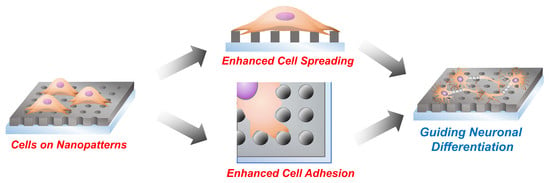
Enhancing Neurogenesis of Neural Stem Cells Using Homogeneous Nanohole Pattern-Modified Conductive Platform
Abstract
1. Introduction
2. Results and Discussion
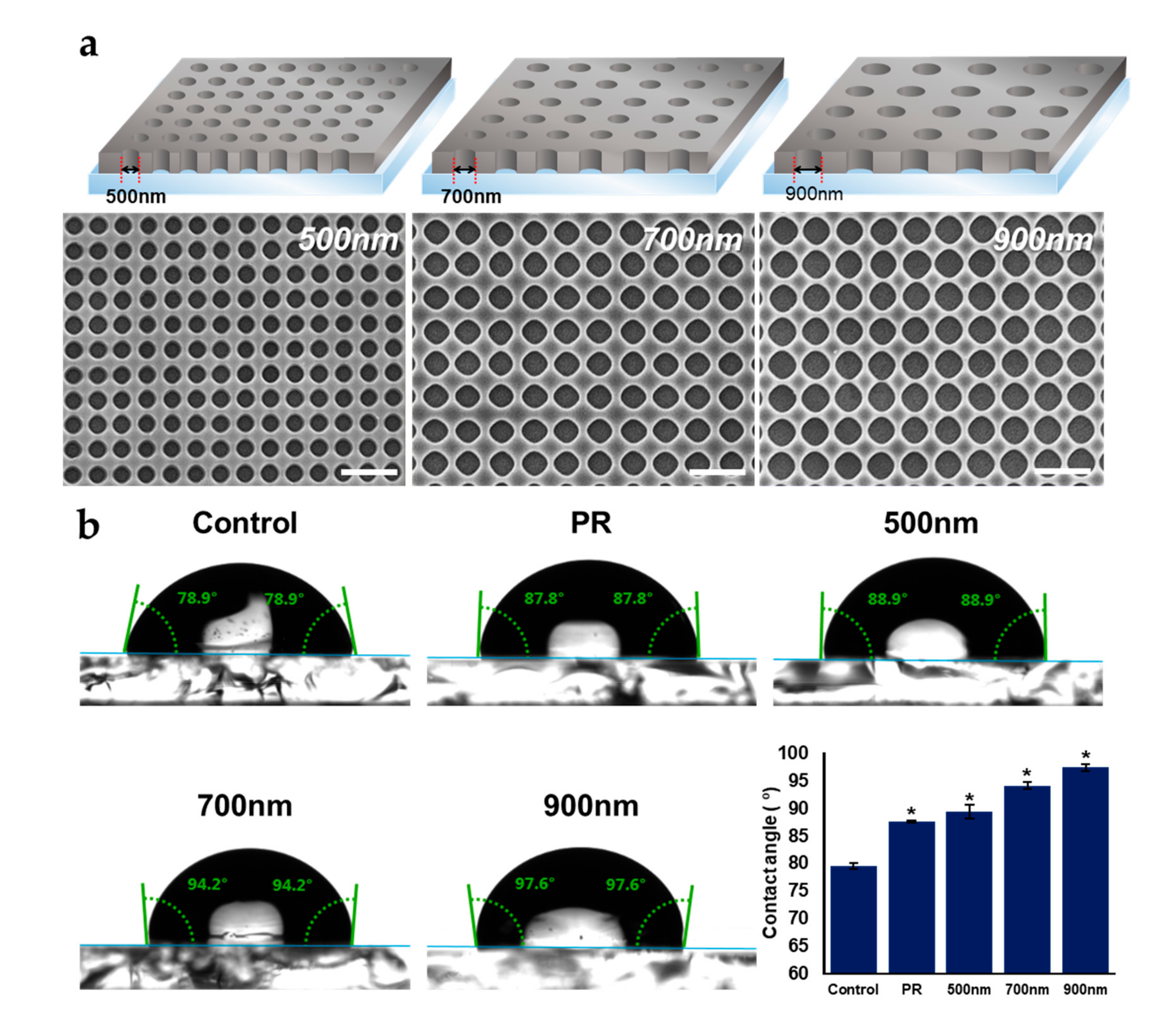
2.1. Fabrication and Characterization of Nanohole Pattern
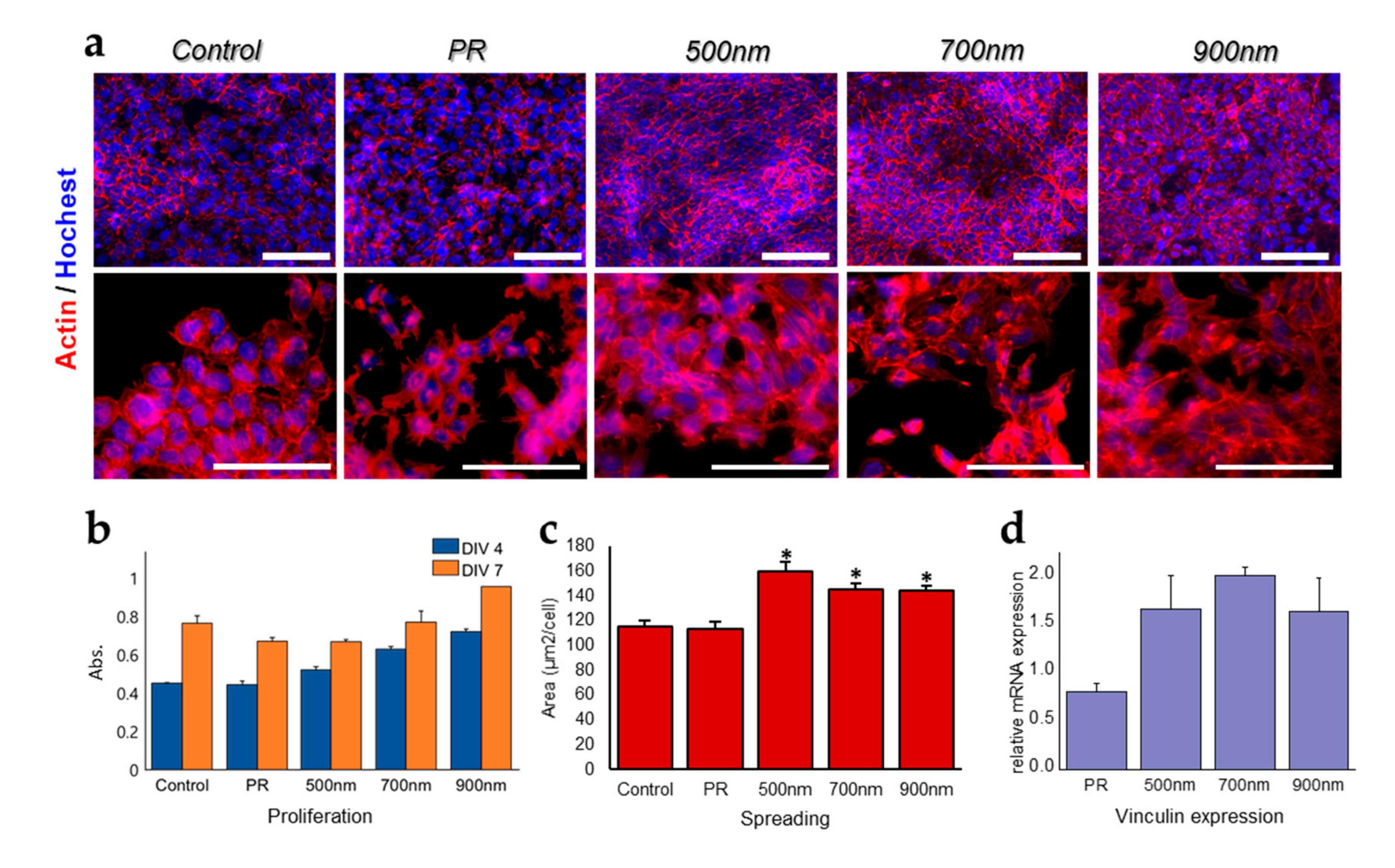
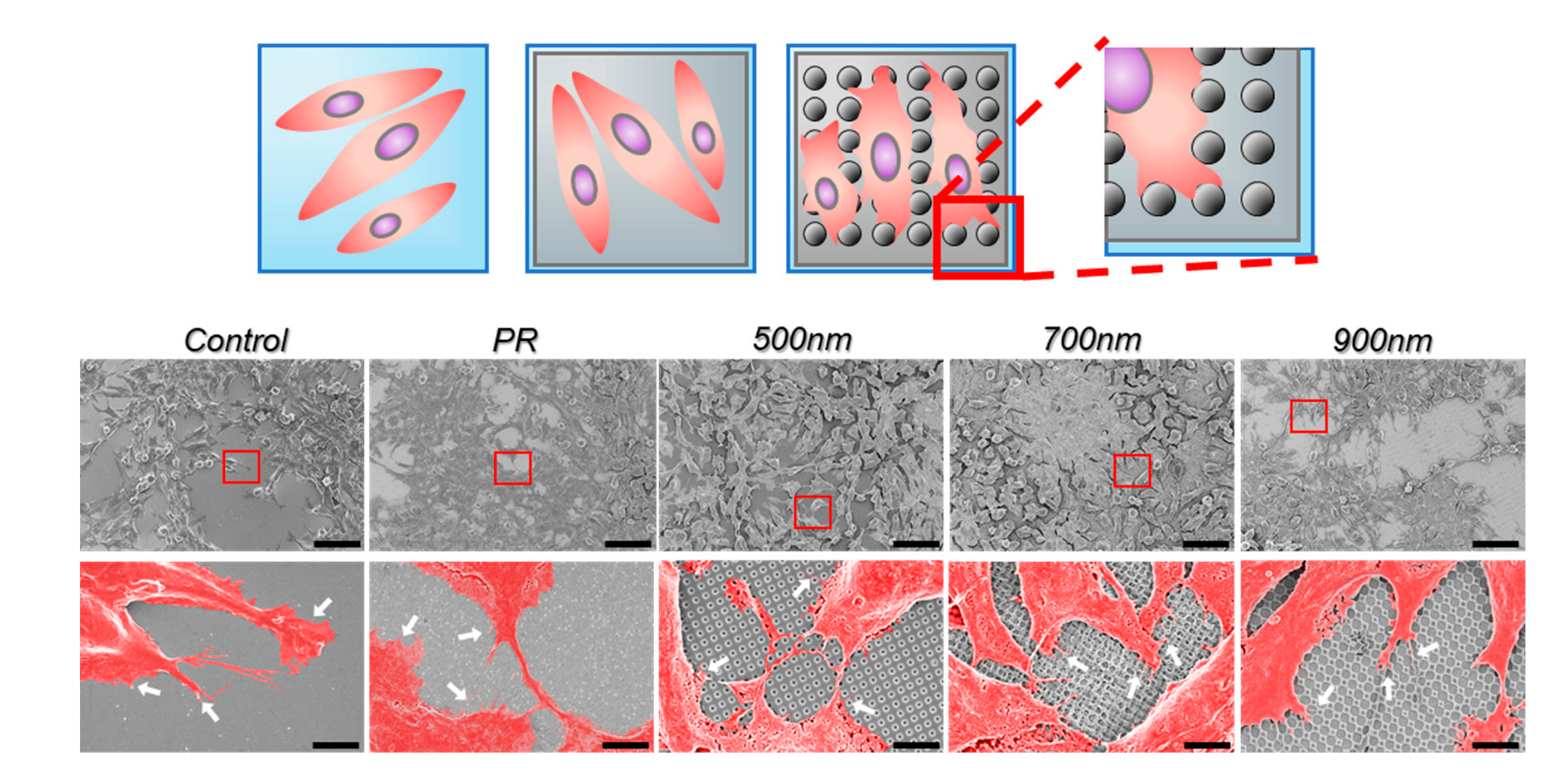
2.2. The Effects of Nanohole Patterns on mNSCs Adhesion and Growth
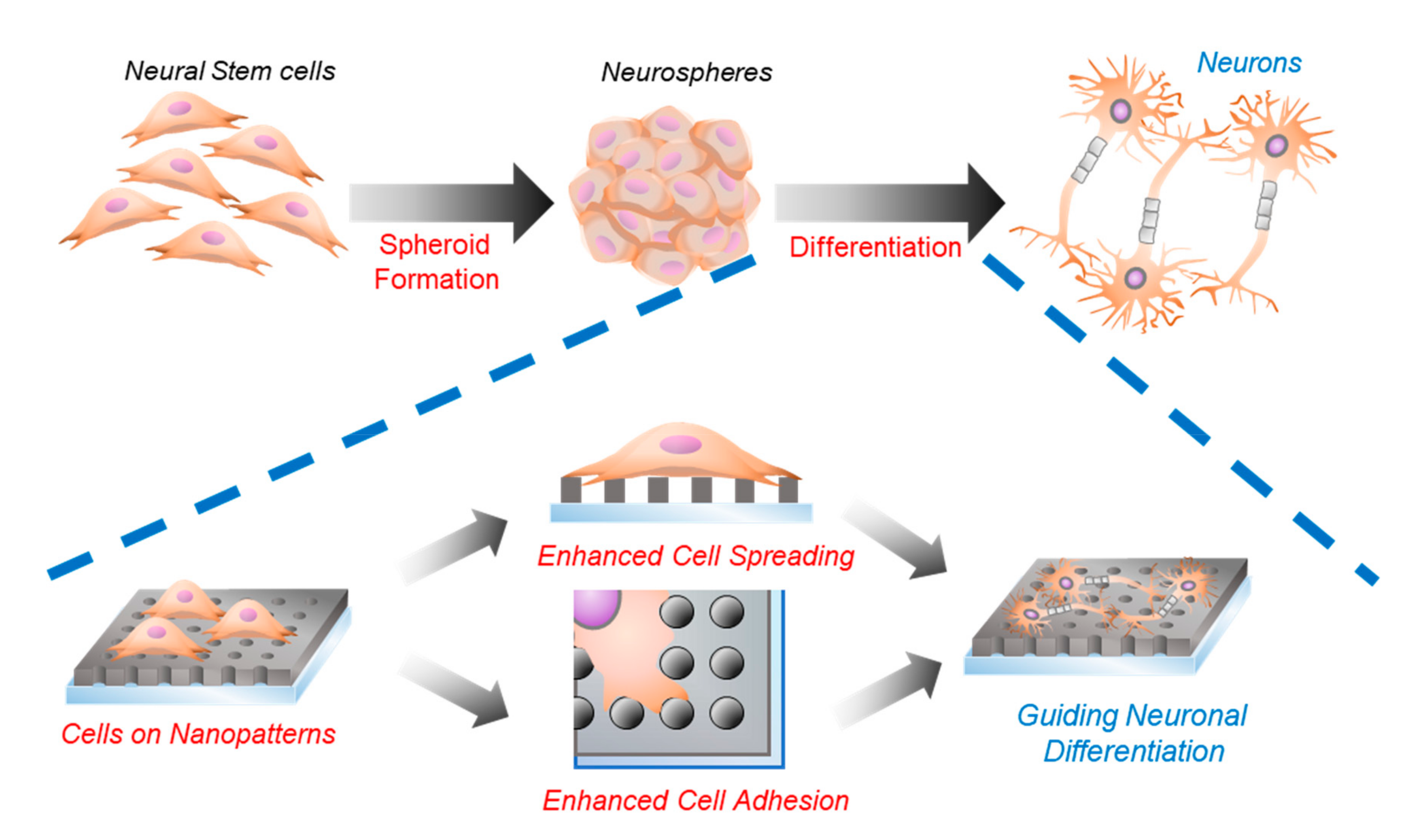
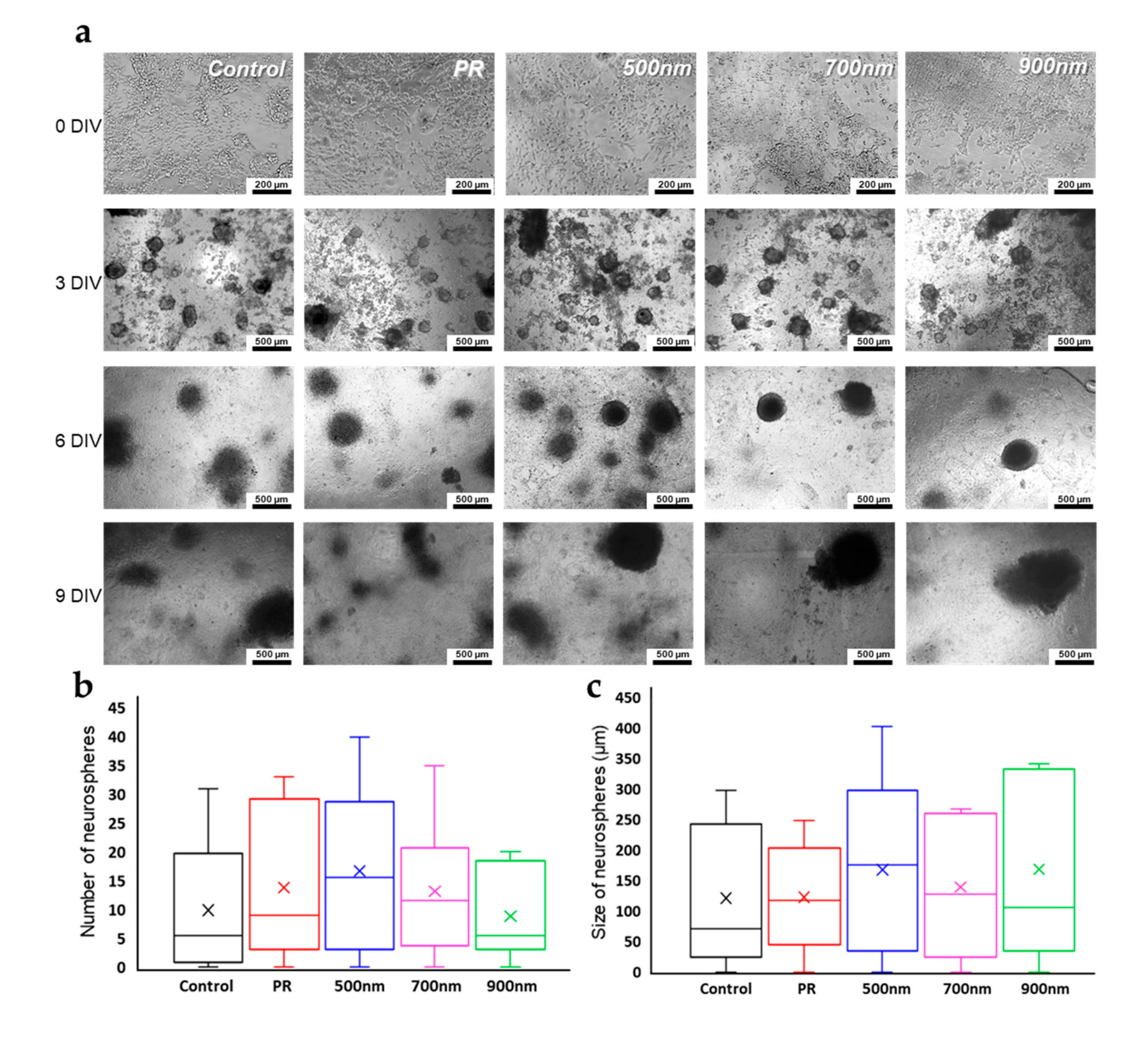
2.3. Neurosphere Formation of mNSCs on Nanohole Pattern Arrays
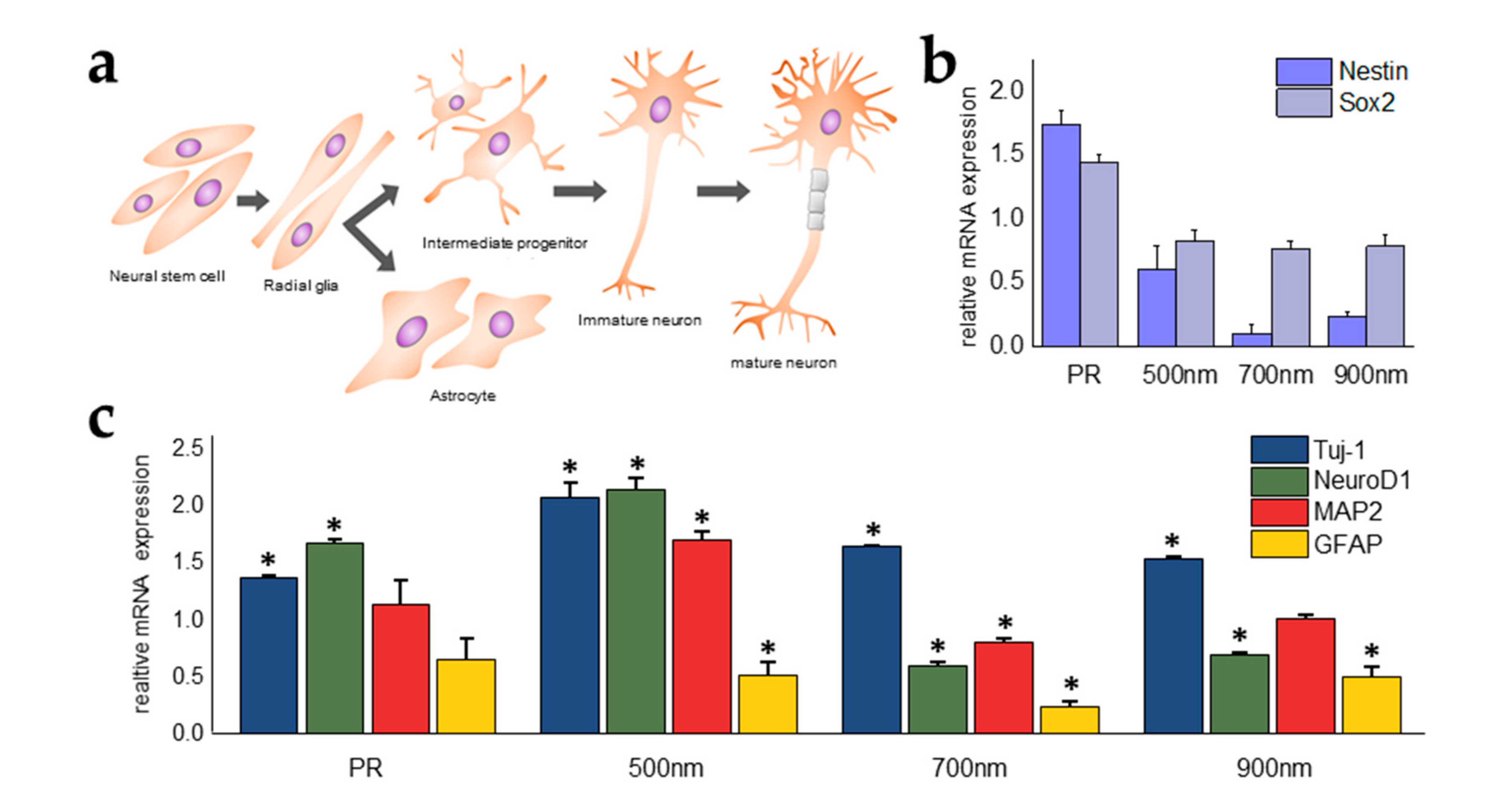
2.4. Investigations of the Effects of Nanohole Pattern Arrays on Neuronal Differentiation of mNSCs
3. Materials and Methods
3.1. Materials
3.2. Fabrication of HNPAs and Surface Characterization
3.3. Cell Culture and Analysis of Cell Growth, Spreading, and Adhesion of mNSCs
3.4. Neural Differentiation and Confirmation of mNSCs
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| mNSC | Mouse neural stem cell |
| RA | Retinoic acid |
| HNPAs | Homogeneous nanohole pattern arrays |
| ECM | Extracellular matrix |
| CNS | Central neural system |
| LIL | Laser interference lithography |
| ITO | Indium tin oxide |
| PR | Photoresist |
| Abs | Absorbance |
| TuJ1 | Class III beta-tubulin |
| NeuroD1 | Neurogenic differentiation 1 |
| MAP2 | Microtubule-associated protein 2 |
References
- Reynolds, B.; Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Bonaguidi, M.A.; Ming, G.-L.; Song, H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009, 19, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Kang, E.; Liu, C.Y.; Ming, G.-L.; Song, H. Development of neural stem cell in the adult brain. Curr. Opin. Neurobiol. 2008, 18, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Kitazawa, M.; Martinez-Coria, H.; Castello, N.A.; Müller, F.