Molecular Simulation Study on the Microscopic Structure and Mechanical Property of Defect-Containing sI Methane Hydrate
Abstract
:1. Introduction
2. Results and Discussion
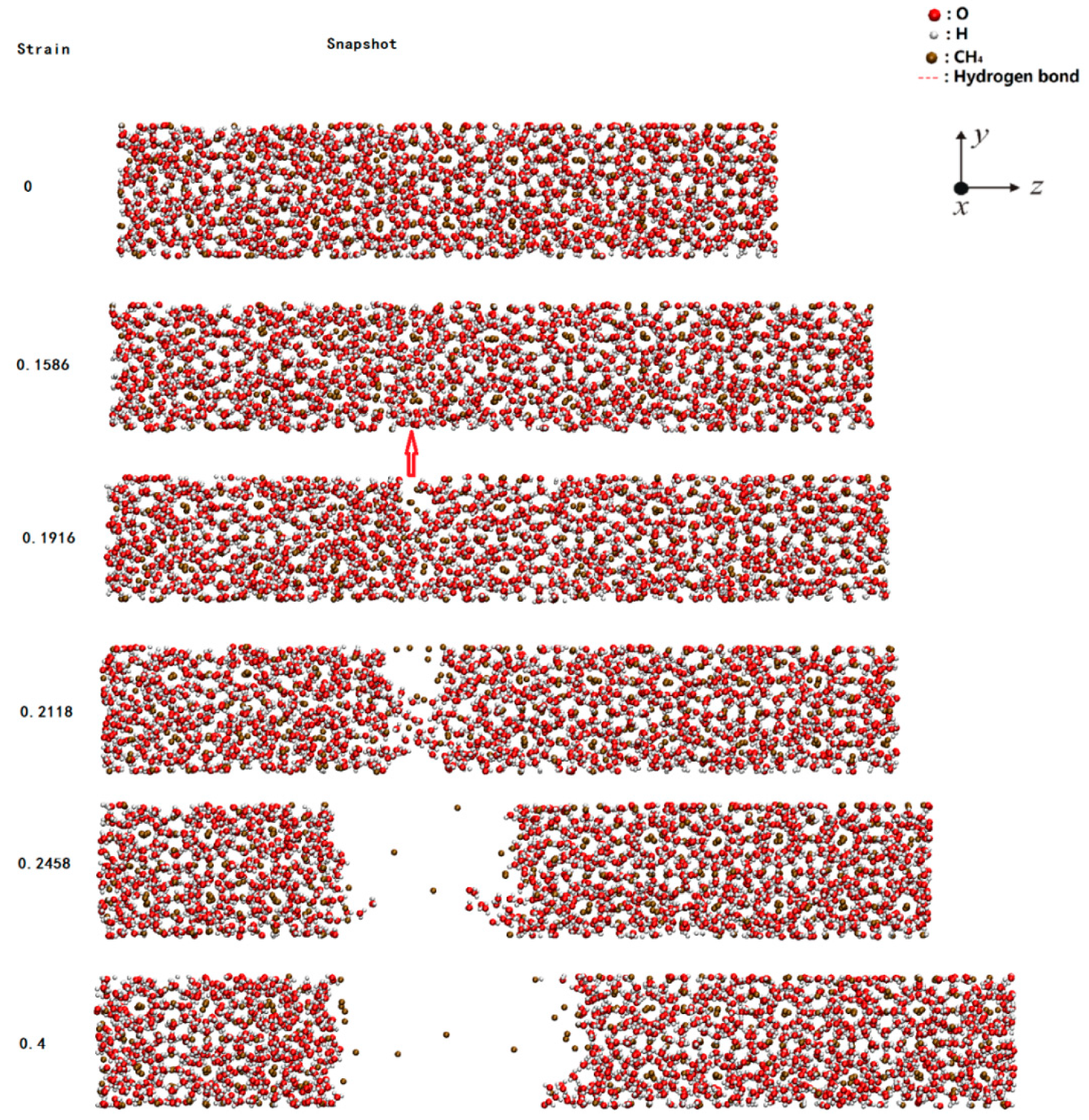
2.1. Failure Process During Stretching
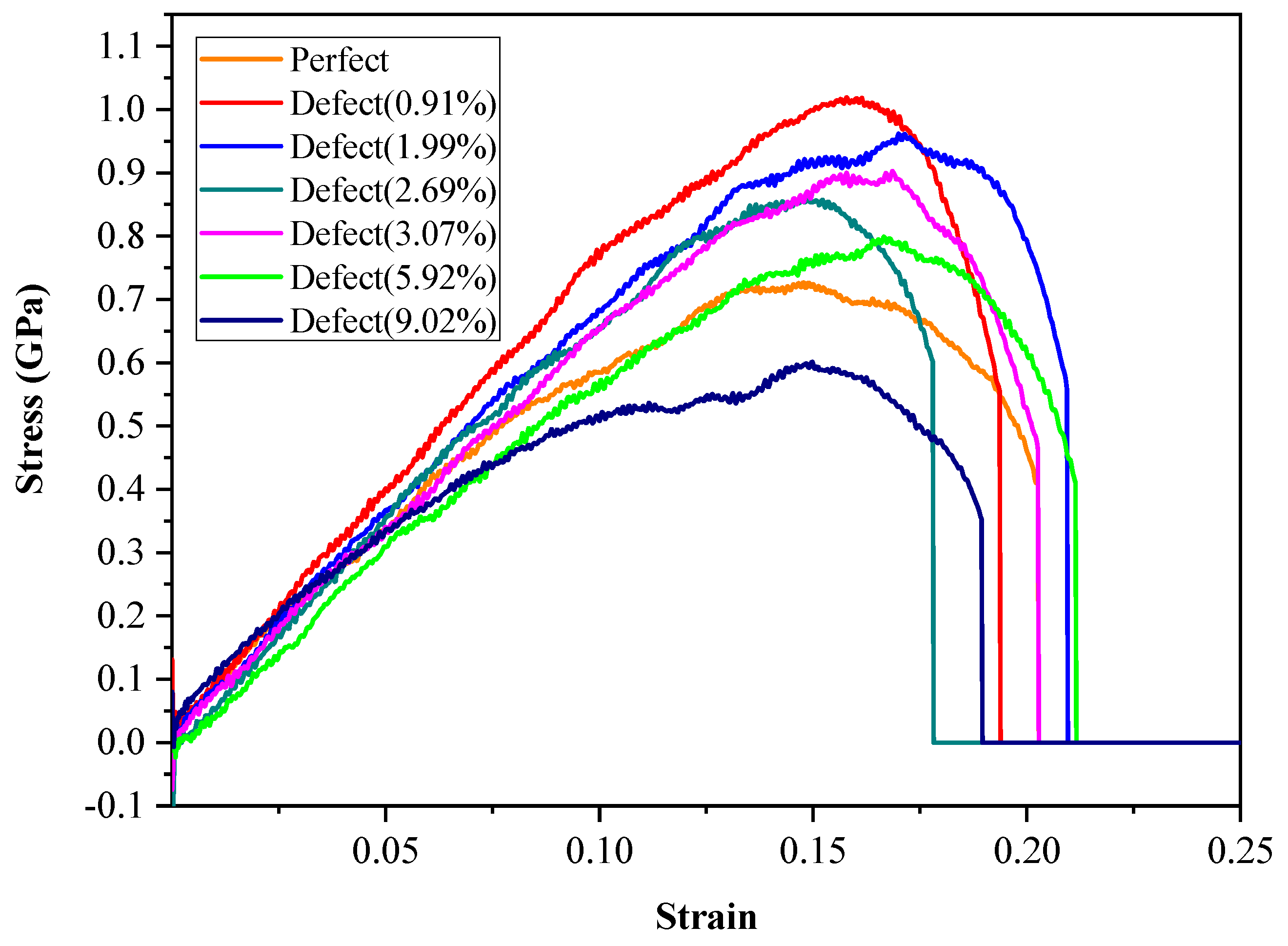
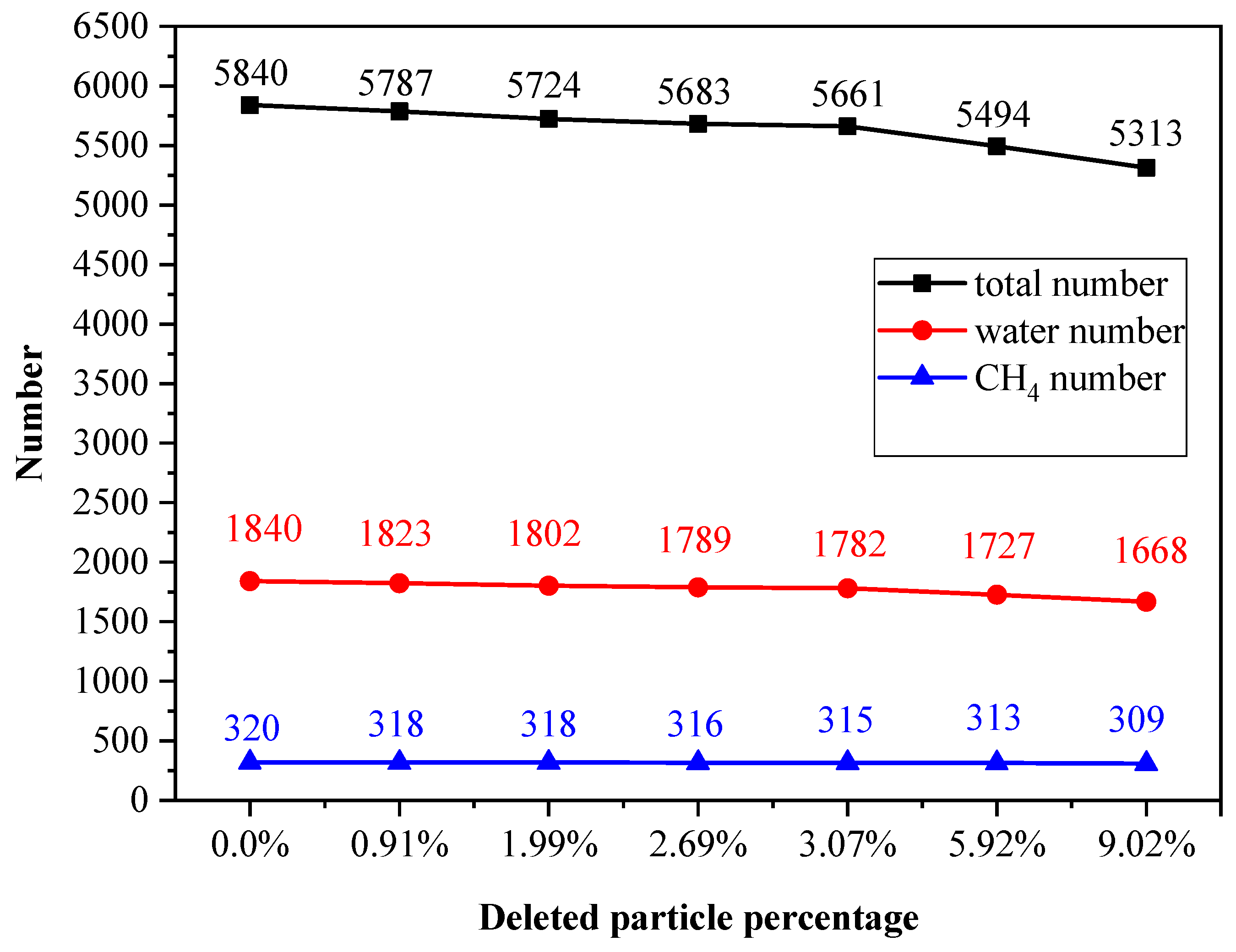
2.2. Strain–Stress Curves with Various Defect Ratios
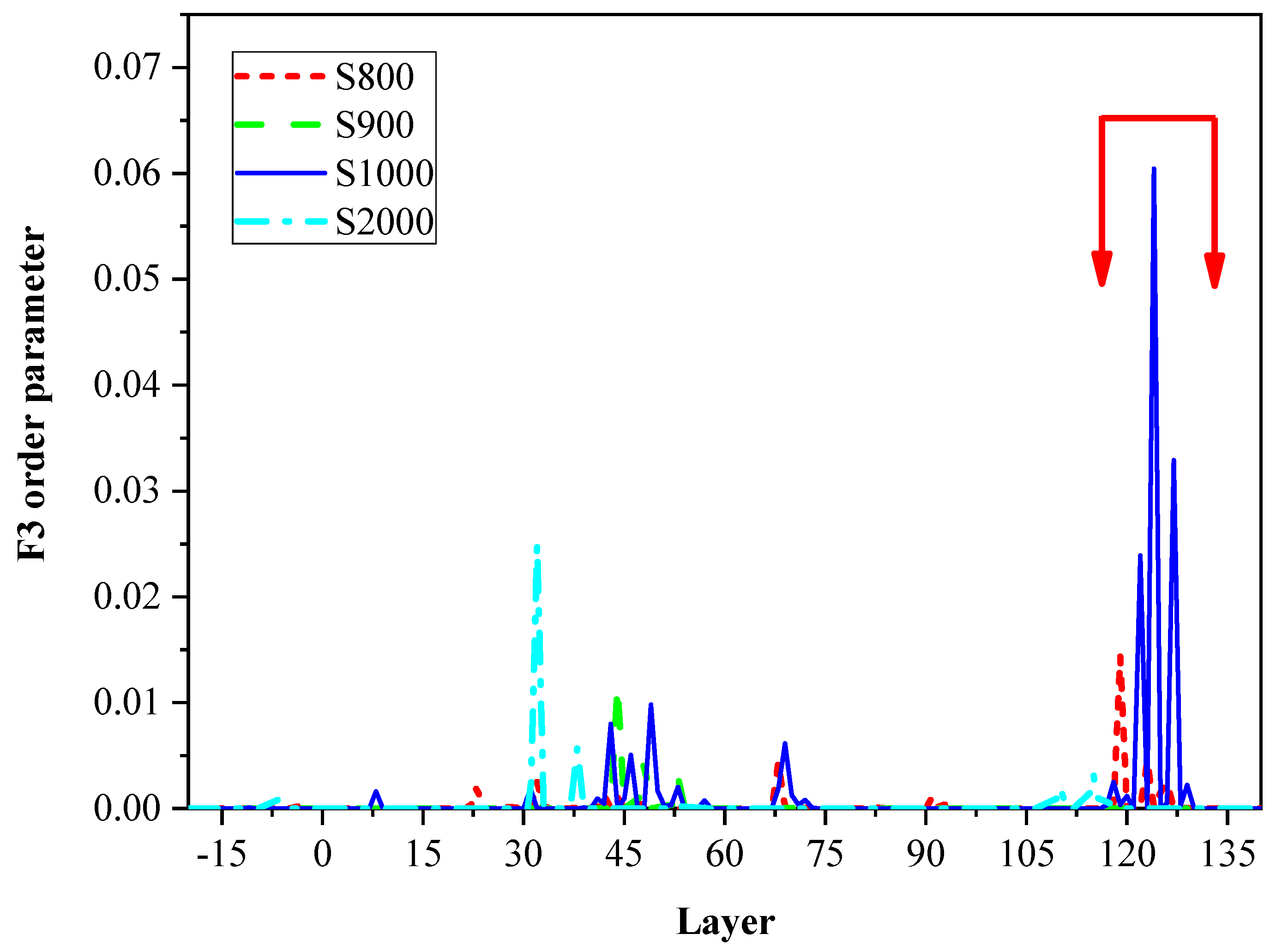
2.3. F3 Order Parameters
3. Materials and Methods
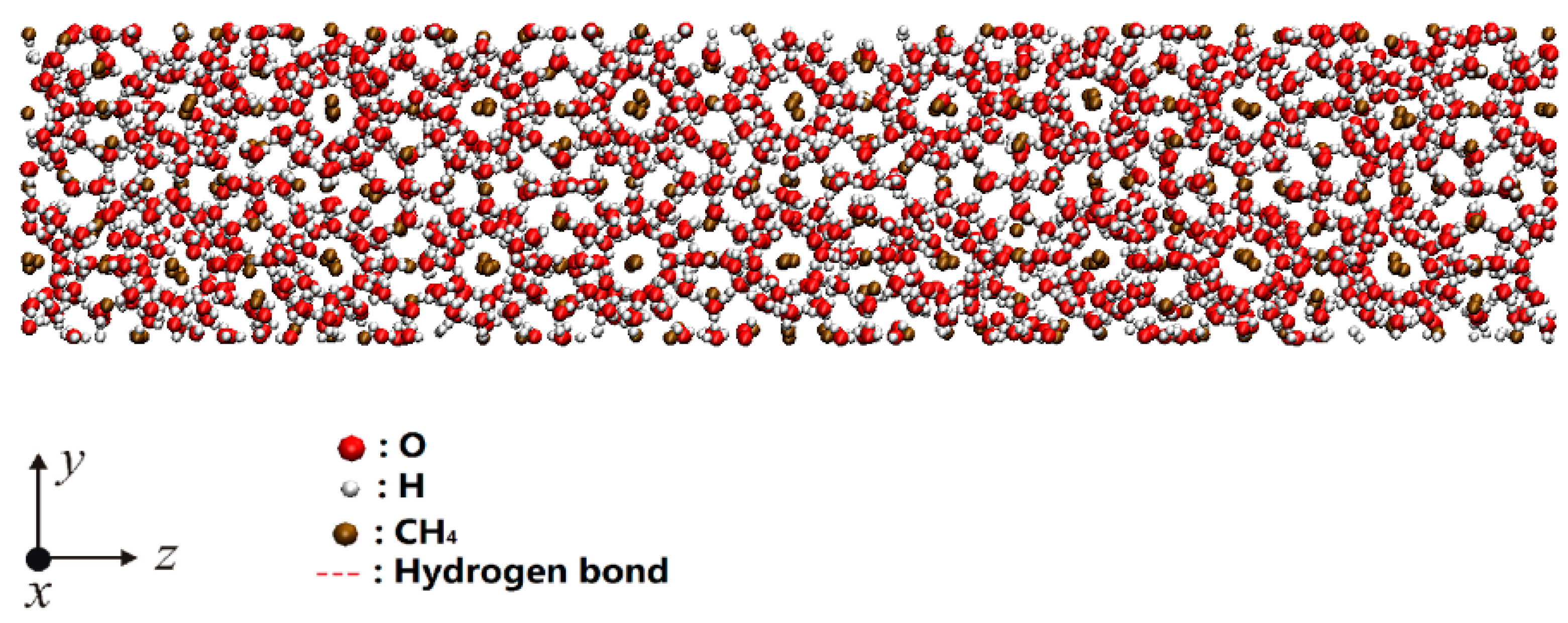
3.1. Simulation Model and Computational Method
3.2. Computational Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D., Jr.; Carolyn, K. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Feng, Y.; Chen, L.; Suzuki, A.; Kogawa, T.; Okajima, J.; Komiya, A.; Maruyama, S. Enhancement of gas production from methane hydrate reservoirs by the combination of hydraulic fracturing and depressurization method. Energy Conv. Manag. 2019, 184, 194–204. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Methane hydrate-A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- McMullan, R.K.; Jeffrey, G.A. Polyhedral Clathrate Hydrates. IX. Structure of Ethylene Oxide Hydrate. J. Chem. Phys. 1965, 42, 2725–2732. [Google Scholar] [CrossRef]
