Effects of the Emitted Light Spectrum of Liquid Crystal Displays on Light-Induced Retinal Photoreceptor Cell Damage
Abstract
1. Introduction
2. Results
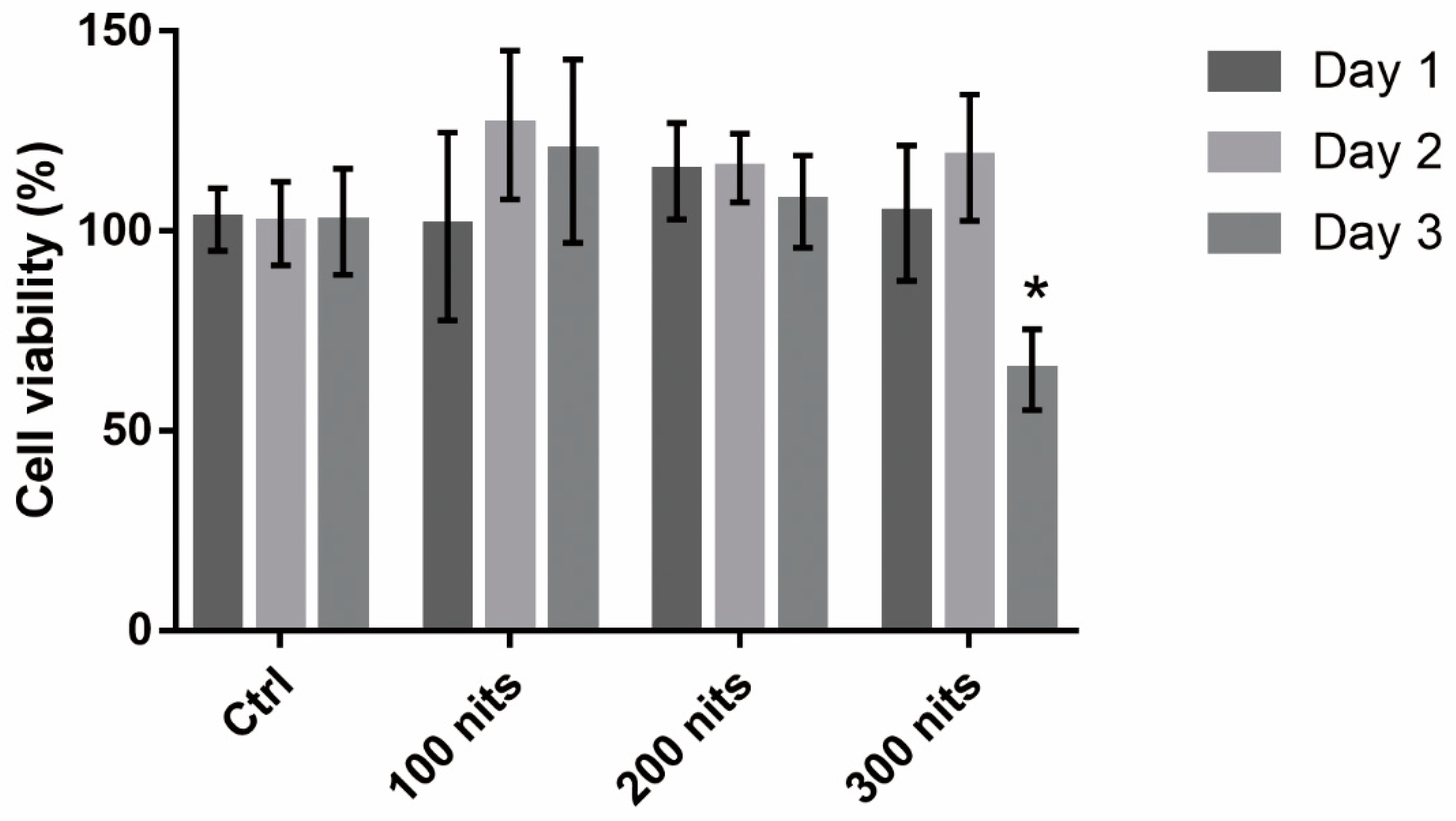
2.1. 661 W Cell Death upon Exposure to Differing-Luminance LCDs
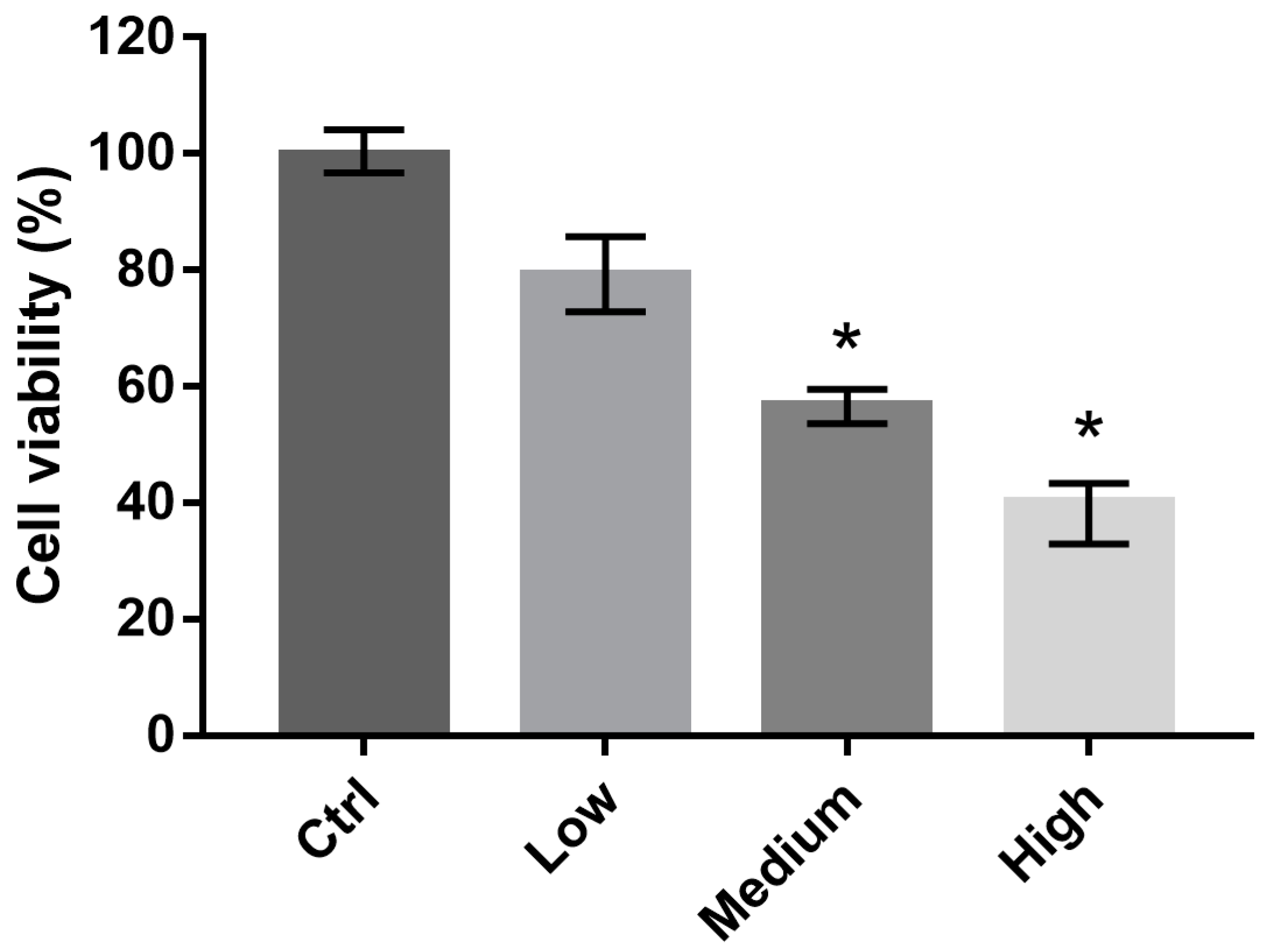
2.2. 661 W Cell Viability upon Exposure to Differing-OEEI LCDs
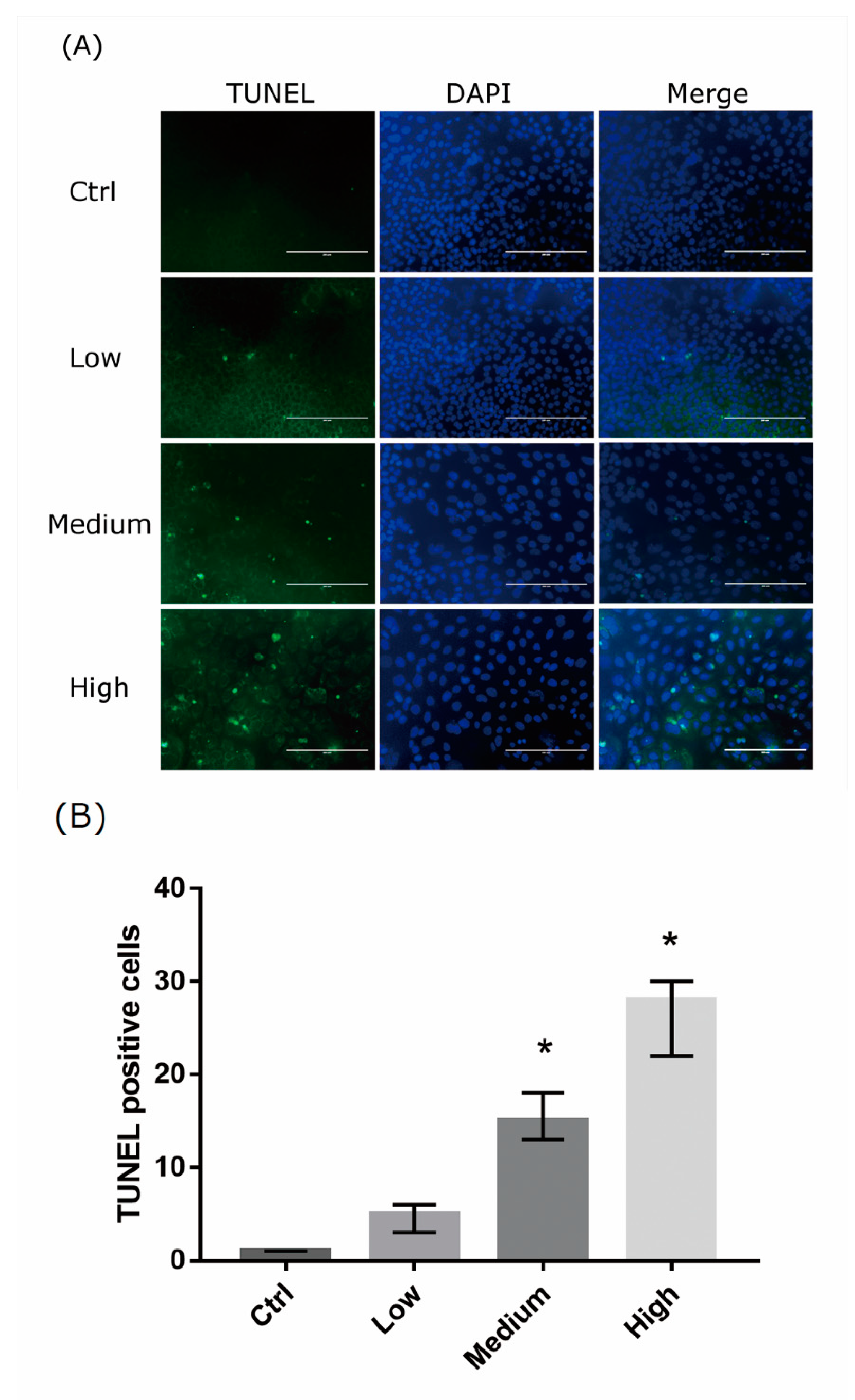
2.3. 661 Cell Apoptosis upon Exposure to Differing-OEEI LCDs
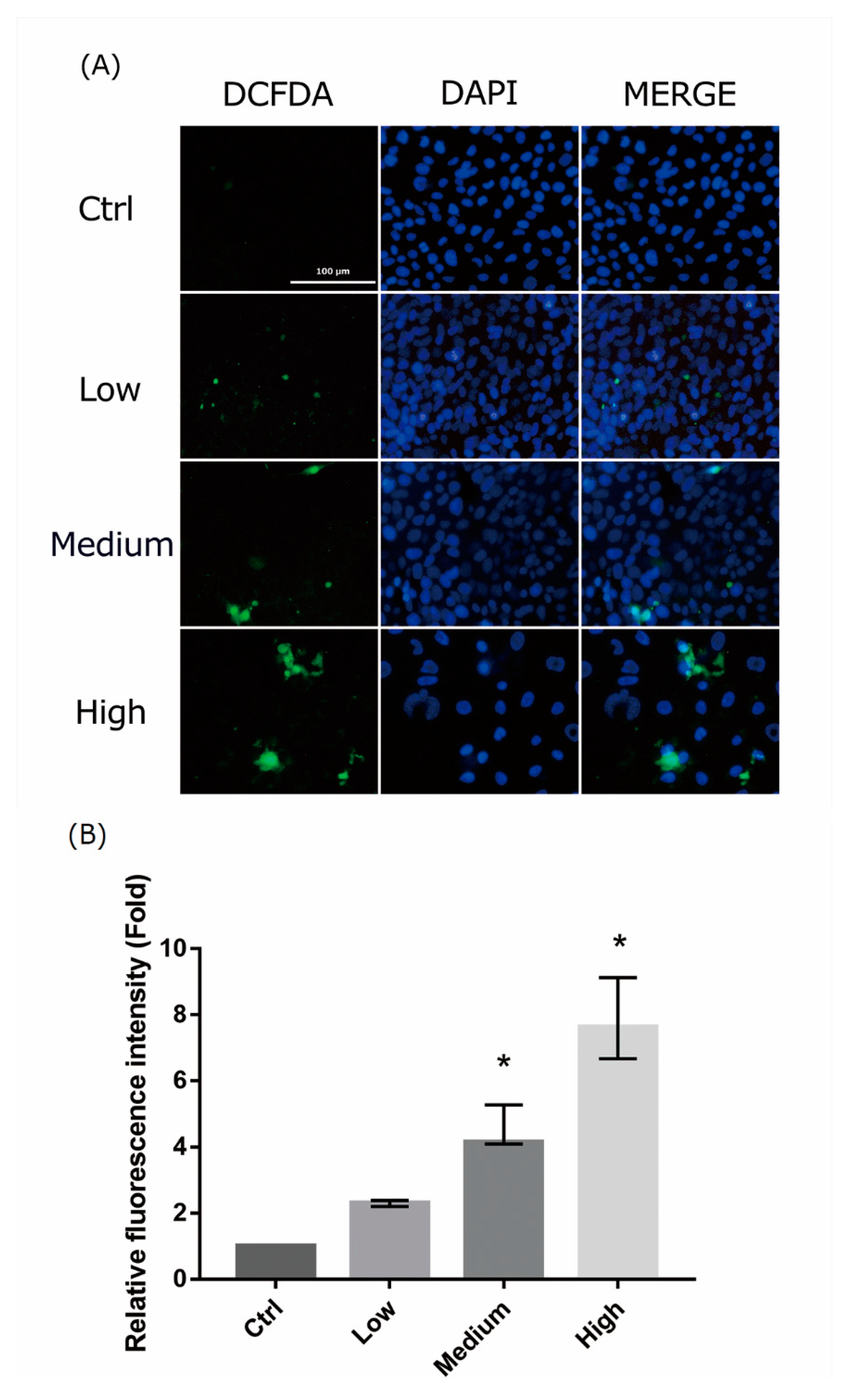
2.4. 661 W Cell ROS Production upon Differing-OEEI LCD Exposure
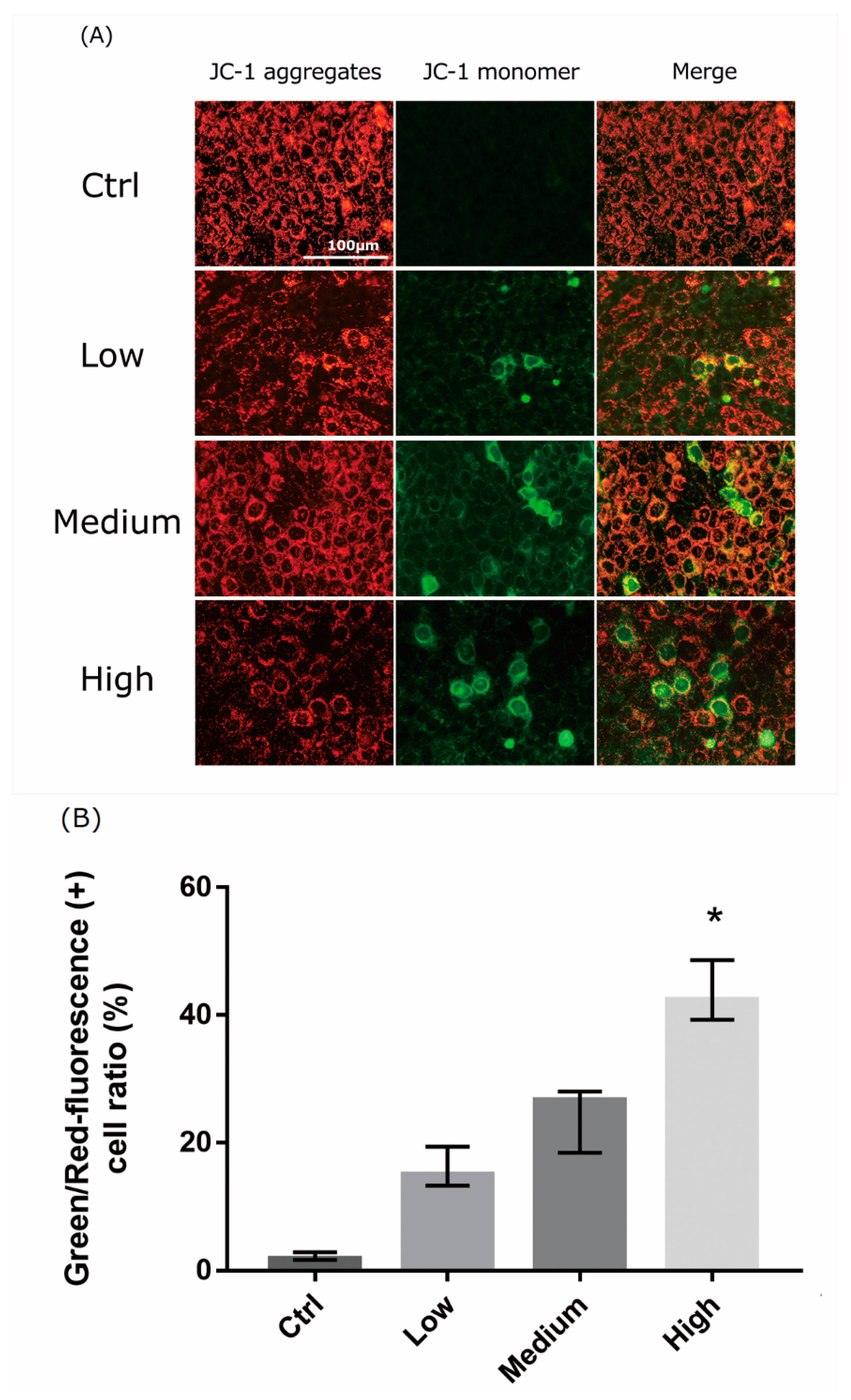
2.5. 661 W Cell Mitochondrial Damage upon Differing-OEEI LCD Exposure
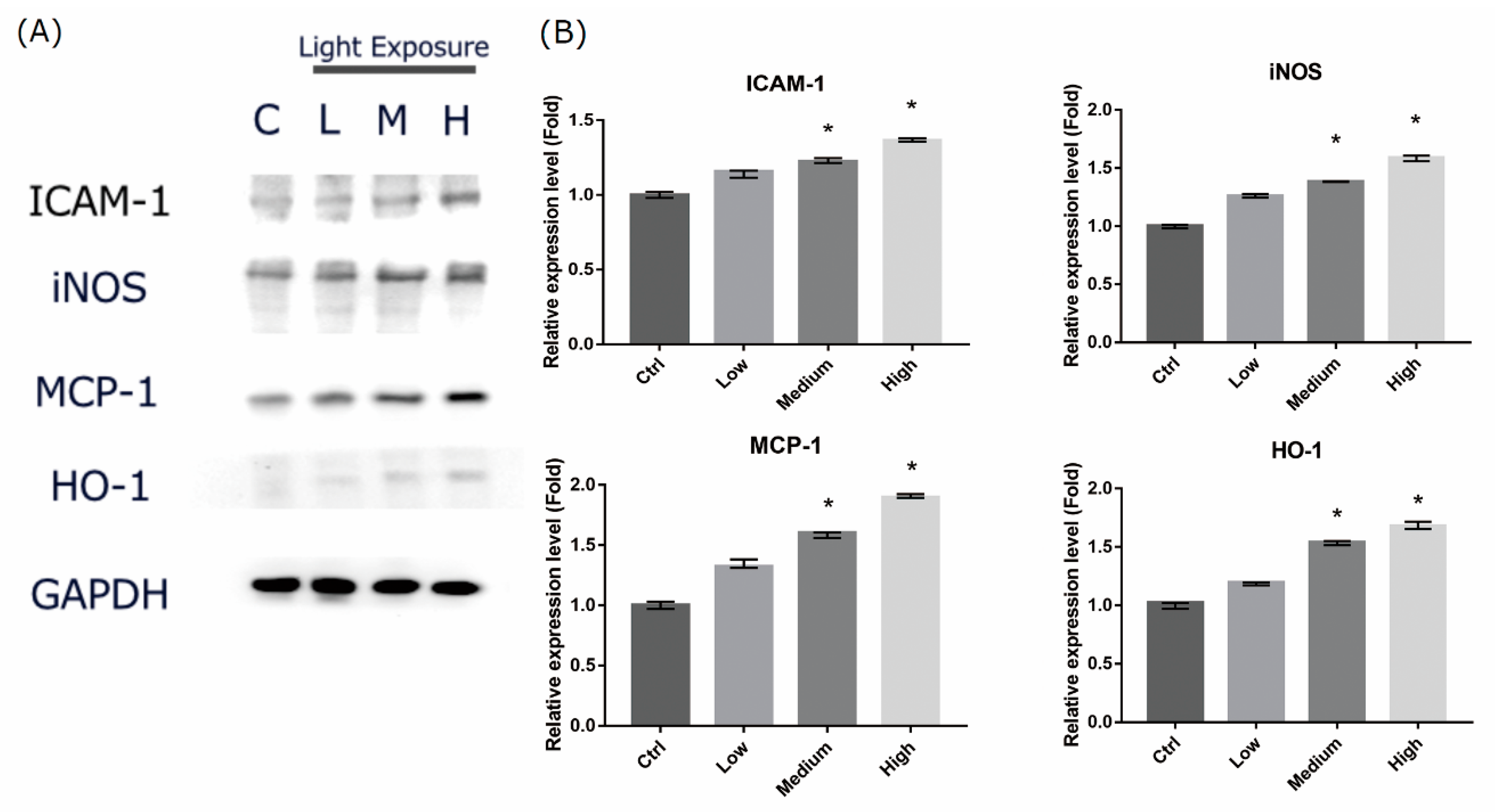
2.6. LCD-exposed 661W Cell Oxidative Stress/Inflammatory Response-associated Protein Expression
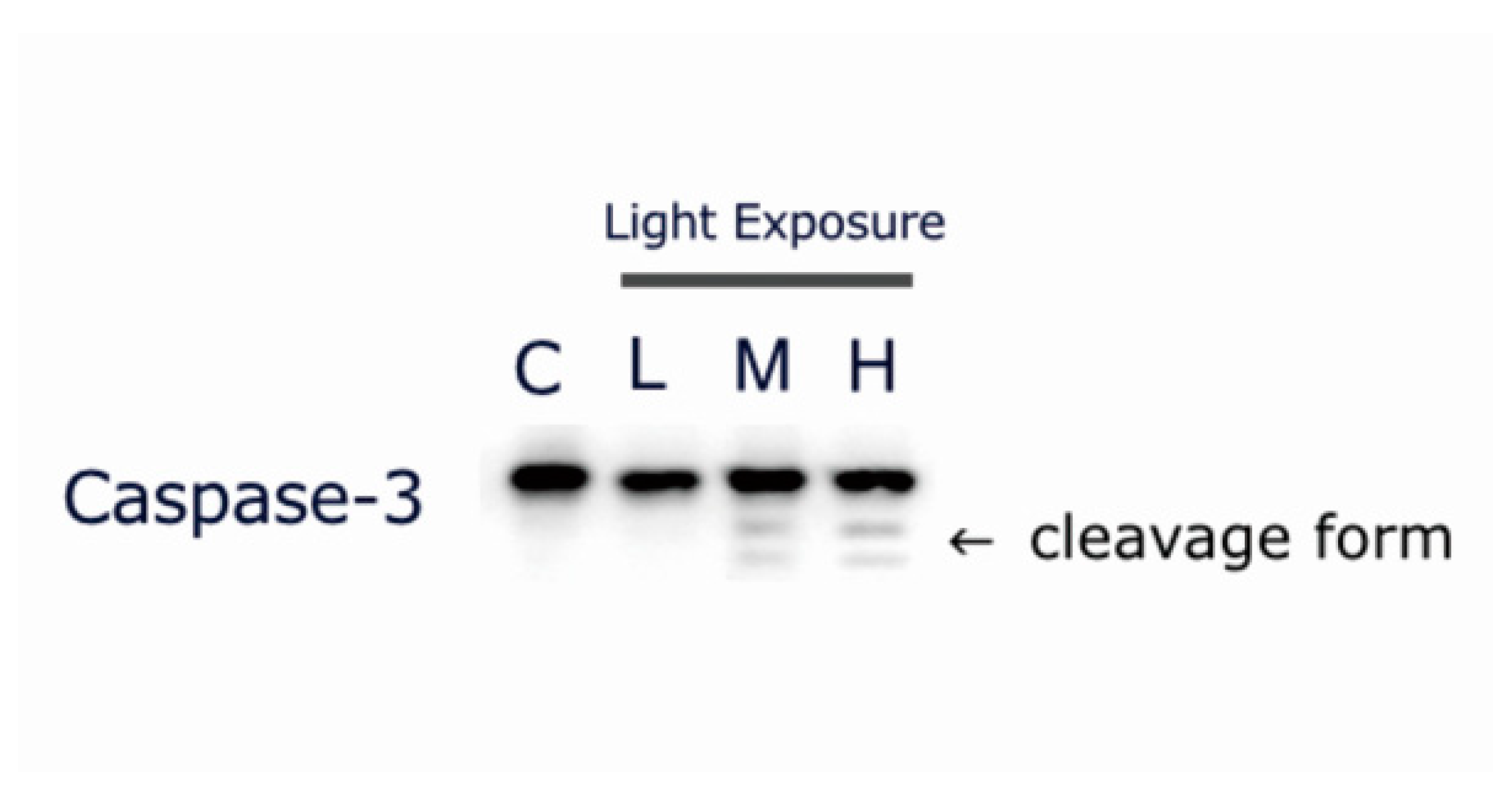
2.7. LCD-exposed 661W Cell Apoptosis-related Protein Cleaved Caspase-3 Expression
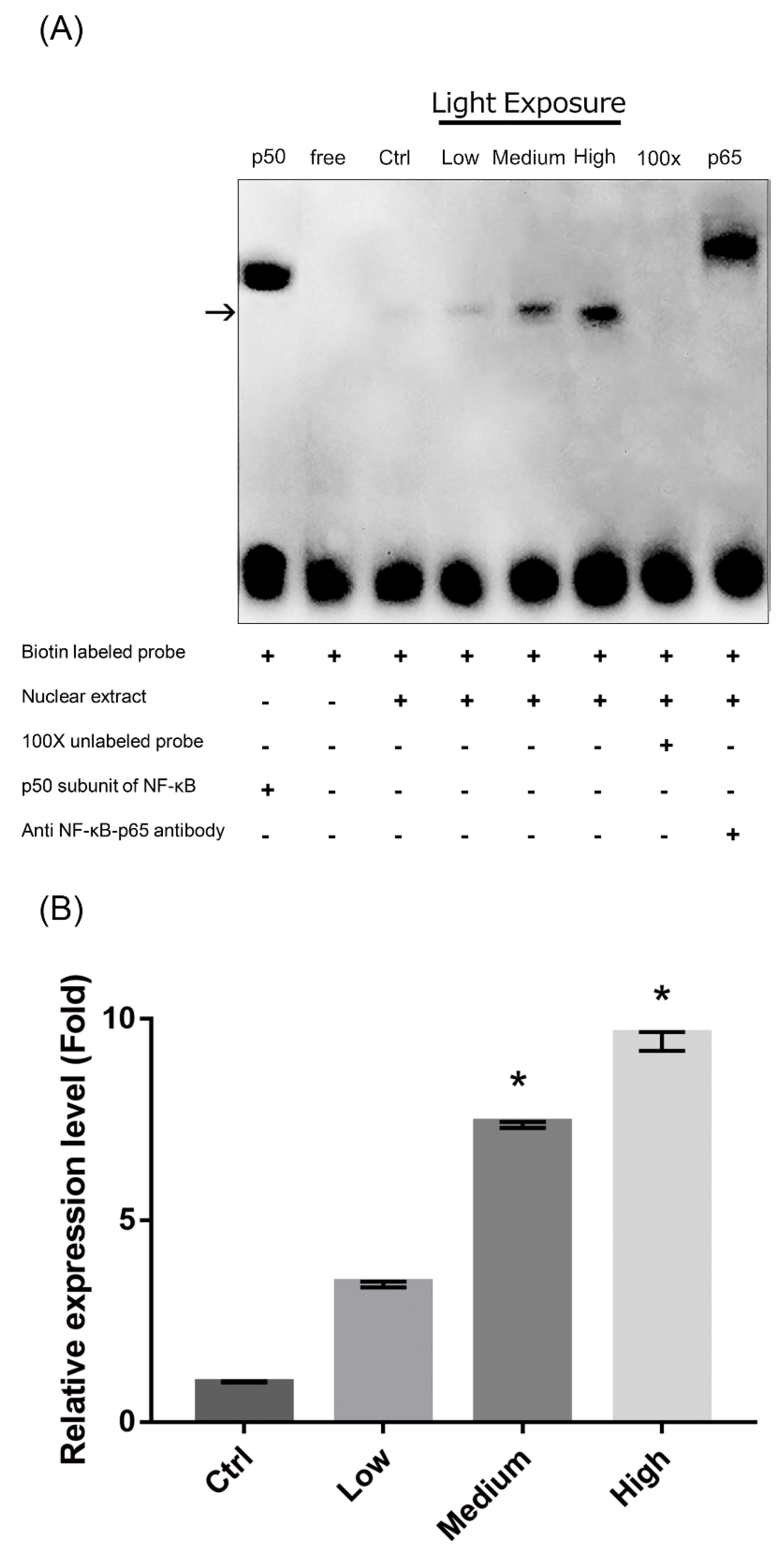
2.8. Nuclear Factor-κB (NF-κB) Pathway Activation in LCD-exposed 661W Cells
3. Discussion
4. Materials and Methods
4.1. 661 W Cell Culture
4.2. LCD Light Exposure
4.3. Cell Viability Assay
4.4. Preparation of LCDs with Different Energy Spectra (OEEI)
4.5. TUNEL Assay
4.6. Measurement of Intracellular ROS
4.7. Mitochondrial Membrane Potential Detection
4.8. Western Blot
4.9. Nuclear Protein Extraction and NF-κB EMSA
4.10. NF-κB EMSA
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LCD | Liquid crystal display |
| LED | Light-emitting diodes |