-J.; Loring, J.F.; Yamasaki, T.R.; Poon, W.W.; Green, K.N.; LaFerla, F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA 2009, 106, 13594–13599. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z. Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol. Sci. 2009, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-W.; Chu, K.; Jung, K.-H.; Kim, S.U.; Kim, M.; Roh, J.-K. Human Neural Stem Cell Transplantation Promotes Functional Recovery in Rats With Experimental Intracerebral Hemorrhage. Stroke 2003, 34, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–105. [Google Scholar] [CrossRef]
- Suhito, I.R.; Han, Y.; Min, J.; Son, H.; Kim, T.-H. In situ label-free monitoring of human adipose-derived mesenchymal stem cell differentiation into multiple lineages. Biomaterials 2018, 154, 223–233. [Google Scholar] [CrossRef]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef]
- Teti, A. Regulation of cellular functions by extracellular matrix. J. Am. Soc. Nephrol. 1992, 2, S83. [Google Scholar]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, D.; Kim, K. Engineered Co-culture Strategies Using Stem Cells for Facilitated Chondrogenic Differentiation and Cartilage Repair. Biotechnol. Bioprocess Eng. 2018, 23, 261–270. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-C.; Ling, J.-Y.; Chen, W.-C.; Lin, H.-H.; Tang, M.-J. Mechanotransduction of matrix stiffness in regulation of focal adhesion size and number: Reciprocal regulation of caveolin-1 and β1 integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef] [PubMed]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.-Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Ding, M.; Luo, G.; Liang, Q. A Microfluidic Hydrogel Chip with Orthogonal Dual Gradients of Matrix Stiffness and Oxygen for Cytotoxicity Test. Biochip. J. 2018, 12, 93–101. [Google Scholar] [CrossRef]
