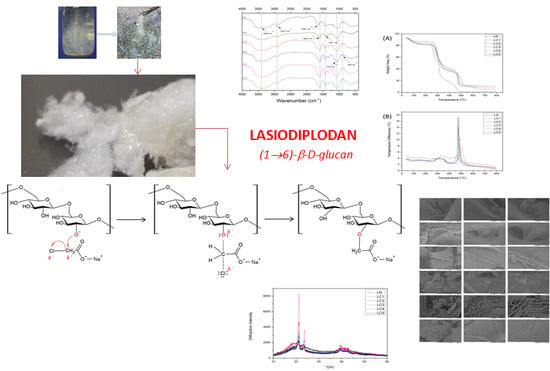
Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Degree of Carboxymethylation
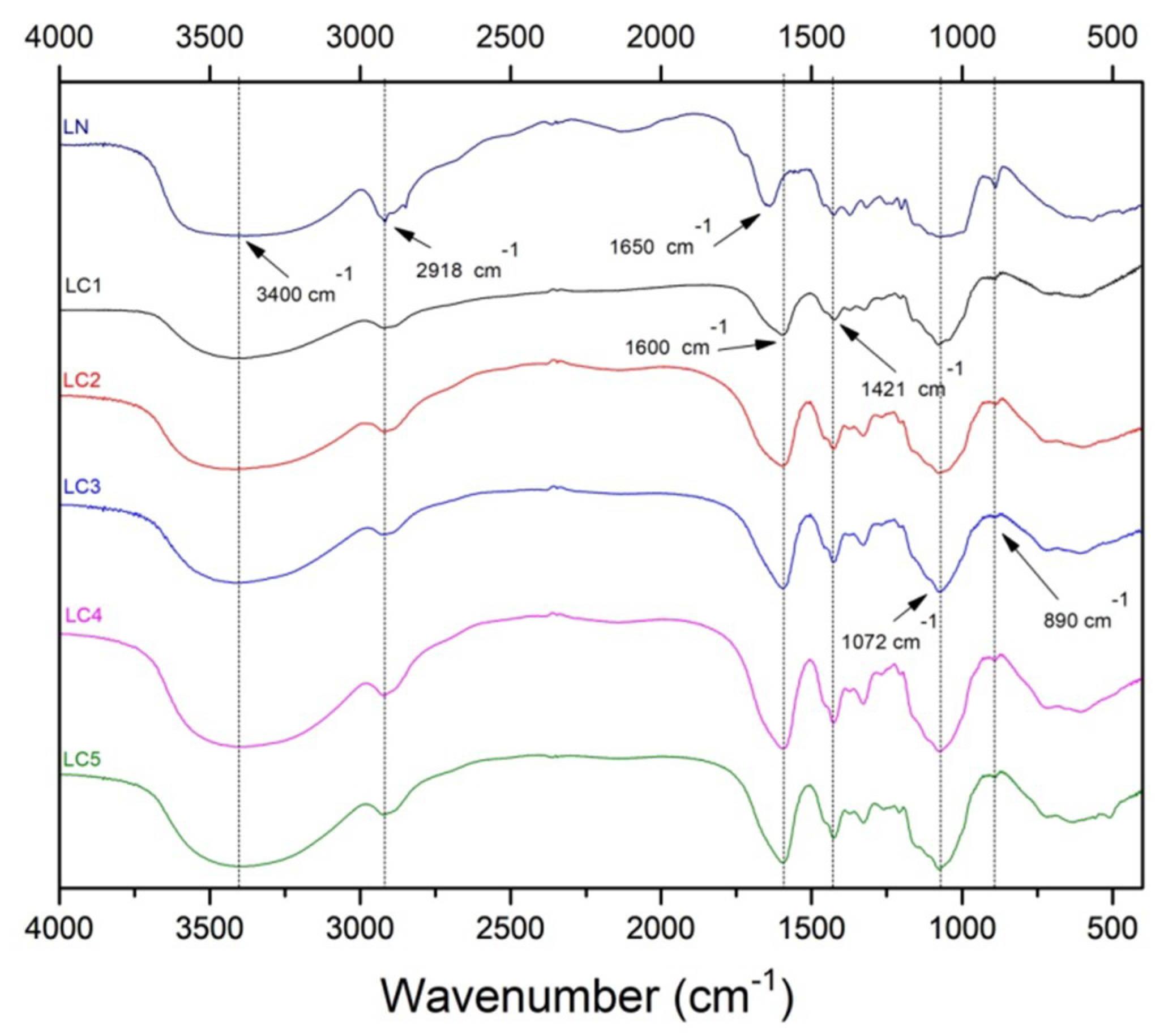
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
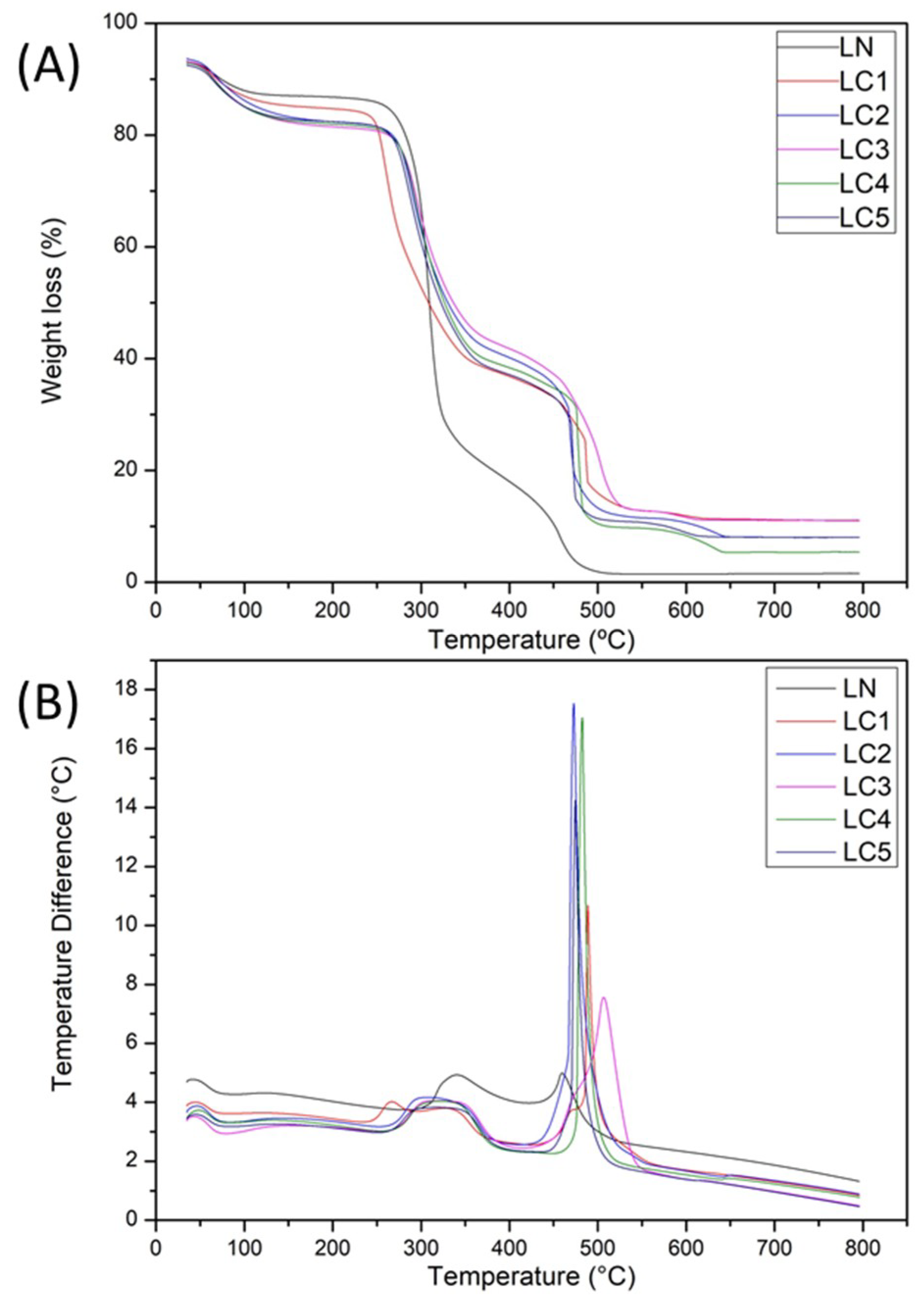
2.3. Thermal Characterization
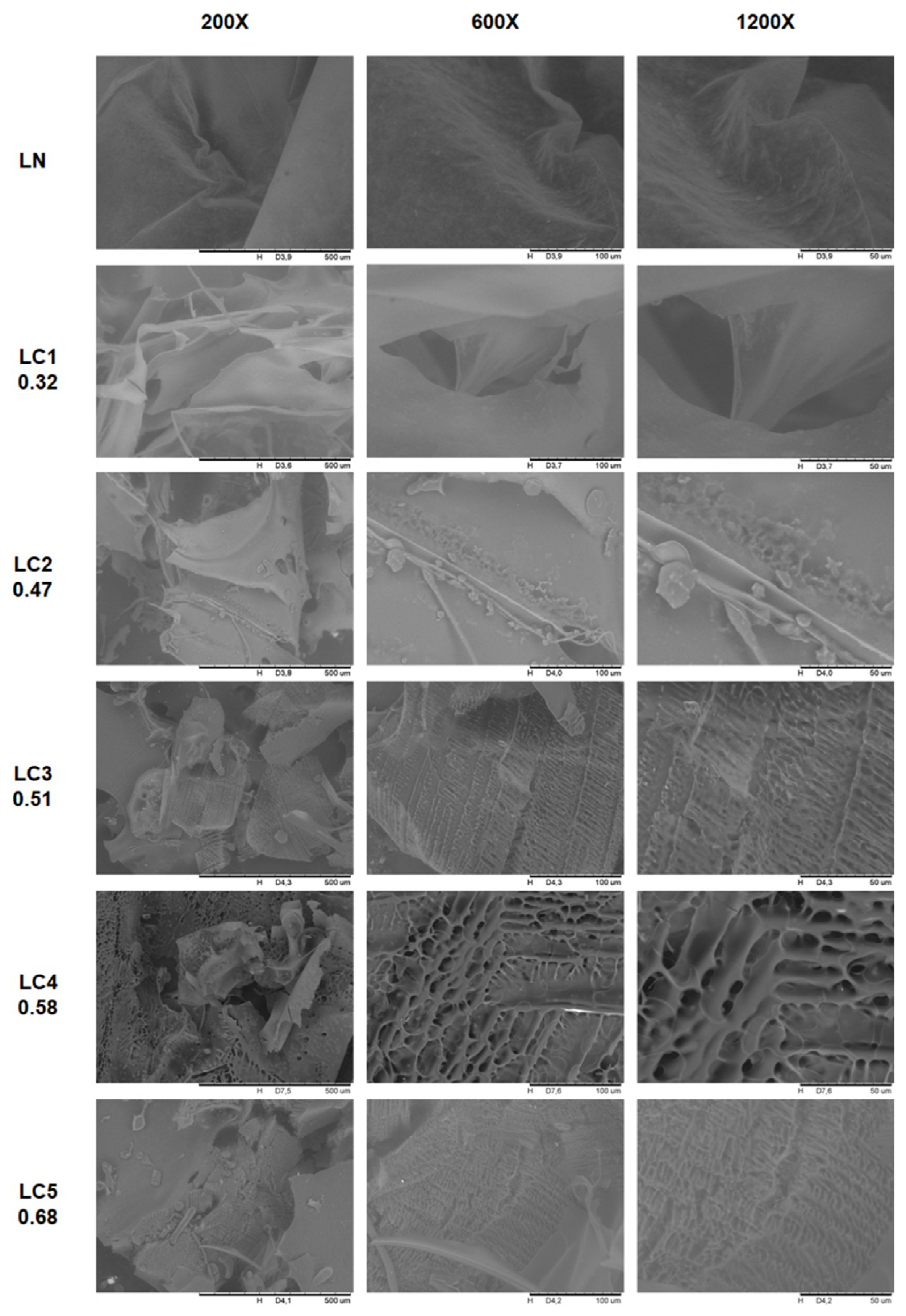
2.4. Morphological Characteristics of Native and Carboxymethylated Lasiodiplodan
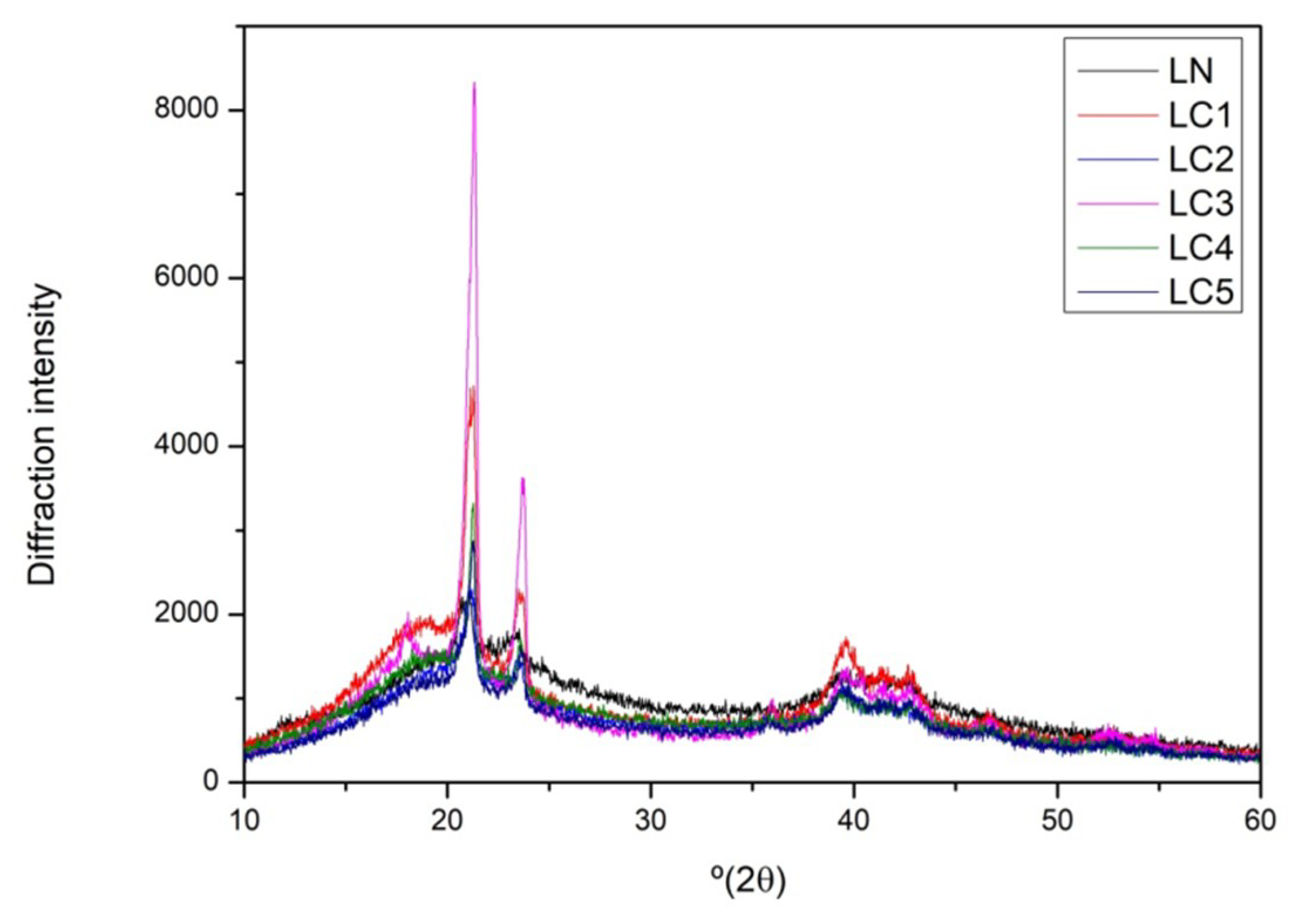
2.5. X-ray Diffraction
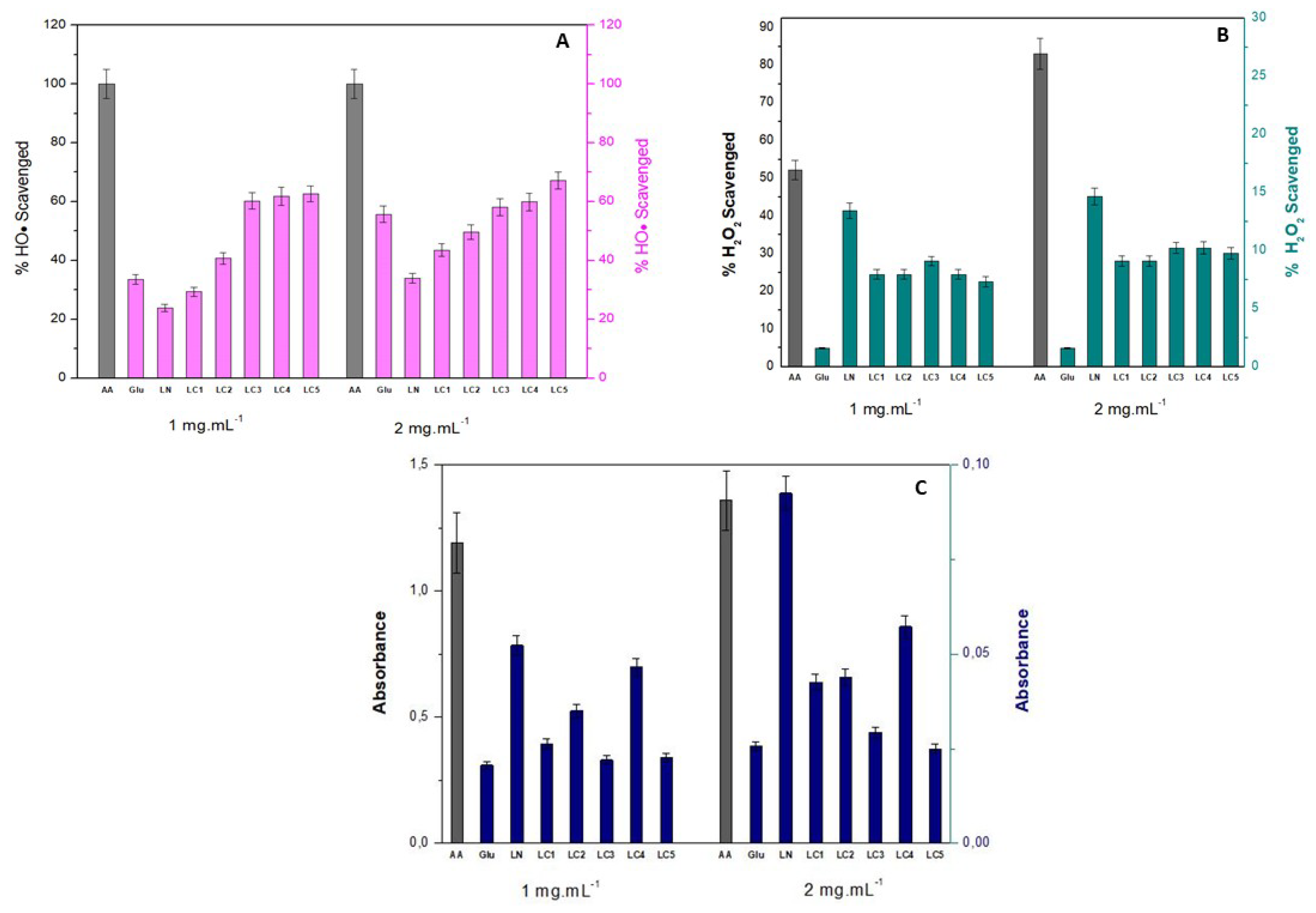
2.6. Antioxidant Ability
2.6.1. Hydroxyl Radical Scavenging Activity (OH•)
2.6.2. Hydrogen Peroxide Removal Scavenging Ability (H2O2)
2.6.3. Reducing Power
3. Materials and Methods
3.1. Biotechnological Process for Obtaining Lasiodiplodan
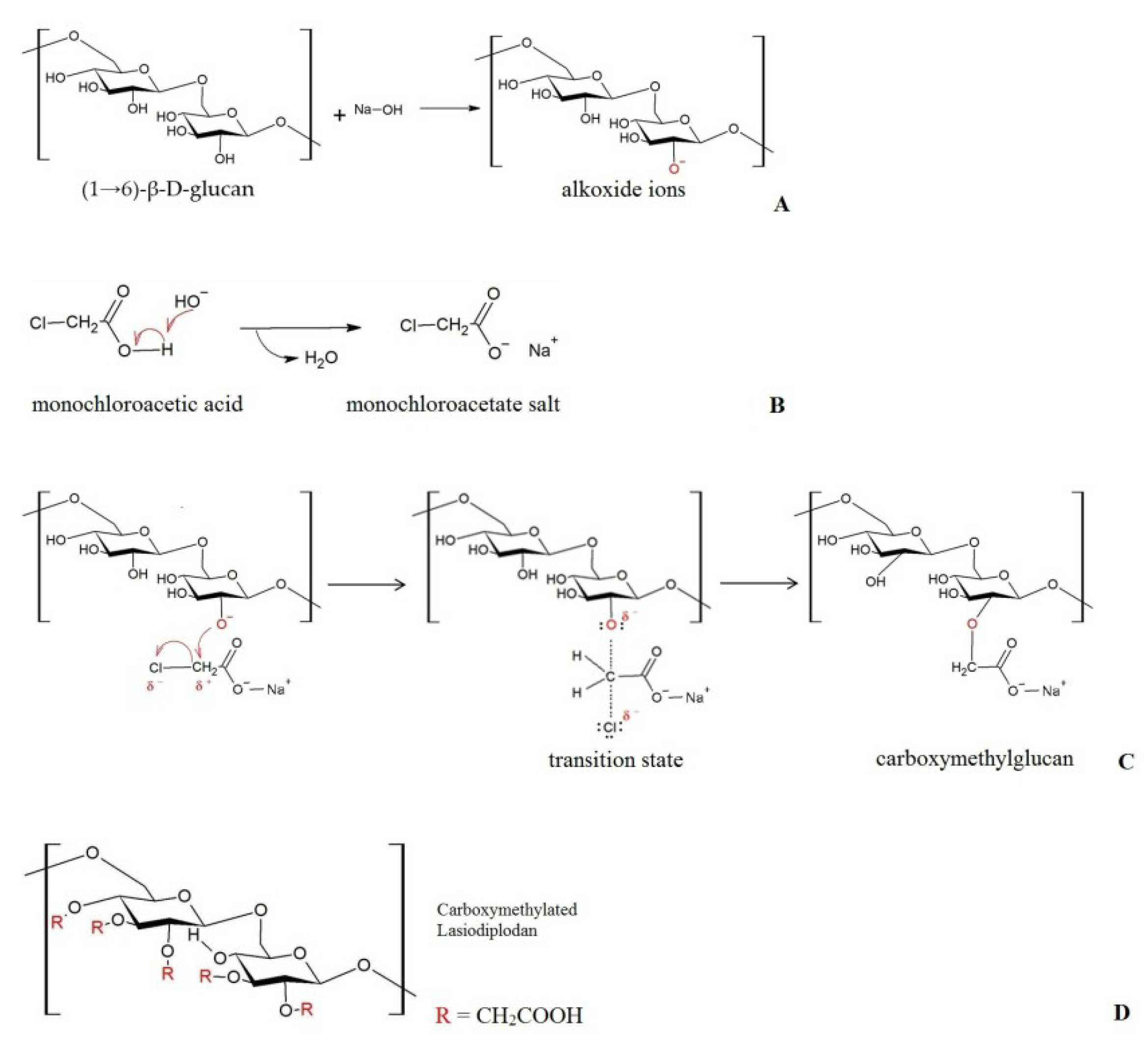
3.2. Carboxymethylation and the Degree of Substitution (DS)
3.3. Characterization of Native and Carboxymethylated Lasiodiplodan
3.3.1. Evaluation of Water Solubility
3.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.3. Thermal Analysis
3.3.4. Morphological Analysis by Scanning Electron Microscopy
3.3.5. X-ray Diffraction Analysis
3.4. In-Vitro Antioxidant Activity
3.4.1. Hydroxyl Radical Scavenging Activity Assay
3.4.2. Hydrogen Peroxide Scavenging Activity Assay
3.4.3. Assay of Reducing Power
