Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice
Abstract
1. Introduction
2. Results
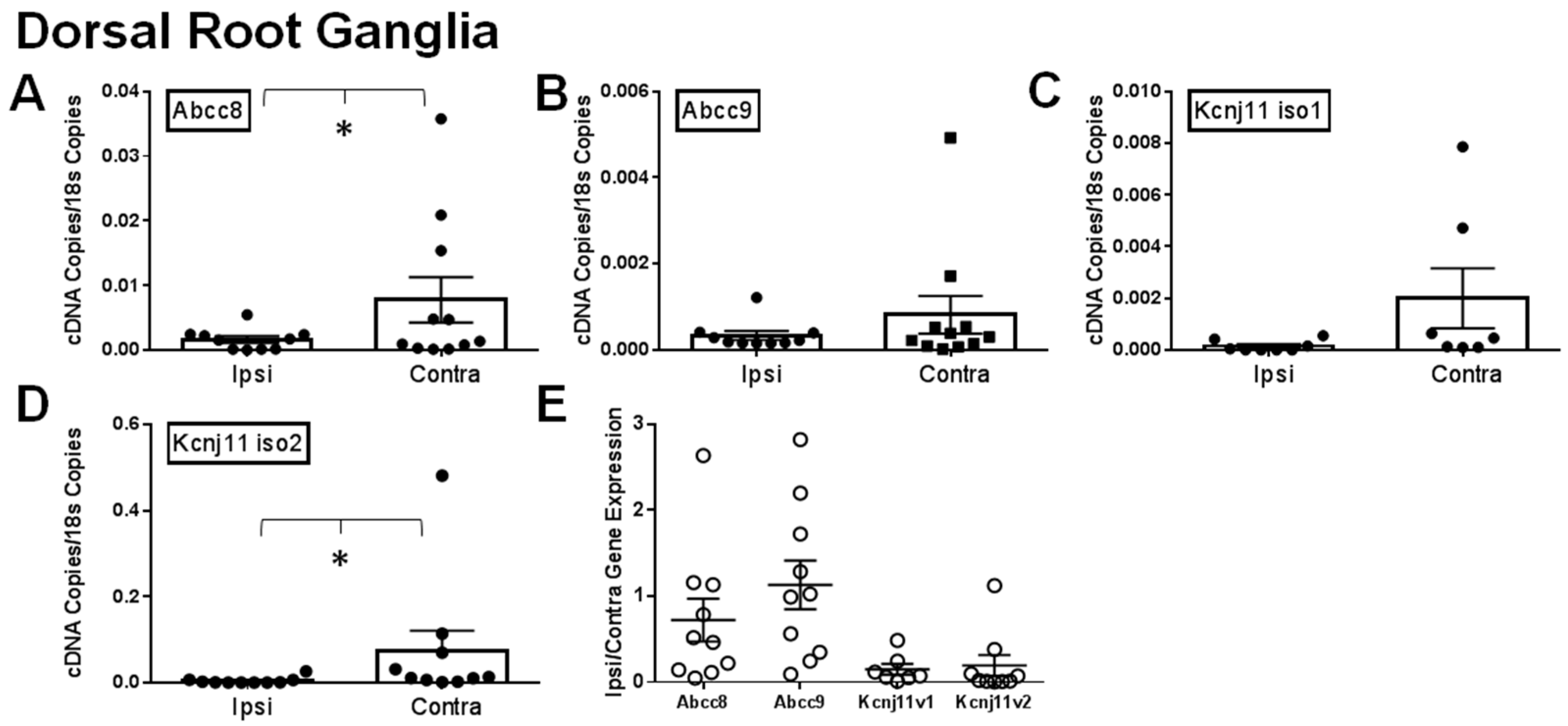
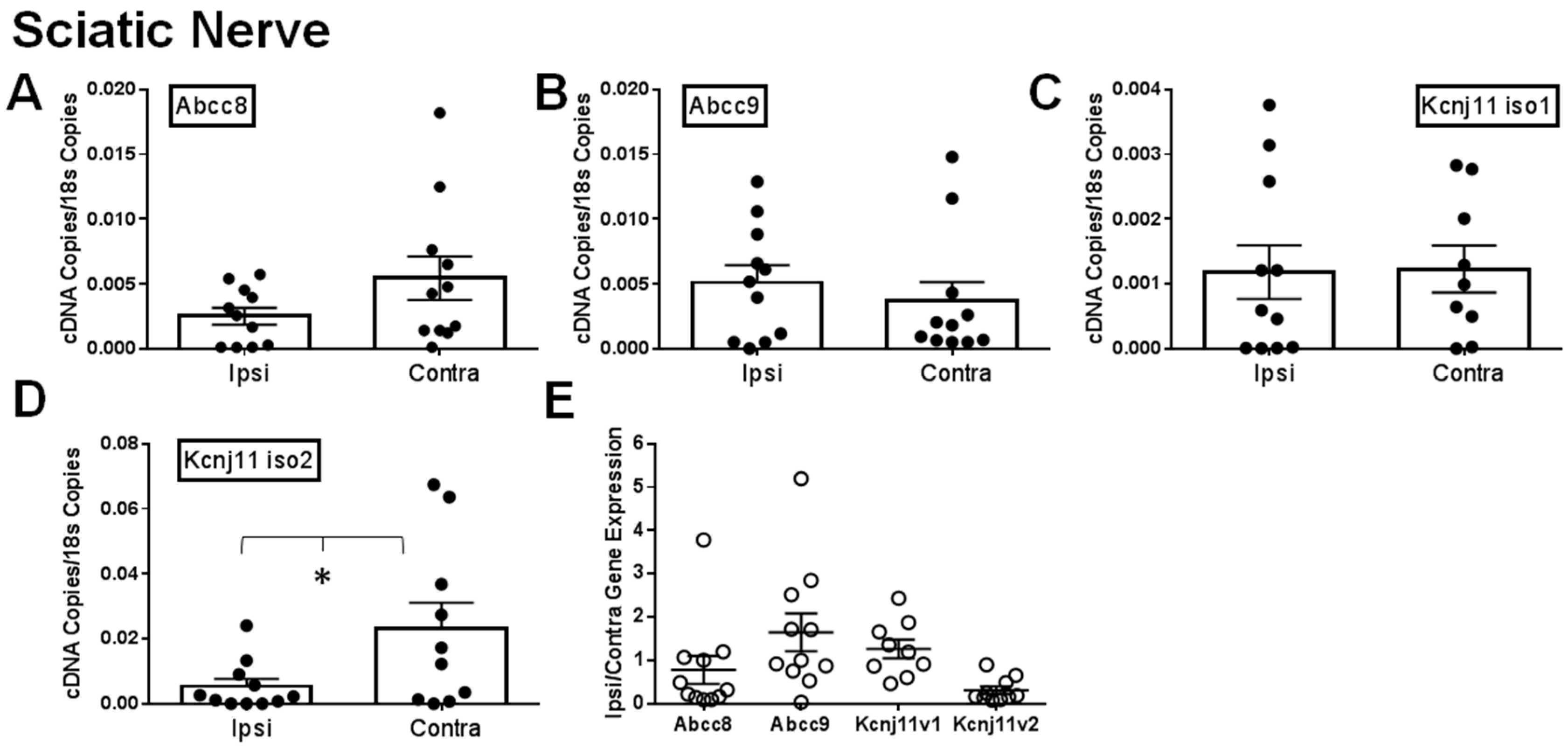
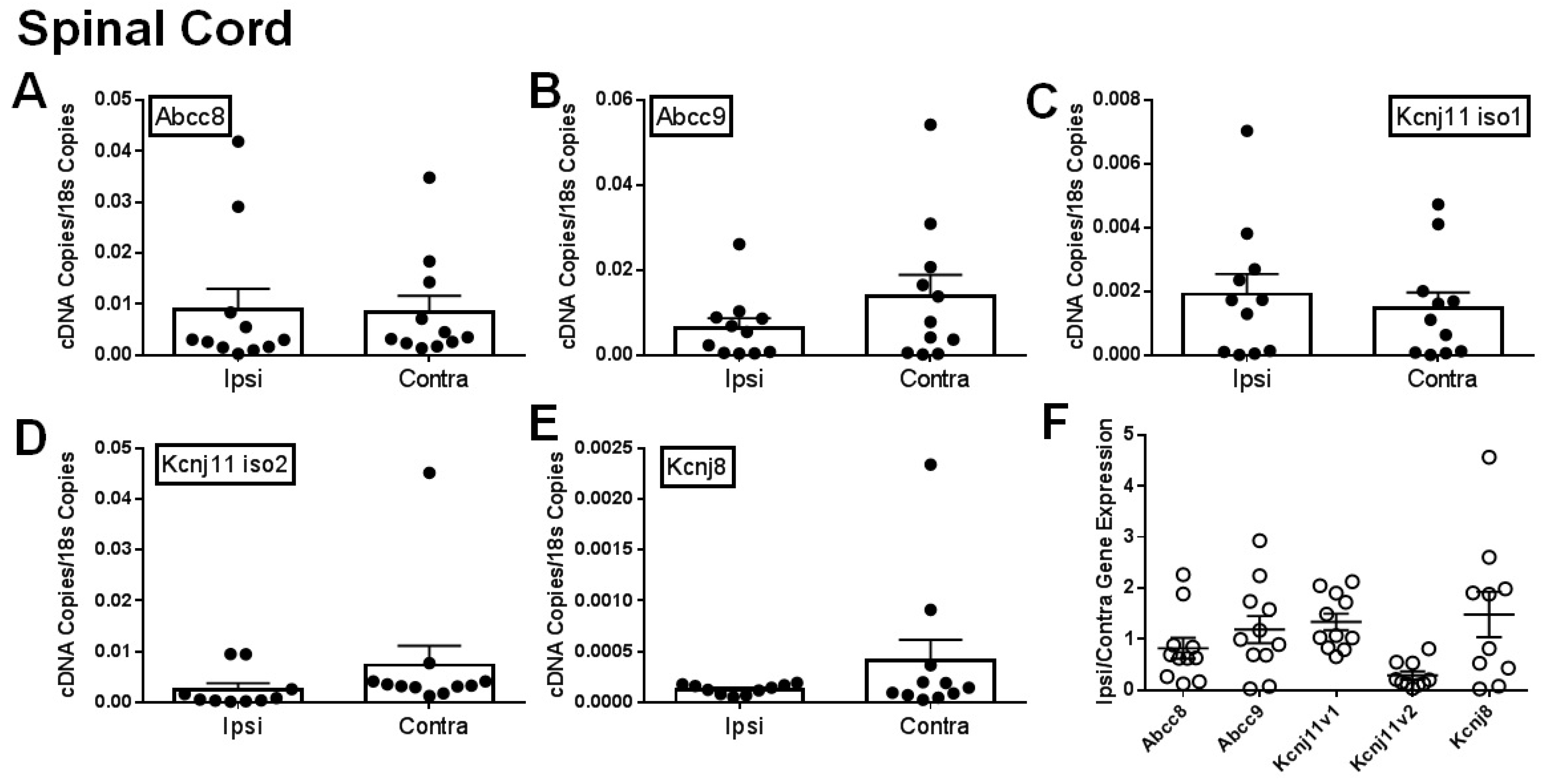
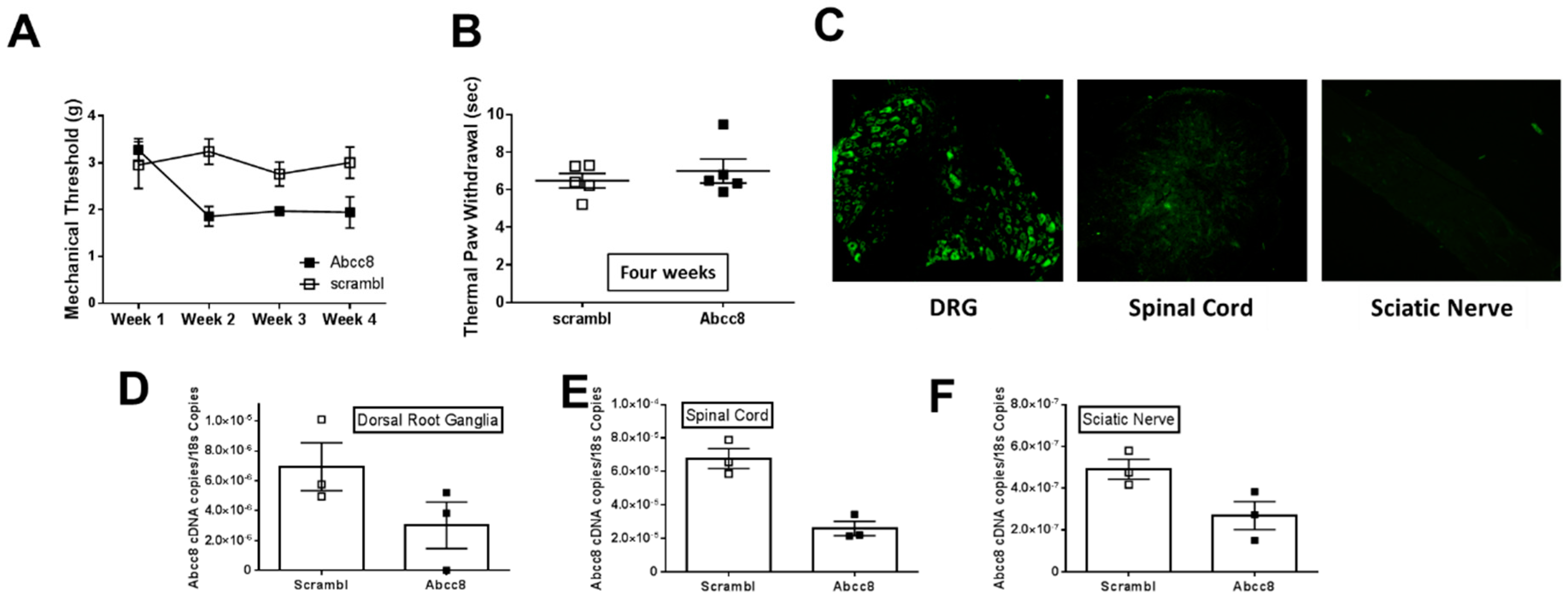
