R genes lead to different types of host cell death during resistance to Xanthomonas oryzae in rice. Front. Plant Sci. 2018, 9, 1711. [Google Scholar] [CrossRef]
- Mur, L.A.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef]
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schiff, M.; Czymmek, K.; Talloczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005, 121, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dinesh-Kumar, S.P. Arabidopsis ATG6 is required to limit the pathogen-associated cell death response. Autophagy 2008, 4, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hofius, D.; Schultz-Larsen, T.; Joensen, J.; Tsitsigiannis, D.I.; Petersen, N.H.; Mattsson, O.; Jorgensen, L.B.; Jones, J.D.; Mundy, J.; Petersen, M. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 2009, 137, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Jones, J.D.; Dangl, J.L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wendehenne, D.; Lamotte, O.; Frachisse, J.M.; Barbier-Brygoo, H.; Pugin, A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell 2002, 14, 1937–1951. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Levine, A.; Pennell, R.I.; Alvarez, M.E.; Palmer, R.; Lamb, C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 1996, 6, 427–437. [Google Scholar] [CrossRef]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zeng, X.; Gu, K.; Qiu, C.; Yang, X.; Zhou, Z.; Goh, M.; Luo, Y.; Muratahori, M. The rice TAL effector-dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell 2014, 26, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Cao, Y.L.; Zhang, Q.L.; Li, X.H.; Wang, S.P. A cytosolic triosephosphate isomerase is a key component in XA3/XA26-mediated resistance. Plant Physiol. 2018, 178, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Cao, Y.L.; Yang, Z.F.; Xu, C.G.; Li, X.H.; Wang, S.P.; Zhang, Q.F. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004, 37, 517–527. [Google Scholar] [PubMed]
- Zhang, J.; Chen, L.L.; Xing, F.; Kudrna, D.A.; Yao, W.; Copetti, D.; Mu, T.; Li, W.; Song, J.M.; Xie, W.; et al. Extensive sequence divergence between the reference genomes of two elite indica rice varieties Zhenshan 97 and Minghui 63. Proc. Natl. Acad. Sci. USA 2016, 113, 5163–5171. [Google Scholar] [CrossRef]
- Xiang, Y.; Cao, Y.L.; Xu, C.G.; Li, X.H.; Wang, S.P. Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor. Appl. Genet. 2006, 113, 1347–1355. [Google Scholar] [CrossRef]
- Li, H.J.; Li, X.H.; Xiao, J.H.; Wang, S.P. Ortholog alleles at Xa3/Xa26 locus confer conserved race-specific resistance against Xanthomonas oryzae in rice. Mol. Plant. 2012, 5, 281–290. [Google Scholar] [CrossRef]
- Cao, Y.L.; Ding, X.H.; Cai, M.; Zhao, J.; Lin, Y.S.; Li, X.H.; Xu, C.G.; Wang, S.P. The expression pattern of a rice disease resistance gene Xa3/Xa26 is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics 2007, 177, 523–533. [Google Scholar] [CrossRef]
- Xu, L.; Li, X.H.; Wang, S.P. Analysis on expression patterns of the family members of rice bacterial blight resistance gene Xa3/Xa26. Chin. J. Rice Sci. 2008, 22, 559–563. [Google Scholar]
- Zhang, H.T.; Cao, Y.L.; Jing, Z.; Li, X.H.; Xiao, J.H.; Wang, S.P. A pair of orthologs of a leucine-rich repeat receptor kinase-like disease resistance gene family regulates rice response to raise temperature. BMC Plant Biol. 2011, 11, 1285–1290. [Google Scholar] [CrossRef]
- Shin, J.H.; Yoshimoto, K.; Ohsumi, Y.; Jeon, J.S.; An, G. OsATG10b, an autophagosome component, is needed for cell survival against oxidative stresses in rice. Mol. Cells 2009, 27, 67–74. [Google Scholar] [CrossRef]