- Mak, T.C.W.; McMullan, R.K. Polyhedral Clathrate Hydrates. X. Structure of the Double Hydrate of Tetrahydrofuran and Hydrogen Sulfide. J. Chem. Phys. 1965, 42, 2732–2737. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Gas Hydrates-Geological Perspective and Global Change. Rev. Geophys. 1993, 32, 173–187. [Google Scholar] [CrossRef]
- Englezos, P.; Lee, J. Gas hydrates: A cleaner source of energy and opportunity for innovative technologies. Korean J. Chem. Eng. 2005, 22, 671–681. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Englezos, P. Gas hydrate formation from hydrogen/carbon dioxide and nitrogen/carbon dioxide gas mixtures. Chem. Eng. Sci. 2007, 62, 4268–4276. [Google Scholar] [CrossRef]
- Ning, F.; Yu, Y.; Kjelstrup, S.; Vlugt, T.J.H.; Glavatskiy, K. Mechanical properties of clathrate hydrates: Status and perspectives. Energy Environ. Sci. 2012, 5, 6779–6795. [Google Scholar] [CrossRef]
- Gladis, A.; Gundersen, M.T.; Neerup, R.; Fosbol, P.L.; Woodley, J.M.; Sloms, N.V. CO2 mass transfer model for carbonic anhydrase-enhanced aqueous MDEA solutions. Chem. Eng. J. 2018, 335, 197–208. [Google Scholar] [CrossRef]
- Luis, D.P.; García-González, A.; Saint-Martin, H. A Theoretical Study of the Hydration of Methane, from the Aqueous Solution to the sI Hydrate-Liquid Water-Gas Coexistence. Int. J. Mol. Sci. 2016, 17, 378. [Google Scholar] [CrossRef]