2.1. Gene Expression of SUR1 and Kir6.2 Decreases in the Peripheral Nervous System after Spinal Nerve Ligation
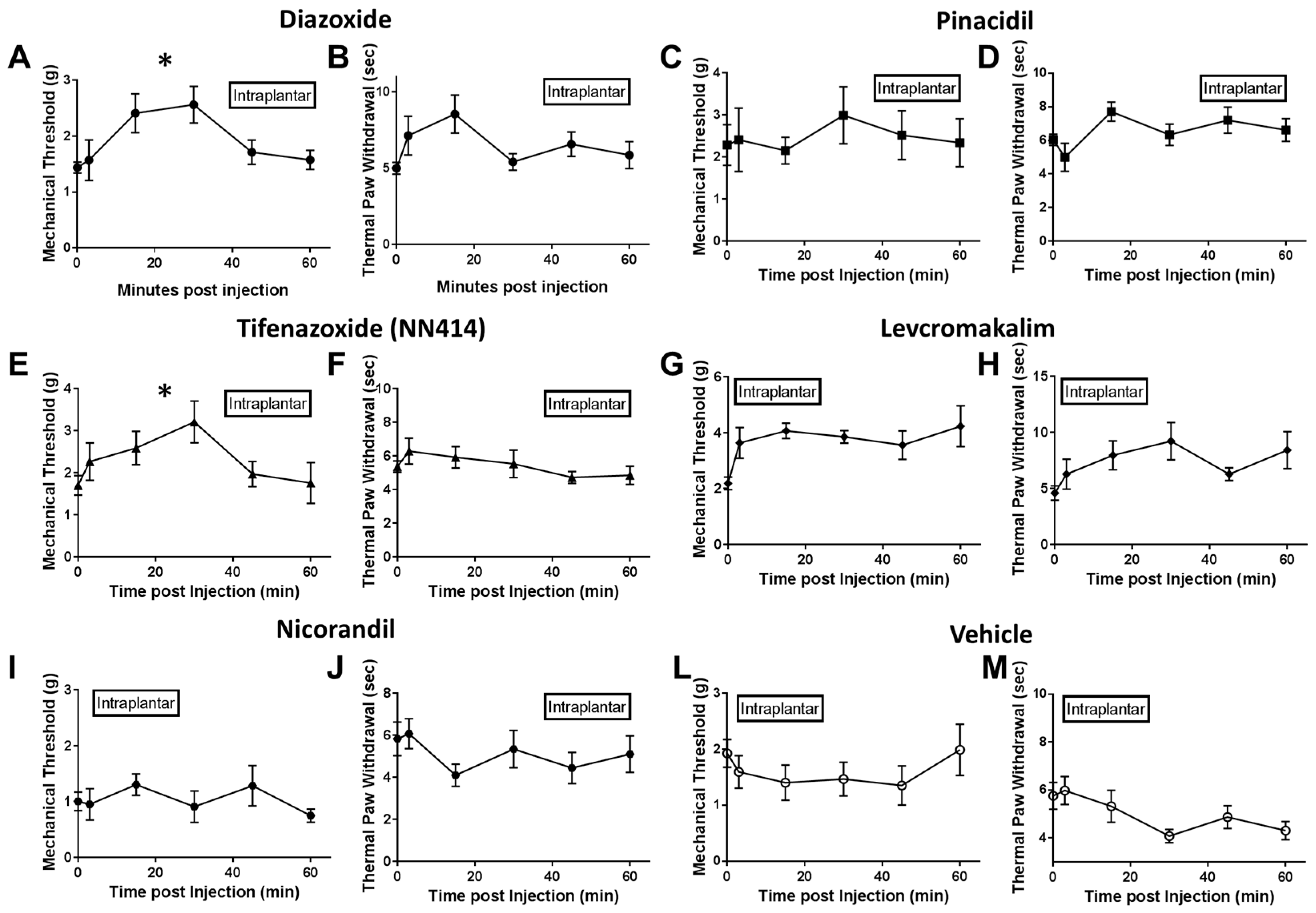
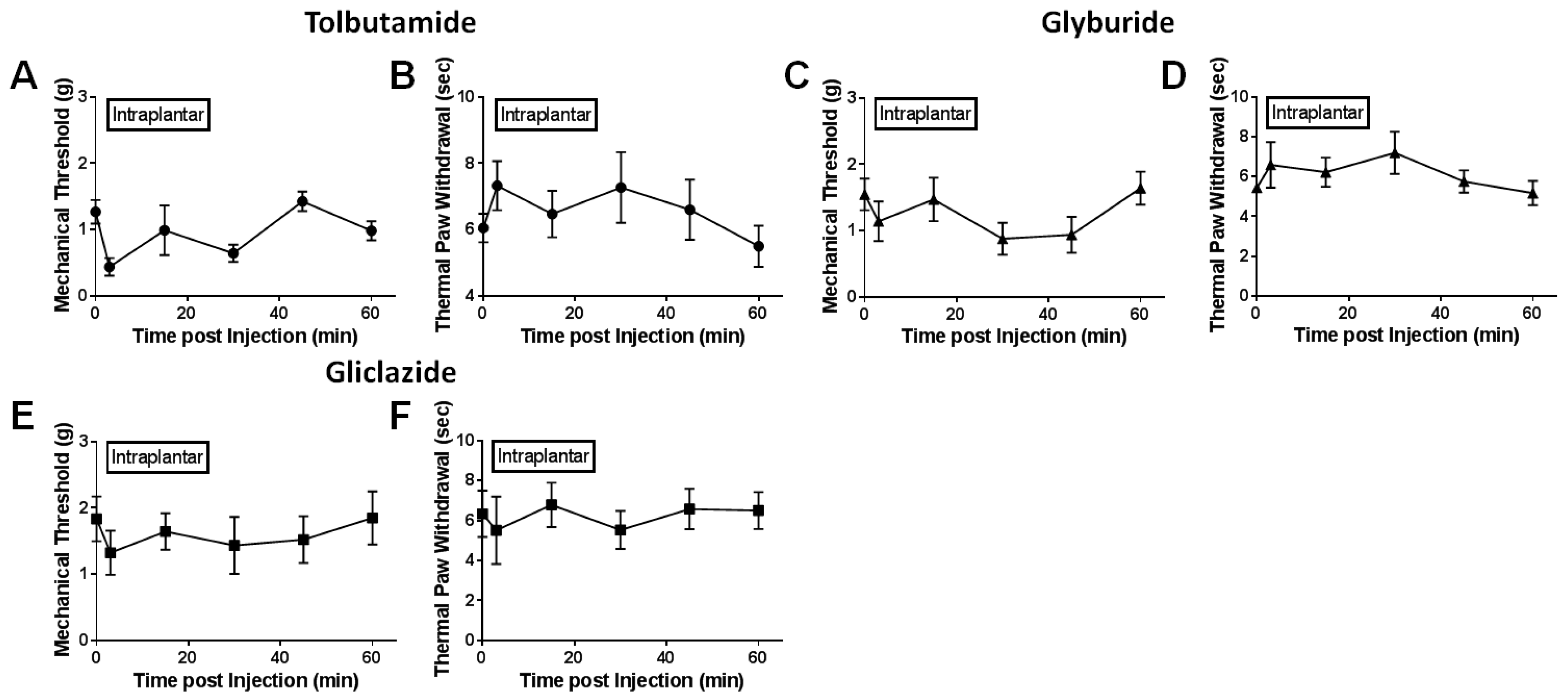
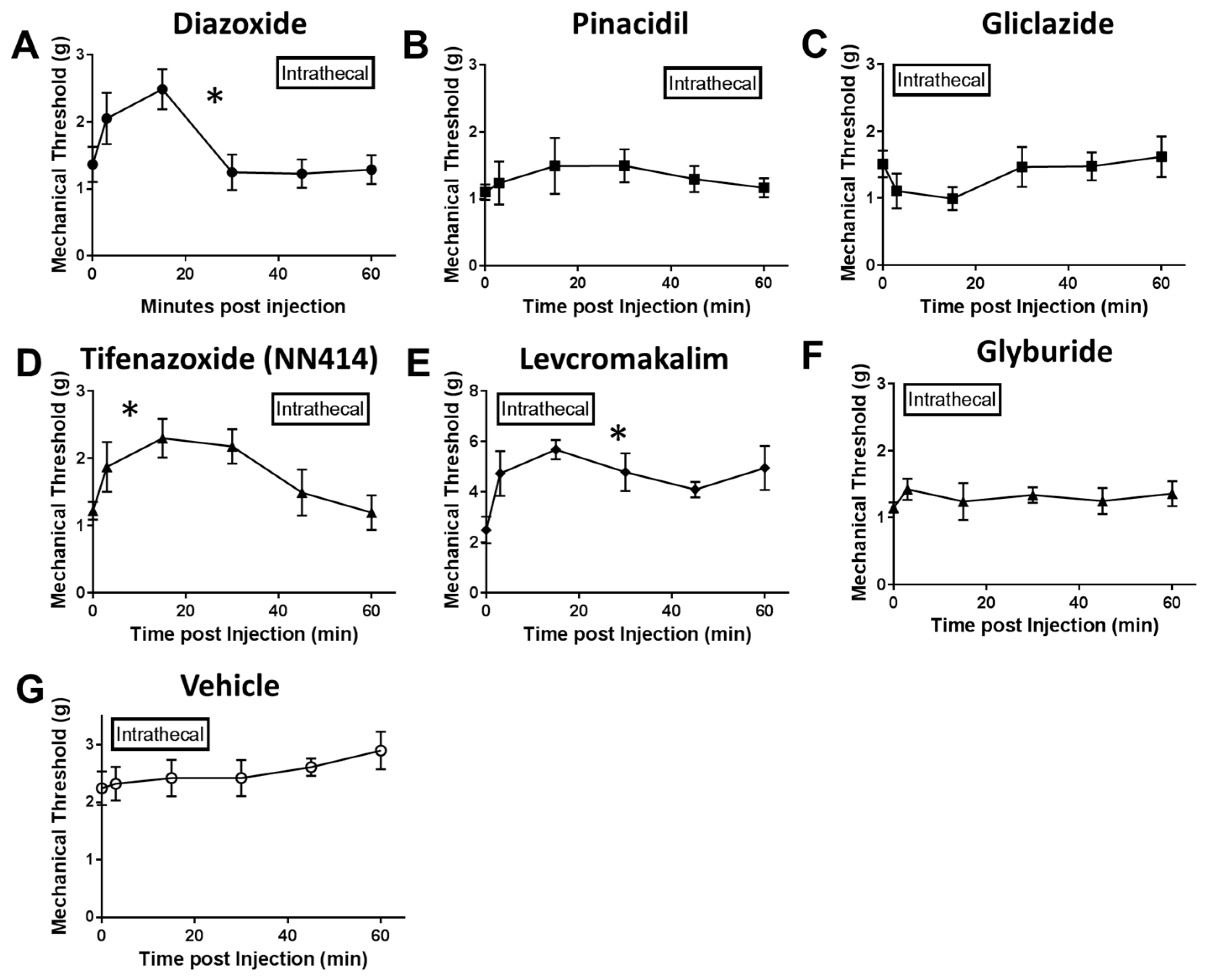