- Min, S.K.; Shim, H.J.; Shin, H.S. 3D Astrogliosis Model with bFGF and GFAP Expression Profiles Corresponding to an MCAO-injured Brain. Biotechnol. Bioprocess. Eng. 2018, 23, 588–597. [Google Scholar] [CrossRef]
- Venkatesan, J.; Rekha, P.D.; Anil, S.; Bhatnagar, I.; Sudha, P.N.; Dechsakulwatana, C.; Kim, S.-K.; Shim, M.S. Hydroxyapatite from Cuttlefish Bone: Isolation, Characterizations, and Applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.B.; Cho, S.-G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Shah, S.; Yang, L.; Yin, P.T.; Hossain, M.K.; Conley, B.; Choi, J.-W.; Lee, K.-B. Controlling Differentiation of Adipose-Derived Stem Cells Using Combinatorial Graphene Hybrid-Pattern Arrays. ACS Nano 2015, 9, 3780–3790. [Google Scholar] [CrossRef]
- Suhito, I.R.; Han, Y.; Kim, D.-S.; Son, H.; Kim, T.-H. Effects of two-dimensional materials on human mesenchymal stem cell behaviors. Biochem. Biophys. Res. Commun. 2017, 493, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Song, I.; Kim, D.-S.; Lee, U.; Kim, J.-K.; Son, H.; Min, J.; Kim, T.-H. Size-dependent effects of graphene oxide on the osteogenesis of human adipose-derived mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 169, 20–29. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Kim, T.-H. Nanomaterial-modified Hybrid Platforms for Precise Electrochemical Detection of Dopamine. BioChip J. 2019, 13, 20–29. [Google Scholar] [CrossRef]
- Tran, T.D.; Kim, M.I. Organic-Inorganic Hybrid Nanoflowers as Potent Materials for Biosensing and Biocatalytic Applications. BioChip J. 2018, 12, 268–279. [Google Scholar] [CrossRef]
- Kang, Y.J.; Cutler, E.G.; Cho, H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018, 5, 35. [Google Scholar] [CrossRef]