- Kobayashi, N.; Minami, T.; Tani, A.; Nakagoshi, M.; Sugahara, T.; Takeya, K.; Ohgaki, K. Intermolecular Hydrogen Transfer in Isobutane Hydrate. Energies 2012, 5, 1705–1712. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A Kinetic-Study of Methane Hydrate Formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Waite, W.F.; Kneafsey, T.J.; Winters, W.J.; Mason, D.H. Physical property changes in hydrate-bearing sediment due to depressurization and subsequent repressurization. J. Geophys. Res. Sol. Ea. 2008, 113. [Google Scholar] [CrossRef]
- Winters, W.J.; Waite, W.F.; Mason, D.H.; Gilbert, L.Y.; Pecher, I.A. Methane gas hydrate effect on sediment acoustic and strength properties. J. Pet. Sci. Eng. 2007, 56, 127–135. [Google Scholar] [CrossRef]
- Yun, T.S.; Francisca, F.M.; Santamarina, J.C.; Ruppel, C. Compressional and shear wave velocities in uncemented sediment containing gas hydrate. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Francisca, F.; Yun, T.S.; Ruppel, C.; Santamarina, J.C. Geophysical and geotechnical properties of near-seafloor sediments in the northern Gulf of Mexico gas hydrate province. Earth Planet. Sci. Lett. 2005, 237, 924–939. [Google Scholar] [CrossRef]
- Lee, J.Y.; Santamarina, J.C.; Ruppel, C. Mechanical and electromagnetic properties of northern Gulf of Mexico sediments with and without THF hydrates. Mar. Pet. Geol. 2008, 25, 884–895. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Aoki, K.; Sakamoto, Y.; Yamaguchi, T.; Okubo, S. Strain-Rate Dependence of Triaxial Compressive Strength of Artificial Methane-Hydrate-Bearing Sediment. Int. J. Offshore Polar Eng. 2010, 20, 9. [Google Scholar]