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LN | Native lasiodiplodan |
| LC | Carboxymethylated lasiodiplodan |
| °C | Degree Celsius |
| θ | Theta |
| DS | Degree of substitution |
References
- Cunha, M.A.A.; Albornoz, S.L.; Santos, V.A.Q.; Sanchez, W.N.; Barbosa-Dekker, A.M.; Dekker, R.F.H. Structure and biological functions of d-glucans and their applications. In Studies in Natural Products Chemistry, 53rd ed.; Atta-ur-Rahmanc, Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 53, pp. 309–333. [Google Scholar]
- Chen, X.; Zhang, L.; Cheung, P.C.K. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated β-(1→3)-d-glucan from Poria cocos. Int. Immunopharmacol. 2010, 10, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kagimura, F.Y.; Cunha, M.A.A.; Barbosa, A.M.; Dekker, R.F.H.; Malfatti, C.R.M. Biological activities of derivatized d-glucans: A review. Int. J. Biol. Macromol. 2015, 72, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohyd. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Machová, E.; Bystrický, P.; Malovíková, A.; Bystrický, S. Preparation and characterization of carboxymethyl derivatives of yeast mannans in aqueous solutions. Carbohyd. Polym. 2014, 110, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheung, P.C.K.; Zhang, L.; Chiu, C.