| RPE | Retinal pigment epithelial |
| AMD | Age-related macular degeneration |
| ROS | Reactive oxygen species |
| OEEI | Ocular energy exposure index |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP-biotinide end labeling |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| ICAM-1 | Intercellular adhesion molecule 1 |
| iNOS | Inducible nitric oxide synthase |
| MCP-1 | Monocyte chemoattractant protein 1 |
| HO-1 | Heme oxygenase-1 |
| NF-κB | Nuclear factor-κB |
| EMSA | Electrophoretic mobility shift assay |
| DMEM | Dulbecco’s modified Eagle’s media |
| PBS | Phosphate buffer solution |
| H2DCF | 2′,7′-dichlorodihydrofluorescein |
| DCF | Dichlorofluorescein |
| EDTA | Ethylenediaminetetraacetic acid |
| PBST | PBS containing 0.1% Tween-20 |
| DTT | Dithiothreitol |
| PMSF | Phenylmethylsulfonyl fluoride |
References
- Holzman, D.C. What’s in a color? The unique human health effect of blue light. Environ. Health Perspect. 2010, 118, A22–A27. [Google Scholar] [CrossRef]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Adverse health effects of nighttime lighting. Am. J. Prev. Med. 2013, 45, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.O. Photic maculopathy in rhesus monkey. A light and electron microscopic study. Investig. Ophthalmol. 1973, 12, 17–34. [Google Scholar]
- Parver, L.M.; Auker, C.R.; Fine, B.S. Observations on monkey eyes exposed to light from an operating microscope. Ophthalmolgy 1983, 90, 964–972. [Google Scholar] [CrossRef]