- Hyodo, M.; Wu, Y.; Nakashima, K.; Kajiyama, S.; Nakata, Y. Influence of Fines Content on the Mechanical Behavior of Methane Hydrate-Bearing Sediments. J. Geophys. Res. Sol. Ea. 2017, 122, 7511–7524. [Google Scholar] [CrossRef]
- Rehder, G.; Kirby, S.H.; Durham, W.B.; Stern, L.A.; Peltzer, E.T.; Pinkston, J.; Brewer, P.G. Dissolution rates of pure methane hydrate and carbon-dioxide hydrate in undersaturated seawater at 1000-m depth. Geochim. Cosmochim. Acta 2004, 68, 285–292. [Google Scholar] [CrossRef]
- Hu, J.; Liu, C.; Li, Q.; Shi, X. Molecular simulation of thermal energy storage of mixed CO2/IRMOF-1 nanoparticle nanofluid. Int. J. Heat Mass Transf. 2018, 125, 1345–1348. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C. Molecular dynamics simulation of heat transfer with effects of fluid–lattice interactions. Int. J. Heat Mass Transf. 2012, 55, 8088–8092. [Google Scholar] [CrossRef]
- Zhan, H.; Gu, Y. A fundamental numerical and theoretical study for the vibrational properties of nanowires. J. Appl. Phys. 2012, 111, 124303. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Chen, X. Molecular dynamics simulation of sulphur nucleation in S–H2S system. Mol. Phys. 2014, 112, 947–955. [Google Scholar] [CrossRef]