-M.; Ooi, V.E.C. Carboxymethylated β-glucans from mushroom sclerotium of Pleurotus tuber-regiumas novel water-soluble anti-tumor agent. Carbohyd Polym. 2004, 57, 319–325. [Google Scholar] [CrossRef]
- Machová, E.; Čížová, A.; Bystrický, P. Effect of carboxymethylation on antioxidant properties and radical degradation of mannans and glucans. Carbohyd Polym. 2014, 112, 603–607. [Google Scholar] [CrossRef]
- Theis, T.V.; Calegari, G.C.; Santos, V.A.Q.; Zorel-Júnior, H.E.; Barbosa, A.M.; Dekker, R.F.H.; Cunha, M.A.A. Exocellular (1→6)-β-d-glucan (lasiodiplodan): Carboxymethylation, thermal behavior, antioxidant and antimicrobial activity. Am. J. Immunol. 2017, 3, 19–33. [Google Scholar] [CrossRef]
- Vasconcelos, A.F.D.; Monteiro, N.K.; Dekker, R.F.H.; Barbosa, A.M.; Carbonero, E.R.; Silveira, J.L.M.; Sassaki, G.L.; Silva, R.; Silva, M.L.C. Three exopolysaccharides of the β-(1→6)-d-glucan type and a β-(1→3;1→6)-d-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit. Carbohyd Res. 2008, 343, 2481–2485. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.H.; Barbosa, A.M.; Teixeira, S.D.; Salomé, K. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): Preparation, characterization and antioxidant evaluation. Carbohyd Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef]