- Ham, W.T., Jr.; Mueller, H.A.; Sliney, D.H. Retinal sensitivity to damage from short wavelength light. Nature 1976, 260, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Rozanowska, M.; Rozanowski, B. Retinal photodamage. J. Photochem. Photobiol. B 2001, 64, 144–161. [Google Scholar] [CrossRef]
- Chamorro, E.; Bonnin-Arias, C.; Pérez-Carrasco, M.J.; Luna, J.M.; Vázquez, D.; Sánchez-Ramose, C. Effects of light-emitting diode radiations on human retinal pigment epithelial cells in vitro. Photochem. Photobiol. 2013, 89, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. Solar radiation and age-related macular degeneration. Surv. Ophthalmol. 1988, 32, 252–269. [Google Scholar] [CrossRef]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef]
- Hafezi, F.; Marti, A.; Munz, K.; Reme, C.E. Light-induced apoptosis: Differential timing in the retina and pigment epithelium. Exp. Cell Res. 1997, 64, 963–970. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.; Yang, C.H.; Lee, L.L. White light-emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Glazer-Hockstein, C.; Dunaief, J.L. Could blue light-blocking lenses decrease the risk of age-related macular degeneration? Retina 2006, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Margrain, T.H.; Boulton, M.; Marshall, J.; Sliney, D.H. Do blue light filters confer protection against age-related macular degeneration? Prog. Retin. Eye Res. 2004, 23, 523–531. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.; Yang, C.H.; Lee, L.L. Light-emitting-diode induced retinal damage and its wavelength dependency in vivo. Int. J. Ophthalmol. 