- Peng, T.; Li, Q.; Liu, C. Accelerated aqueous nano-film rupture and evaporation induced by electric field: A molecular dynamics approach. Int. J. Heat Mass Transf. 2016, 94, 39–48. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, Y.; Shi, X.; Song, S. Rapid evaporation of water on graphene/graphene-oxide: A molecular dynamics study. Nanomaterials 2017, 7, 265. [Google Scholar] [CrossRef]
- Hu, J.; Liu, C.; Liu, L.; Li, Q. Thermal energy storage of R1234yf, R1234ze, R134a and R32/MOF-74 nanofluids: A molecular simulation study. Materials 2018, 11, 1164. [Google Scholar] [CrossRef]
- Li, Q.; Yu, Y.; Liu, Y.; Liu, C.; Lin, L. Thermal properties of the mixed n-octadecane/Cu nanoparticle nanofluids during phase transition: A molecular dynamics study. Materials 2017, 10, 38. [Google Scholar] [CrossRef]
- Rodger, P.M.; Forester, T.R.; Smith, W. Simulations of the methane hydrate/methane gas interface near hydrate forming conditions. Fluid Phase Equilib. 1996, 116, 326–332. [Google Scholar] [CrossRef]
- Rodger, P.M. Methane Hydrate: Melting and Memory. Ann. N. Y. Acad. Sci. 2000, 912, 474–482. [Google Scholar] [CrossRef]
- Moon, C.; Taylor, P.C.; Rodger, P.M. Molecular dynamics study of gas hydrate formation. J. Am. Chem. Soc. 2003, 125, 4706–4707. [Google Scholar] [CrossRef]