- Lee, J.S.; Yang, K.; Cho, A.-N.; Cho, S.-W. Ferritin nanoparticles for improved self-renewal and differentiation of human neural stem cells. Biomater Res. 2018, 22, 5. [Google Scholar] [CrossRef]
- Kawano, T.; Sato, M.; Yabu, H.; Shimomura, M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs): Uniform porous polymer scaffolds prepared by the breath figure technique. Biomater. Sci. 2014, 2, 52–56. [Google Scholar] [CrossRef]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Choo, S.-S.; Kim, S.-J.; Song, I.; Kim, T.-H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef]
- Choo, S.-S.; Kang, E.-S.; Song, I.; Lee, D.; Choi, J.-W.; Kim, T.-H.J.S. Electrochemical detection of dopamine using 3D porous graphene oxide/gold nanoparticle composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef]
- Suhito, I.R.; Lee, W.; Baek, S.; Lee, D.; Min, J.; Kim, T.-H. Rapid and sensitive electrochemical detection of anticancer effects of curcumin on human glioblastoma cells. Sens. Actuators B Chem. 2019, 288, 527–534. [Google Scholar] [CrossRef]
- Chun, S.H.; Yuk, J.S.; Um, S.H. Regulation of cellular gene expression by nanomaterials. Nano Converg. 2018, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.J.; Marei, H.E.-S. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Yang, S.-T.; He, T.; Yang, S.; Tang, X.-H. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. Int. J. Mol. Sci. 2017, 18, 2562. [Google Scholar] [CrossRef]
- Santos-Rasera, J.R.; Sant’Anna Neto, A.; Rosim Monteiro, R.T.; van Gestel, C.A.M.; Pereira de Carvalho, H.W. Toxicity, bioaccumulation and biotransformation of Cu oxide nanoparticles in Daphnia magna. Environ. Sci. Nano 2019, 6, 2897–2906. [Google Scholar] [CrossRef]
- Mendes, P.M.; Yeung, C.L.; Preece, J.A. Bio-nanopatterning of Surfaces. Nanoscale Res. Lett. 2007, 2, 373–384. [Google Scholar] [CrossRef]