2.2. Local Delivery of SUR1 Agonists Alleviate Mechanical Hypersensitivity after Spinal Nerve Ligation
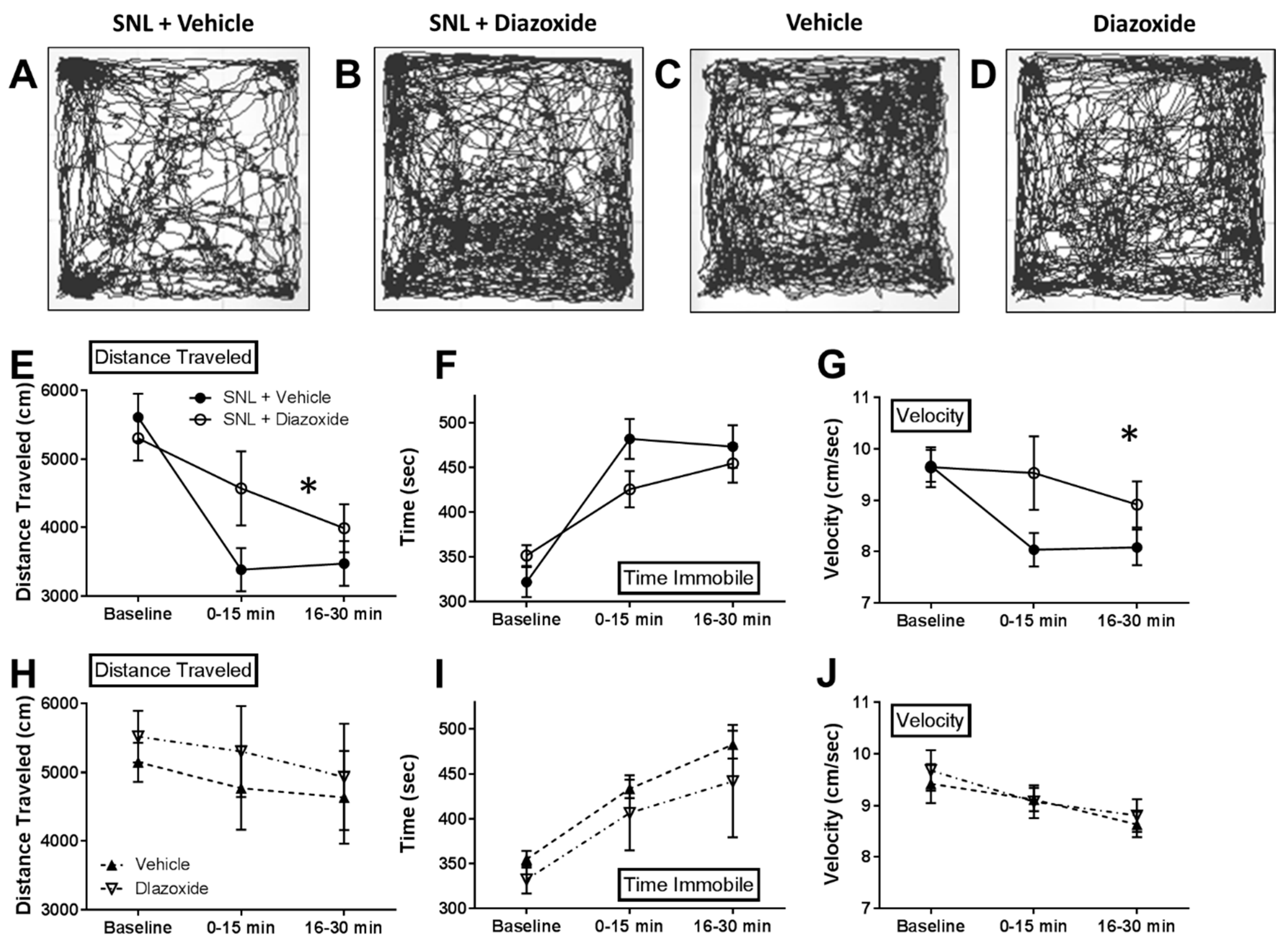
2.3. Local Diazoxide Administration Alters General Movement in Open Field Testing
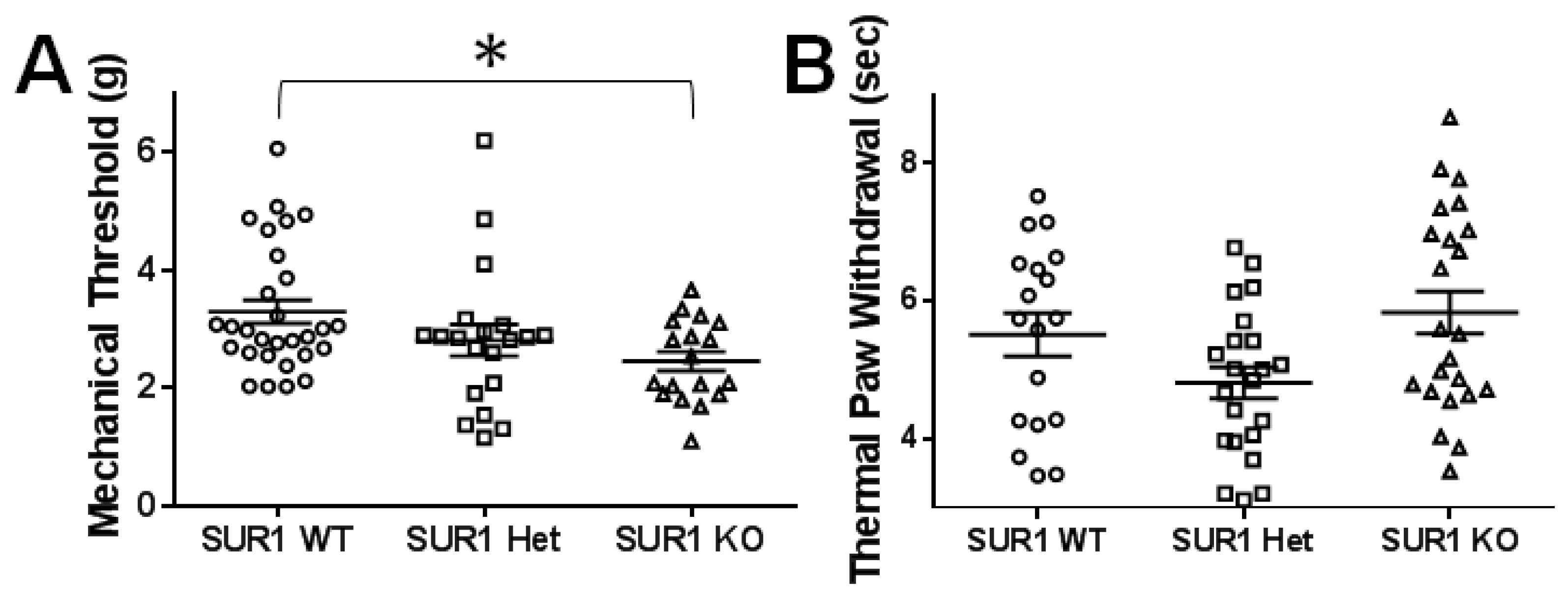