- Storr, M.T.; Taylor, P.C.; Monfort, J.; Roger, P.M. Kinetic Inhibitor of Hydrate Crystallization. J. Am. Chem. Soc. 2004, 126, 1569–1576. [Google Scholar] [CrossRef]
- Moon, C.; Hawtin, R.W.; Rodger, P.M. Nucleation and control of clathrate hydrates: Insights from simulation. Faraday Discuss. 2007, 136, 367–382. [Google Scholar] [CrossRef]
- Yan, K.; Li, X.; Chen, Z.; Li, G.; Tang, G.; Fan, S. Molecular dynamics simulation of methane hydrate dissociation by thermal stimulation. Acta Phys. Sin. 2007, 56, 4994–5002. [Google Scholar]
- Yan, K.; Li, X.; Chen, Z.; Li, G.; Li, Z. Molecular dynamics simulation of methane hydrate dissociation by thermal stimulation in conjunction with chemical injection method. Acta Phys. Sin. 2007, 56, 6727–6735. [Google Scholar]
- Wan, L.; Yan, K.; Li, X.; Fan, S. Molecular Dynamics Simulation of Methane Hydrate Dissociation Process in the Presence of Thermodynamic Inhibitor. Acta Phys. Chim. Sin. 2009, 25, 486–494. [Google Scholar]
- Wu, J.; Ning, F.; Trinh, T.T.; Kjelstrup, S.; Vlugt, T.J.H.; He, J.; Skallerud, B.H.; Zhang, Z. Mechanical instability of monocrystalline and polycrystalline methane hydrates. Nat. Commun. 2015, 6, 8743. [Google Scholar] [CrossRef]
- Wan, L.; Liang, D.; Wu, N.; Guan, J. Molecular dynamics simulations of the mechanisms of thermal conduction in methane hydrates. Sci. China Chem. 2012, 55, 167–174. [Google Scholar] [CrossRef]