- Kaga, N.; Akasaka, T.; Horiuchi, R.; Yoshida, Y.; Yokoyama, A. Adhesion of Human Osteoblast-like Cells (Saos-2 cells) on Micro/nanopatterned Structures Sputter-Coated with Titanium. Nano Biomed. 2016, 8, 74–82. [Google Scholar]
- Yang, K.; Yu, S.J.; Lee, J.S.; Lee, H.-R.; Chang, G.-E.; Seo, J.; Lee, T.; Cheong, E.; Im, S.G.; Cho, S.-W. Electroconductive nanoscale topography for enhanced neuronal differentiation and electrophysiological maturation of human neural stem cells. Nanoscale 2017, 9, 18737–18752. [Google Scholar] [CrossRef]
- Jeong, H.S.; Jeon, H.-J.; Kim, Y.H.; Oh, M.B.; Kumar, P.; Kang, S.-W.; Jung, H.-T. Bifunctional ITO layer with a high resolution, surface nano-pattern for alignment and switching of LCs in device applications. NPG Asia Mater. 2012, 4, e7. [Google Scholar] [CrossRef][Green Version]
- Kim, M.S.; Kim, A.Y.; Jang, K.J.; Kim, J.H.; Kim, J.B.; Suh, K.Y. Effect of nanogroove geometry on adipogenic differentiation. Nanotechnology 2011, 22, 494017. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.G.; Liang, J. Atomic force microscope nanolithography: Dip-pen, nanoshaving, nanografting, tapping mode, electrochemical and thermal nanolithography. J. Phys. Condens. Matter 2009, 21, 483001. [Google Scholar] [CrossRef]
- Xu, S.; Liu, G.-Y. Nanometer-Scale Fabrication by Simultaneous Nanoshaving and Molecular Self-Assembly. Langmuir 1997, 13, 127–129. [Google Scholar] [CrossRef]
- Xu, S.; Miller, S.; Laibinis, P.E.; Liu, G.-Y. Fabrication of Nanometer Scale Patterns within Self-Assembled Monolayers by Nanografting. Langmuir 1999, 15, 7244–7251. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Choo, S.-S.; Woo, H.Y.; Paik, T.; Lee, T.; Kim, T.-H. Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation. Sensors 2018, 18, 2755. [Google Scholar] [CrossRef]