2017, 10, 191–202. [Google Scholar]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Zhou, J.; Jang, Y.P.; Kim, S.R.; Sparrow, J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2006, 103, 16182–16187. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Nakamura, M.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. The involvement of the oxidative stress in murine blue LED light-induced retinal damage model. Biol. Pharm. Bull. 2017, 40, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Brøndsted, A.E.; Lundeman, J.H.; Kessel, L. Short wavelength light filtering by the natural human lens and IOLs—Implications for entrainment of circadian rhythm. Acta Ophthalmol. 2013, 91, 52–57. [Google Scholar] [CrossRef]
- Downie, L.E.; Wormald, R.; Evans, J.; Virgili, G.; Keller, P.R.; Lawrenson, J.G.; Li, T. Analysis of a systematic review about blue light-filtering intraocular lenses for retinal protection: Understanding the limitations of the evidence. JAMA Ophthalmol. 2019. Published online February 21. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.A.R.; Parhoodeh, S.; Hosseini, M.A.; Arabi, H.; Malakooti, H.; Nematollahi, S.; Mortazavi, G.; Darvish, L.; Mortazavi, S.M.J. Blocking short-wavelength component of the visible light emitted by smartphones’ screens improves human sleep quality. J. Biomed. Phys. Eng. 2018, 8, 375–380. [Google Scholar] [PubMed]