- Bai, N.; Gu, M.; Zhang, W.; Xu, W.; Mai, K. Effects of β-glucan derivatives on the immunity of white shrimp Litopenaeus vannamei and its resistance against white spot syndrome virus infection. Aquaculture 2014, 426–427, 66–73. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Tang, W.; Wang, H.; Nie, S.; Xie, M. Carboxymethylation of polysaccharide from Cyclocarya paliurus and their characterization and antioxidant properties evaluation. Carbohyd Polym. 2016, 136, 988–994. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, G.; Wu, X. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2011, 49, 1124–1130. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, Q.; Li, Z.; Lai, H.; Lou, J.; Liu, S.; Mao, J. Effects of degree of carboxymethylation on physicochemical and biological properties of pachyman. Int. J. Biol. Macromol. 2012, 51, 1052–1056. [Google Scholar] [CrossRef]
- Sharma, R.; Rana, V. Effect of carboxymethylation on rheological and drug release characteristics of Terminalia catappa gum. Carbohyd Polym. 2017, 175, 728–738. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 4th ed.; Cengage: São Paulo, Brazil, 2010. [Google Scholar]
- Xu, J.; Liu, W.; Yao, W.; Pang, X.; Yin, D.; Gao, X. Carboxymethylation of a polysaccharide extracted from Ganoderma lucidum enhances its antioxidant activities in vitro. Carbohyd Polym. 2009, 78, 227–234. [Google Scholar] [CrossRef]
- Abureesha, M.A.; Oladipo, A.A.; Gazi, M. Facile synthesis of glucose-sensitive chitosan–poly(vinyl alcohol) hydrogel: Drug release optimization and swelling properties. Int. J. Biol. Macromol. 2016, 90, 75–80. [Google Scholar] [CrossRef]
- Devièsea, T.; Ribechini, E.; Castex, D.; Stuart, B.; Regert, M.; Colombini, M.P. A multi-analytical approach using FTIR, GC/MS and Py-GC/MS revealed early evidence of embalming practices in Roman catacombs. Microchem J. 2017, 133, 49–59. [Google Scholar] [CrossRef]
- Ashori, A.; Babaee, M.; Jonoobi, M.; Hamzeh, Y. Solvent-free acetylation of cellulose nanofibers for improving compatibility and dispersion. Carbohyd Polym. 2014, 102, 369–375. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G.; Zhao, F.; Zhou, L.; Huang, S.; Li, H. The antioxidant activities of six (1→3)-β-d-glucan derivatives prepared from yeast cell wall. Int. J. Biol. Macromol. 2017, 98, 216–221. [Google Scholar] [CrossRef]
- Li, X.; Gao, W.; Huang, L.; Wang, Y.; Huang, L.; Liu, C. Preparation and physicochemical properties of carboxymethyl Fritillaria ussuriensis Maxim. starches. Carbohyd Polym. 2010, 80, 768–773. [Google Scholar] [CrossRef]