- Kim, T.-H.; Yea, C.-H.; Chueng, S.-T.D.; Yin, P.T.-T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.-W.; Lee, K.-B. Large-Scale Nanoelectrode Arrays to Monitor the Dopaminergic Differentiation of Human Neural Stem Cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef]
- Nam, B.; Ko, T.-K.; Hyun, S.-K.; Lee, C. NO2 sensing properties of WO3-decorated In2O3 nanorods and In2O3-decorated WO3 nanorods. Nano Converg. 2019, 6, 40. [Google Scholar] [CrossRef]
- Noh, J.-Y.; Kim, J.-I.; Chang, Y.W.; Park, J.-M.; Song, H.-W.; Kang, M.-J.; Pyun, J.-C. Gold nanoislands chip for laser desorption/ionization (LDI) mass spectrometry. BioChip J. 2017, 11, 246–254. [Google Scholar] [CrossRef]
- Jeong, I.; Lee, J.; Joseph, K.V.; Lee, H.I.; Kim, J.K.; Yoon, S.; Lee, J.J.N.E. Low-cost electrospun WC/C composite nanofiber as a powerful platinum-free counter electrode for dye sensitized solar cell. Nano Energy 2014, 9, 392–400. [Google Scholar] [CrossRef]
- Moreau, W.M. Semiconductor Lithography: Principles, Practices, and Materials; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Moore, T.I.; Aaron, J.; Chew, T.-L.; Springer, T.A. Measuring Integrin Conformational Change on the Cell Surface with Super-Resolution Microscopy. Cell Rep. 2018, 22, 1903–1912. [Google Scholar] [CrossRef]
- Gupta, S.N.; Bagchi, A.K.; Kundu, N.N. Defects in photomasks. Microelectron. J. 1985, 16, 22–40. [Google Scholar] [CrossRef]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ye, T.; Lee, S.-J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L.G. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, S.; Xu, J.; Li, W.; Ge, D.; Sun, C.; Liu, T.; Ma, X. Neural stem cell proliferation and differentiation in the conductive PEDOT-HA/Cs/Gel scaffold for neural tissue engineering. Biomater. Sci. 2017, 5, 2024–2034. [Google Scholar] [CrossRef]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264. [Google Scholar] [CrossRef]