- Xia, K.; Liu, T.; Ouyang, J.; Wang, R.; Fan, T.; Zhang, M. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L.). DNA Res. 2011, 18, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, B.; Zhao, J.; Guo, J.; Li, Y.; Han, S.; Huang, L.; Du, Y.; Hong, Y.; Tang, D.; et al. Autophagy contributes to leaf starch degradation. Plant Cell 2013, 25, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Yu, L.; Xu, C. Dual role for autophagy in lipid metabolism in Arabidopsis. Plant Cell 2019, 31, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Suzuki, T.; Hattori, M.; Yoshimoto, K.; Ohsumi, Y.; Moriyasu, Y. AtATG genes, homologs of yeast autophagy genes, are involved in constitutive autophagy in Arabidopsis root tip cells. Plant Cell Physiol. 2006, 47, 1641–1652. [Google Scholar] [CrossRef]
- ThordalChristensen, H.; Zhang, Z.G.; Wei, Y.D.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Bestwick, C.S.; Brown, I.R.; Bennett, M.H.; Mansfield, J.W. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 1997, 9, 209–221. [Google Scholar]
- Yin, S.; Wang, C.; Jiao, M.; Li, F.; Han, Q.; Huang, L.; Zhang, H.; Kang, Z. Subcellular localization of calcium in the incompatible and compatible interactions of wheat and Puccinia striiformis f. sp. tritici. Protoplasma 2014, 252, 103–116. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, F.; Zheng, Z.; Fan, B.; Chen, Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011, 66, 953–968. [Google Scholar] [CrossRef]
- Minina, E.A.; Moschou, P.N.; Vetukuri, R.R.; Sanchez-Vera, V.; Cardoso, C.; Liu, Q.S.; Elander, P.H.; Dalman, K.; Beganovic, M.; Yilmaz, J.L.; et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018, 69, 1415–1432. [Google Scholar] [CrossRef]
- Takatsuka, C.; Inoue, Y.; Higuchi, T.; Hillmer, S.; Robinson, D.G.; Moriyasu, Y. Autophagy in tobacco BY-2 Cells cultured under sucrose starvation conditions: Isolation of the autolysosome and its characterization. Plant Cell Physiol. 2011, 52, 2074–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.M.; Hong, Q.; Li, Y.; Li, Q.; Wang, M. Autophagy contributes to regulate the ROS levels and PCD progress in TMV-infected tomatoes. Plant Sci. 2018, 269, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Jikumaru, Y.; Kamiya, Y.; Kusano, M.; Consonni, C.; Panstruga, R.; Ohsumi, Y.; Shirasu, K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 2009, 21, 2914–2927. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Li, X.H.; Xiao, J.H.; Wang, S.P. A convenient method for simultaneous quantification of multiple phytohormones and metabolites: Application in study of rice-bacterium interaction. Plant Methods 2012, 8, 2. [Google Scholar] [CrossRef]
- Xiong, Y.; Contento, A.L.; Bassham, D.C. Disruption of autophagy results in constitutive oxidative stress in Arabidopsis. Autophagy 2007, 3, 257–258. [Google Scholar] [CrossRef]
- Ma, W.; Berkowitz, G.A. The grateful dead: Calcium and cell death in plant innate immunity. Cell Microbiol. 2010, 9, 2571–2585. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Mcainsh, M.R.; Brownlee, C.; Hetherington, A.M. Visualizing changes in cytosolic-free Ca2+ during the response of stomatal guard cells to abscisic acid. Plant Cell 1992, 4, 1113–1122. [Google Scholar] [CrossRef]
- Ali, G.S.; Reddy, V.S.; Lindgren, P.B.; Jakobek, J.L.; Reddy, A.S. Differential expression of genes encoding calmodulin-binding proteins in response to bacterial pathogens and inducers of defense responses. Plant Mol. Biol. 2003, 51, 803–815. [Google Scholar] [CrossRef]