- BÁEz, L.A.; Clancy, P. Computer Simulation of the Crystal Growth and Dissolution of Natural Gas Hydrates. Ann. N. Y. Acad. Sci. 1994, 715, 177–186. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Chen, X. Molecular characteristics of dissociated water with memory effect from methane hydrates. Int. J. Mod. Phys. B 2014, 28, 1450062. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Q.; Peng, T.; Zhang, X.; Liu, C.; Shi, X. Molecular characteristics of H2O in hydrate/ice/liquid water mixture. Int. J. Mod. Phys. B 2015, 29, 1550185. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Delhommelle, J.; MilliÉ, P. Inadequacy of the Lorentz-Berthelot combining rules for accurate predictions of equilibrium properties by molecular simulation. Mol. Phys. 2001, 99, 619–625. [Google Scholar] [CrossRef]
- Sandberg, T.; Eklund, P.; Hotokka, M. Conformational Solvation Studies of LIGNOLs with Molecular Dynamics and Conductor-Like Screening Model. Int. J. Mol. Sci. 2012, 13, 9845–9863. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Academic Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Ewald, P.P. Die berechnung optischer und elektrostatischer gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, S.; Peng, T. Molecular Simulations of Sputtering Preparation and Transformation of Surface Properties of Au/Cu Alloy Coatings Under Different Incident Energies. Metals 2019, 9, 259. [Google Scholar] [CrossRef]