- Oh, J.H.; Yoo, H.; Park, H.K.; Do, Y.R. Analysis of circadian properties and healthy levels of blue light from smartphones at night. Sci. Rep. 2015, 5, 11325. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.B.; Khazova, M.; Price, L.L. Low-energy light bulbs, computers, tablets and the blue light hazard. Eye (Lond) 2016, 30, 230–233. [Google Scholar] [CrossRef]
- Santos, A.M.; Lopez-Sanchez, N.; Martin-Oliva, D.; de la Villa, P.; Cuadros, M.A.; Frade, J.M. Sortilin participates in light-dependent photoreceptor degeneration in vivo. PLoS ONE 2012, 7, e36243. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, X.A.; Tso, M.O. Role of NF-kappaB and MAPKs in light-induced photoreceptor apoptosis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4766–4776. [Google Scholar] [CrossRef]
- Chen, W.J.; Wu, C.; Xu, Z.; Kuse, Y.; Hara, H.; Duh, E.J. Nrf2 protects photoreceptor cells from photo-oxidative stress induced by blue light. Exp. Eye Res. 2017, 154, 151–158. [Google Scholar] [CrossRef]
- Ooe, E.; Kuse, Y.; Yako, T.; Sogon, T.; Nakamura, S.; Hara, H.; Shimazawa, M. Bilberry extract and anthocyanins suppress unfolded protein response induced by exposure to blue LED light of cells in photoreceptor cell line. Mol. Vis. 2018, 24, 621–632. [Google Scholar]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef]
- Freeman, B.A.; Crapo, J.D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981, 256, 10986–10992. [Google Scholar]