- Alekhina, M.; Mikkonen, K.S.; Alén, R.; Tenkanen, M.; Sixta, H. Carboxymethylation of alkaline extracted xylan for preparation of bio-based packing films. Carbohyd Polym. 2014, 100, 89–96. [Google Scholar] [CrossRef]
- Mittal, N.; Mattu, P.; Kaur, G. Extraction and derivatization of Leucaena leucocephala (Lam.) galactomannan: Optimization and characterization. Int. J. Biol. Macromol. 2016, 92, 831–841. [Google Scholar] [CrossRef]
- Bhatia, M.; Ahuja, M. Psyllium arabinoxylan: Carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Wang, M.; Zhang, L.; Xiao, S.; Fei, C.; Wang, X.; Zhang, K.; Zheng, W.; Wang, C.; Yang, R.; et al. Effects of low molecular weight yeast β-Glucan on antioxidant and immunological activities in mice. Int. J. Mol. Sci. 2015, 16, 21575–21590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohyd Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar]
- Shi, M.; Wei, X.; Xu, J.; Chen, B.I.; Zhao, D.; Cui, S.; Zhou, T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017, 215, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohyd Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohyd Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S.; Maity, S.; et al. Antioxidant and immunostimulant β-glucan from edible mushroom Russula albonigra (Krombh.) Fr. Carbohyd Polym. 2014, 99, 774–782. [Google Scholar] [CrossRef]