- Huang, S.; Ingber, D.E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1999, 1, E131–E138. [Google Scholar] [CrossRef]
- Carisey, A.; Ballestrem, C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011, 90, 157–163. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Faivre, B.; Le Clainche, C. Integrating actin dynamics, mechanotransduction and integrin activation: The multiple functions of actin binding proteins in focal adhesions. Eur. J. Cell Biol. 2013, 92, 339–348. [Google Scholar] [CrossRef]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef]
- Yang, K.; Jung, K.; Ko, E.; Kim, J.; Park, K.I.; Kim, J.; Cho, S.-W. Nanotopographical Manipulation of Focal Adhesion Formation for Enhanced Differentiation of Human Neural Stem Cells. ACS Appl. Mater. Interfaces 2013, 5, 10529–10540. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kang, E.-S.; Baek, S.; Choo, S.-S.; Chung, Y.-H.; Lee, D.; Min, J.; Kim, T.-H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 14049. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, Y.; Lu, S. Direct role of interrod spacing in mediating cell adhesion on Sr-HA nanorod-patterned coatings. Int. J. Nanomed. 2014, 9, 1243–1260. [Google Scholar]
- Zhou, C.; Wang, Q.; Zhang, D.; Cai, L.; Du, W.; Xie, J. Compliant substratum modulates vinculin expression in focal adhesion plaques in skeletal cells. Int. J. Oral Sci. 2019, 11, 18. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Guo, Y.; Cheng, C.; Yang, L.; Jiang, L.; Yu, G.; Hu, W.; Liu, Y.; Zhu, D. Reduction of graphene oxide to highly conductive graphene by Lawesson’s reagent and its electrical applications. J. Mater. Chem. C 2013, 1, 3104–3109. [Google Scholar] [CrossRef]