- Ali, R.; Ma, W.; Lemtiri-Chlieh, F.; Tsaltas, D.; Leng, Q.; Bodman, S.V.; Berkowitz, G.A. Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 2007, 19, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Volotovski, I.D.; Sokolovsky, S.G.; Molchan, O.V.; Knight, M.R. Second messengers mediate increases in cytosolic calcium in tobacco protoplasts. Plant Physiol. 1998, 117, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Wang, S.P.; Zhang, Q.F. New gene for bacterial blight resistance in rice located on chromosome 12 identified from Minghui 63, an elite restorer line. Phytopathology 2002, 92, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Grogan, R.G.; Misaghi, I.J.; Kimble, K.A.; Greathead, A.S.; Ririe, D.; Bardin, R. Varnish spot, destructive disease of lettuce in California caused by Pseudomonas cichorii. Phytopathology 1977, 67, 957–960. [Google Scholar] [CrossRef]
- Schaad, N.W.; Wang, Z.K.; Di, M.; Mcbeath, J.; Peterson, G.L.; Bonde, M.R. An improved infiltration technique to test the pathogenicity of Xanthomonas oryzae pv. oryzae in rice seedlings. Seed Sci. Technol. 1996, 24, 449–456. [Google Scholar]
- Sugio, A.; Yang, B.; White, F.F. Characterization of the hrpF pathogenicity peninsula of Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 2005, 18, 546–554. [Google Scholar] [CrossRef]
- Qiu, D.Y.; Xiao, J.; Ding, X.H.; Xiong, M.; Cai, M.; Cao, C.L.; Li, X.H.; Xu, C.G.; Wang, S.P. OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol. Plant Microbe Interact. 2007, 20, 492–499. [Google Scholar] [CrossRef]
- Corpas, F.J.; Sandalio, L.M. Cadmium-induced subcellular accumulation of O2.− and H2O2 in pea leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar]
- Hirano, H.; Yonezawa, H.; Yunoue, S.; Habu, M.; Uchida, H.; Yoshioka, T.; Kishida, S.; Kishida, M.; Oyoshi, T.; Fujio, S.; et al. Immunoreactivity of Wnt5a, Fzd2, Fzd6, and Ryk in glioblastoma: Evaluative methodology for DAB chromogenic immunostaining. Brain Tumor Pathol. 2014, 31, 85–93. [Google Scholar] [CrossRef]
- Wu, A.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.I.; Asensi-Fabado, M.A.; Munné-Bosch, S.; Antonio, C.; Tohge, T. JUNGBRUNNEN1, a reactive oxygen species responsive NAC transcription factor, regulates longevity in Arabidopsis. Plant. Cell 2012, 24, 482–506. [Google Scholar] [CrossRef] [PubMed]
| Plant Inoculation after 3 days 1 | Water-Soaked Symptoms | N 4 (Inoculation Sites) | |
|---|---|---|---|
| Yes (%) 2 | No (%) 3 | ||
| Rb49 PXO61 | 4 | 96 | 51 |
| Rb49 PXO61+3-MA | 65 | 35 | 80 |
| Rb49 3-MA | 0 | 100 | 36 |
| Mudanjiang 8 PXO61 | 100 | 0 | 72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Zhang, M.; Zhu, M.; He, L.; Xiao, J.; Li, X.; Yuan, M. Autophagy-Like Cell Death Regulates Hydrogen Peroxide and Calcium Ion Distribution in Xa3/Xa26-Mediated Resistance to Xanthomonas oryzae pv. oryzae. Int. J. Mol. Sci. 2020, 21, 194. https://doi.org/10.3390/ijms21010194
Cao J, Zhang M, Zhu M, He L, Xiao J, Li X, Yuan M. Autophagy-Like Cell Death Regulates Hydrogen Peroxide and Calcium Ion Distribution in Xa3/Xa26-Mediated Resistance to Xanthomonas oryzae pv. oryzae. International Journal of Molecular Sciences. 2020; 21(1):194. https://doi.org/10.3390/ijms21010194
Chicago/Turabian StyleCao, Jianbo, Meng Zhang, Mengmeng Zhu, Limin He, Jinghua Xiao, Xianghua Li, and Meng Yuan. 2020. "Autophagy-Like Cell Death Regulates Hydrogen Peroxide and Calcium Ion Distribution in Xa3/Xa26-Mediated Resistance to Xanthomonas oryzae pv. oryzae" International Journal of Molecular Sciences 21, no. 1: 194. https://doi.org/10.3390/ijms21010194
APA StyleCao, J., Zhang, M., Zhu, M., He, L., Xiao, J., Li, X., & Yuan, M. (2020). Autophagy-Like Cell Death Regulates Hydrogen Peroxide and Calcium Ion Distribution in Xa3/Xa26-Mediated Resistance to Xanthomonas oryzae pv. oryzae. International Journal of Molecular Sciences, 21(1), 194. https://doi.org/10.3390/ijms21010194