- Vogel, H.J. A convenient growth medium for Neurospora crassa. Genet. Bull. 1956, 13, 42–47. [Google Scholar]
- Cunha, M.A.A.; Túrmina, J.A.; Ivanov, R.C.; Barroso, R.R.; Marques, P.T.; Fonseca, E.A.I.; Fortes, Z.B.; Dekker, R.F.H.; Khaper, E.N.; Barbosa, A.M. Lasiodiplodan, an exocellular (1→6)-β-d-glucan from Lasiodiplodia theobromae MMPI: Production on glucose, fermentation kinetics, rheology and anti-proliferative activity. J. Ind. Microbiol. Biotechnol. 2012, 39, 1179–1188. [Google Scholar] [CrossRef]
- Tatongjai, J.; Lumdubwong, N. Physicochemical properties and textile utilization of low and moderate substituted carboxymethyl rice starches with various amylose content. Carbohyd. Polym. 2010, 81, 377–384. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Ton, J.K.H.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
| Sample | Ratio of Lasiodiplodan/Monochloroacetic Acid (g/g) | Carboxymethyl Groups * (% by mass) | DS # | Solubility ** (%) |
|---|---|---|---|---|
| LN | - | - | - | 4.02 a ± 0.07 |
| LC1 | 1:3 | 10.28 | 0.32 | 67.99 b ± 0.86 |
| LC2 | 1:5 | 14.40 | 0.47 | 54.30 c ± 1.29 |
| LC3 | 1:6.5 | 15.44 | 0.51 | 51.20 cd ± 1.92 |