- Peng, T.; Li, Q.; Xu, L.; He, C.; Luo, L. Surface interaction of nanoscale water film with SDS from computational simulation and film thermodynamics. Entropy 2017, 19, 620. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, S.; Tang, Q.; Tian, S.; Lu, Y.; Gao, X. Molecular Simulation Study on the Microscopic Structure and Mechanical Property of Defect-Containing sI Methane Hydrate. Int. J. Mol. Sci. 2019, 20, 2305. https://doi.org/10.3390/ijms20092305
Cai S, Tang Q, Tian S, Lu Y, Gao X. Molecular Simulation Study on the Microscopic Structure and Mechanical Property of Defect-Containing sI Methane Hydrate. International Journal of Molecular Sciences. 2019; 20(9):2305. https://doi.org/10.3390/ijms20092305
Chicago/Turabian StyleCai, Shouyin, Qizhong Tang, Sen Tian, Yiyu Lu, and Xuechao Gao. 2019. "Molecular Simulation Study on the Microscopic Structure and Mechanical Property of Defect-Containing sI Methane Hydrate" International Journal of Molecular Sciences 20, no. 9: 2305. https://doi.org/10.3390/ijms20092305
APA StyleCai, S., Tang, Q., Tian, S., Lu, Y., & Gao, X. (2019). Molecular Simulation Study on the Microscopic Structure and Mechanical Property of Defect-Containing sI Methane Hydrate. International Journal of Molecular Sciences, 20(9), 2305. https://doi.org/10.3390/ijms20092305