- Fridovich, I. Oxygen toxicity: A radical explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Lin, C.W.; Huang, H.H.; Yang, C.M.; Yang, C.H. Protective effect of chitosan oligosaccharides on blue light light-emitting diode induced retinal pigment epithelial cell damage. J. Funct. Food 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Pilat, A.; Herrnreiter, A.M.; Skumatz, C.M.; Sarna, T.; Burke, J.M. Oxidative stress increases HO-1 expression in ARPE-19 cells, but melanosomes suppress the increase when light is the stressor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 47–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piehl, L.; Capani, F.; Facorro, G.; López, E.M.; de Celis, E.R.; Pustovrh, C.; Hager, A.; Coirini, H.; López-Costa, J.J. Nitric oxide increases in the rat retina after continuous illumination. Brain Res. 2007, 1156, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Pang, X.; Boucher, W.; Letourneau, R.; Reale, M.; Barbacane, R.C.; Thibault, J.; Theoharides, T.C. Monocyte chemotactic protein-1 is a proinflammatory chemokine in rat skin injection sites and chemoattracts basophilic granular cells. Int. Immunol. 1997, 9, 1563–1570. [Google Scholar] [CrossRef]
- Satoh, S.; Nüssler, A.K.; Liu, Z.Z.; Thomson, A.W. Proinflammatory cytokines and endotoxin stimulate ICAM-1 gene expression and secretion by normal human hepatocytes. Immunology 1994, 82, 571–576. [Google Scholar]
- Lee, S.J.; Drabik, K.; Van Wagoner, N.J.; Lee, S.; Choi, C.; Dong, Y.; Benveniste, E.N. ICAM-1-induced expression of proinflammatory cytokines in astrocytes: Involvement of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. J. Immunol. 2000, 165, 4658–4666. [Google Scholar] [CrossRef]