| LC4 | 1:8 | 17.19 | 0.58 | 49.58 d ± 0.39 |
| LC5 | 1:11.5 | 19.58 | 0.68 | 45.50 e ± 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theis, T.V.; Queiroz Santos, V.A.; Appelt, P.; M. Barbosa-Dekker, A.; Vetvicka, V.; F. H. Dekker, R.; A. Cunha, M.A. Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 2337. https://doi.org/10.3390/ijms20092337
Theis TV, Queiroz Santos VA, Appelt P, M. Barbosa-Dekker A, Vetvicka V, F. H. Dekker R, A. Cunha MA. Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity. International Journal of Molecular Sciences. 2019; 20(9):2337. https://doi.org/10.3390/ijms20092337
Chicago/Turabian StyleTheis, Thais Vanessa, Vidiany Aparecida Queiroz Santos, Patrícia Appelt, Aneli M. Barbosa-Dekker, Vaclav Vetvicka, Robert F. H. Dekker, and Mário A. A. Cunha. 2019. "Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity" International Journal of Molecular Sciences 20, no. 9: 2337. https://doi.org/10.3390/ijms20092337
APA StyleTheis, T. V., Queiroz Santos, V. A., Appelt, P., M. Barbosa-Dekker, A., Vetvicka, V., F. H. Dekker, R., & A. Cunha, M. A. (2019). Fungal Exocellular (1-6)-β-d-glucan: Carboxymethylation, Characterization, and Antioxidant Activity. International Journal of Molecular Sciences, 20(9), 2337. https://doi.org/10.3390/ijms20092337