- Fischer, R.S.; Lam, P.-Y.; Huttenlocher, A.; Waterman, C.M. Filopodia and focal adhesions: An integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev. Biol. 2019, 451, 86–95. [Google Scholar] [CrossRef]
- Campos, L.S. Neurospheres: Insights into neural stem cell biology. J. Neuroscien. Res. 2004, 78, 761–769. [Google Scholar] [CrossRef]
- Campos, L.S.; Leone, D.P.; Relvas, J.B.; Brakebusch, C.; Fässler, R.; Suter, U. β1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 2004, 131, 3433–3444. [Google Scholar] [CrossRef]
- Yoshimura, T.; Arimura, N.; Kaibuchi, K. Molecular Mechanisms of Axon Specification and Neuronal Disorders. Ann. N. Y. Acad. Sci. 2006, 1086, 116–125. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-W.; Kim, D.-S.; Suhito, I.R.; Han, D.K.; Lee, T.; Kim, T.-H. Enhancing Neurogenesis of Neural Stem Cells Using Homogeneous Nanohole Pattern-Modified Conductive Platform. Int. J. Mol. Sci. 2020, 21, 191. https://doi.org/10.3390/ijms21010191
Cho Y-W, Kim D-S, Suhito IR, Han DK, Lee T, Kim T-H. Enhancing Neurogenesis of Neural Stem Cells Using Homogeneous Nanohole Pattern-Modified Conductive Platform. International Journal of Molecular Sciences. 2020; 21(1):191. https://doi.org/10.3390/ijms21010191
Chicago/Turabian StyleCho, Yeon-Woo, Da-Seul Kim, Intan Rosalina Suhito, Dong Keun Han, Taek Lee, and Tae-Hyung Kim. 2020. "Enhancing Neurogenesis of Neural Stem Cells Using Homogeneous Nanohole Pattern-Modified Conductive Platform" International Journal of Molecular Sciences 21, no. 1: 191. https://doi.org/10.3390/ijms21010191
APA StyleCho, Y.-W., Kim, D.-S., Suhito, I. R., Han, D. K., Lee, T., & Kim, T.-H. (2020). Enhancing Neurogenesis of Neural Stem Cells Using Homogeneous Nanohole Pattern-Modified Conductive Platform. International Journal of Molecular Sciences, 21(1), 191. https://doi.org/10.3390/ijms21010191