- Jo, N.; Wu, G.S.; Rao, N.A. Up-regulation of chemokine expression in the retinal vasculature in ischemia-reperfusion injury. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4054–4060. [Google Scholar] [CrossRef]
- Freter, R.R.; Alberta, J.A.; Hwang, G.Y.; Wrentmore, A.L.; Stiles, C.D. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-κB and a 90-kDa phosphoprotein coactivator. J. Biol. Chem. 1996, 271, 17417–17424. [Google Scholar] [CrossRef]
- Lemay, S.; Lebedeva, T.V.; Singh, A.K. Inhibition of cytokine gene expression by sodium salicylate in a macrophage cell line through an NF-κB-independent mechanism. Clin. Diagn. Lab. Immunol. 1999, 6, 567–572. [Google Scholar]
- Jacquemin, E.; de Kozak, Y.; Thillaye, B.; Courtois, Y.; Goureau, O. Expression of inducible nitric oxide synthase in the eye fromendotoxin-induced uveitis rats. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1187–1196. [Google Scholar]
- Schreck, R.; Albermann, K.; Baeuerle, P.A. Nuclear factor kappa B: An oxidative stress-responsive transcription factor of eukaryotic cells. Free Radic. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Apoptosis and nuclear factor-kappa B: A tale of association and dissociation. Biochem. Pharmacol. 2000, 60, 1033–1039. [Google Scholar] [CrossRef]
- Thompson, A.F.; Crowe, M.E.; Lieven, C.J.; Levin, L.A. Induction of Neuronal Morphology in the 661W Cone Photoreceptor Cell Line with Staurosporine. PLoS ONE 2015, 10, e0145270. [Google Scholar] [CrossRef]
- Bell, R.A.V.; Megeney, L.A. Evolution of caspase-mediated cell death and differentiation: Twins separated at birth. Cell Death Differ. 2017, 24, 1359–1368. [Google Scholar] [CrossRef]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef]
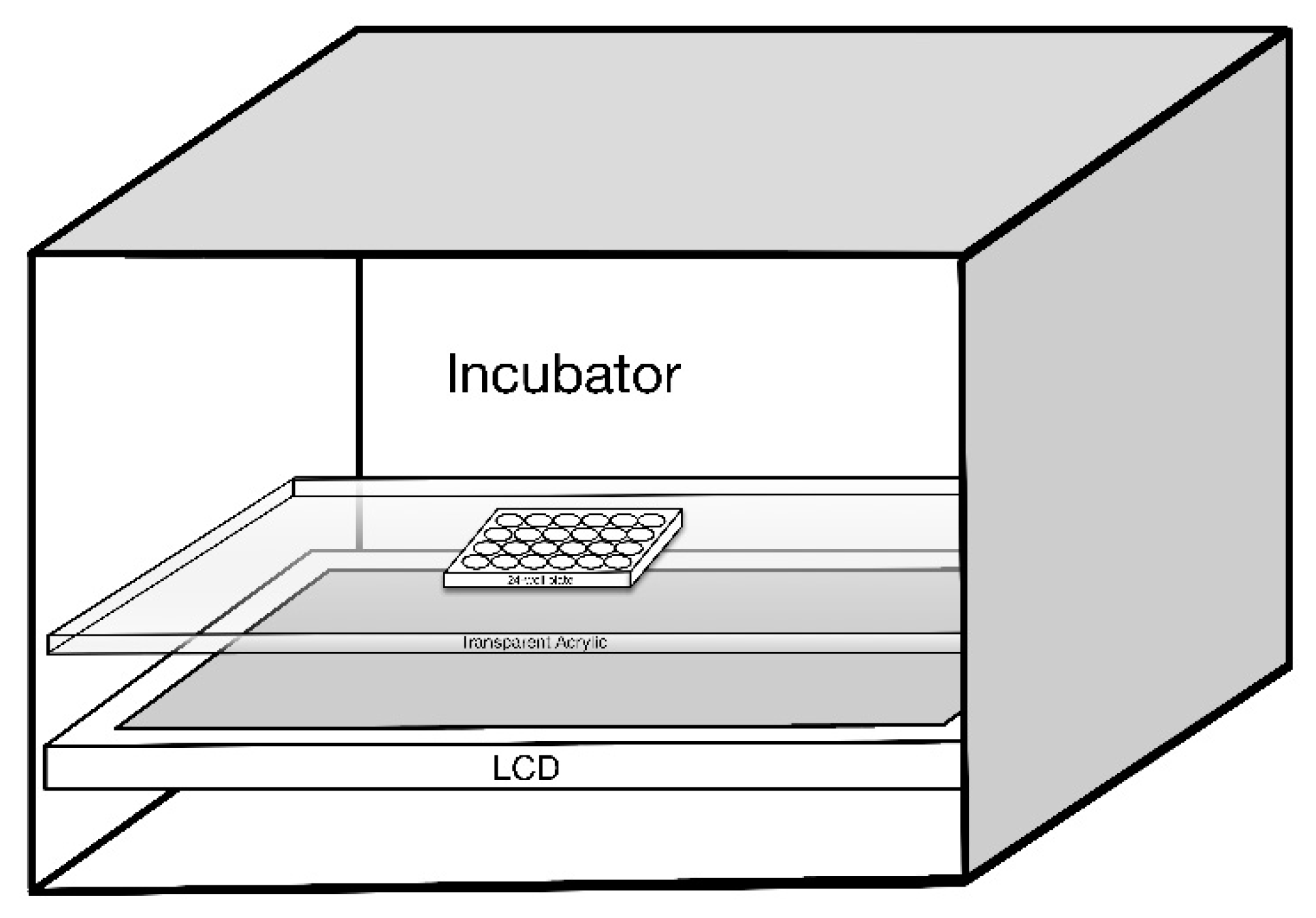
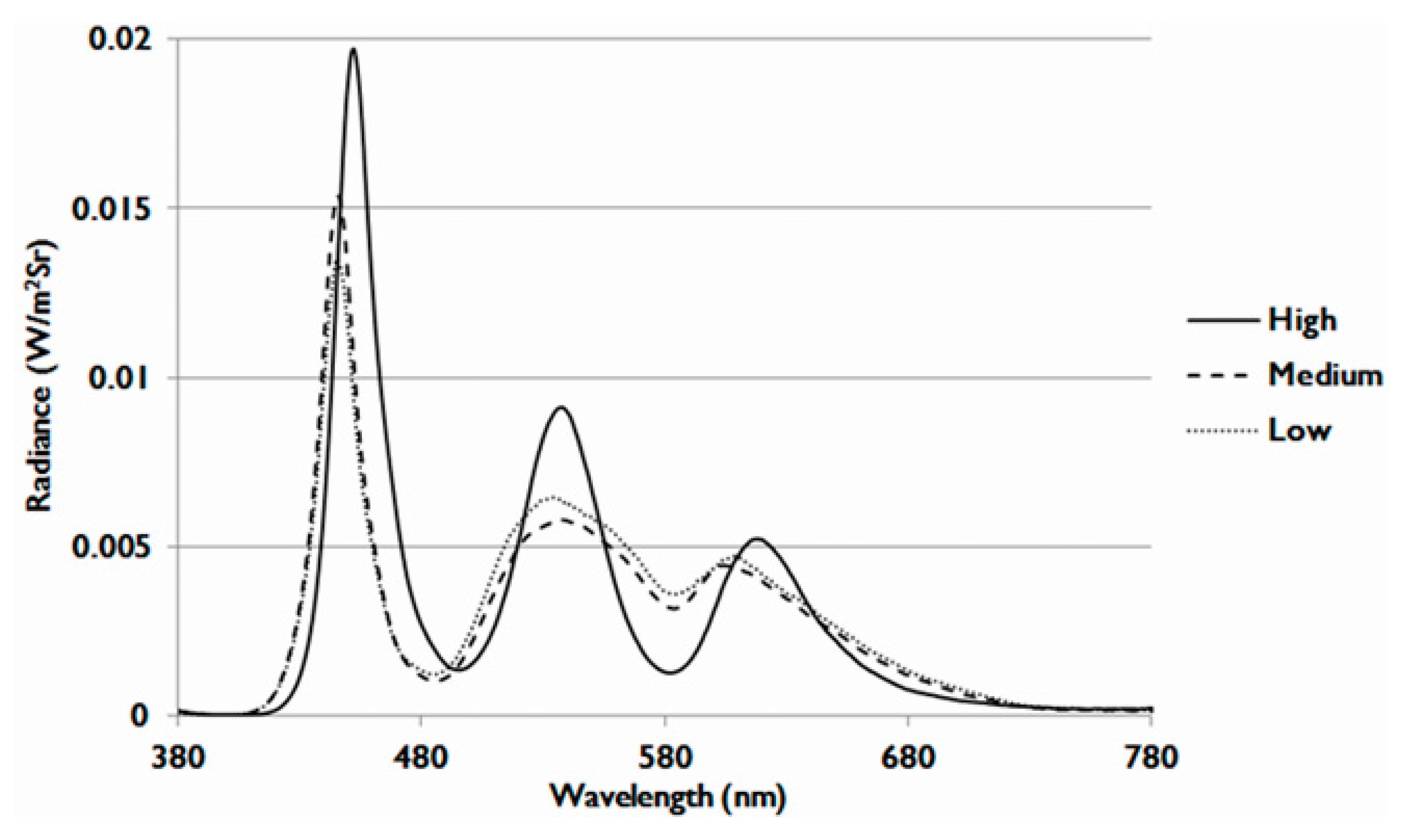
| OEEI 1 | Low | Medium | High |
|---|---|---|---|
| Value | 3.35 × 10−3 (W/lm) | 3.53 × 10−3 (W/lm) | 3.75 × 10−3 (W/lm) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-W.; Yang, C.-M.; Yang, C.-H. Effects of the Emitted Light Spectrum of Liquid Crystal Displays on Light-Induced Retinal Photoreceptor Cell Damage. Int. J. Mol. Sci. 2019, 20, 2318. https://doi.org/10.3390/ijms20092318
Lin C-W, Yang C-M, Yang C-H. Effects of the Emitted Light Spectrum of Liquid Crystal Displays on Light-Induced Retinal Photoreceptor Cell Damage. International Journal of Molecular Sciences. 2019; 20(9):2318. https://doi.org/10.3390/ijms20092318
Chicago/Turabian StyleLin, Chao-Wen, Chung-May Yang, and Chang-Hao Yang. 2019. "Effects of the Emitted Light Spectrum of Liquid Crystal Displays on Light-Induced Retinal Photoreceptor Cell Damage" International Journal of Molecular Sciences 20, no. 9: 2318. https://doi.org/10.3390/ijms20092318
APA StyleLin, C.-W., Yang, C.-M., & Yang, C.-H. (2019). Effects of the Emitted Light Spectrum of Liquid Crystal Displays on Light-Induced Retinal Photoreceptor Cell Damage. International Journal of Molecular Sciences, 20(9), 2318. https://doi.org/10.3390/ijms20092318