2.4. Genetic Ablation or Knockdown of SUR1 Increases Mechanical Hypersensitivity
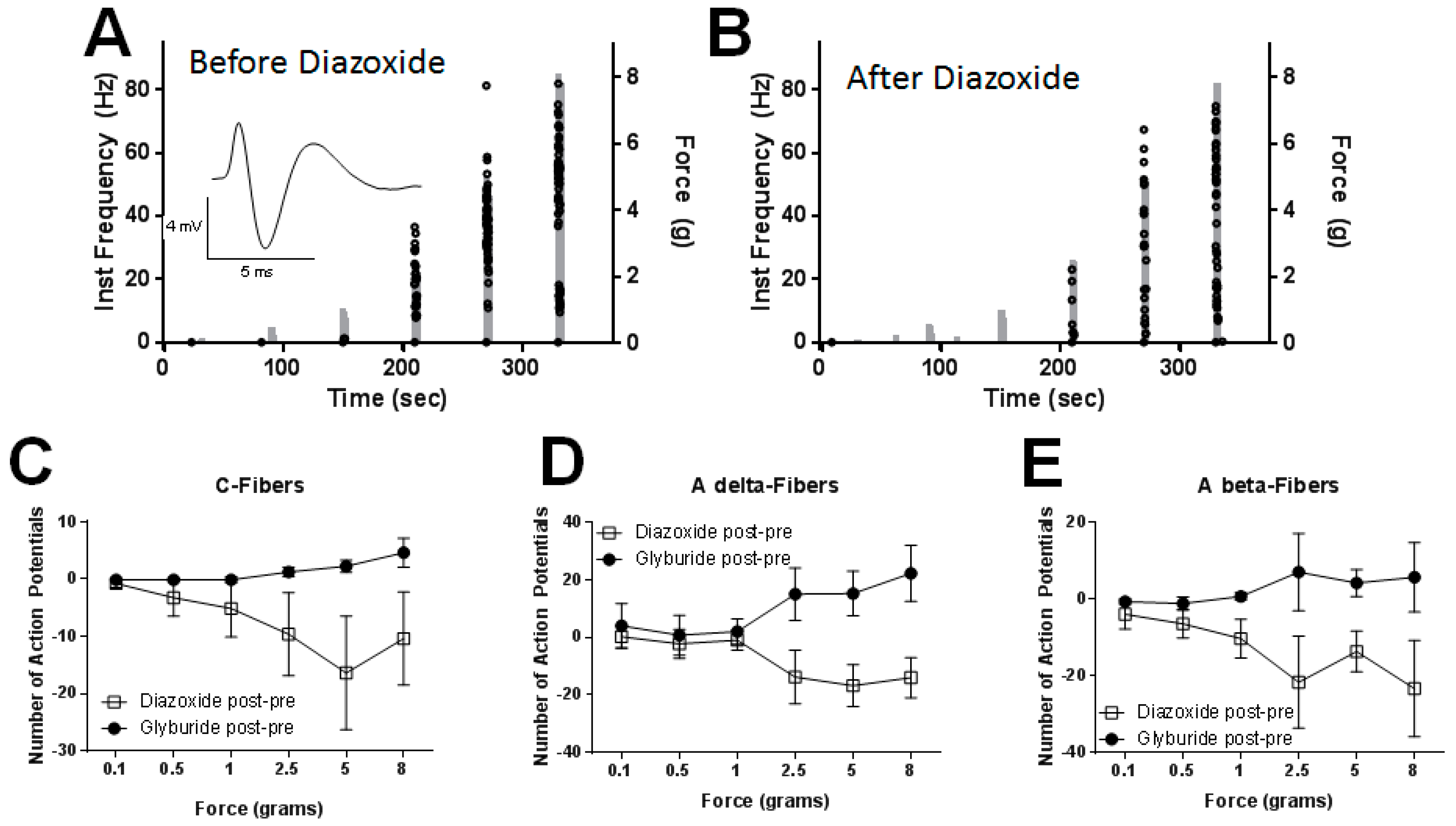
2.5. Peripheral Application of SUR1 Subtype Agonists Decreased Evoked Mechanical Responses to Nerve Fibers Innervating the Hindpaw
3. Discussion
3.1. SUR1-Subtype KATP Channels Contribute to Analgesia in the Peripheral and Central Nervous System after Nerve Injury
3.2. SUR1 Involvement in Mechanical Hypersensitivity after Spinal Nerve Ligation
4. Material and Methods
4.1. Animals and Breeding
4.2. Spinal Nerve Ligation
4.3. Tissue Collection and RNA Extraction
4.4. Quantitative PCR
4.5. Evoked Behavioral Experiments
4.6. Open Field Testing
4.7. Drug Delivery
4.8. shRNA Delivery
4.9. Ex-Vivo Electrophysiology
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D.; Boulanger, A.; Clark, A.J.; Clarke, H.; Dao, T.; Finley, G.A.; Furlan, A.; Gilron, I.; Gordon, A.; Morley-Forster, P.K.; et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Res. Manag. 2014, 19, 328–335. [Google Scholar] [CrossRef]
- Schaefer, C.; Sadosky, A.; Mann, R.; Daniel, S.; Parsons, B.; Tuchman, M.; Anschel, A.; Stacey, B.R.; Nalamachu, S.; Nieshoff, E. Pain severity and the economic burden of neuropathic pain in the United States: BEAT Neuropathic Pain Observational Study. Clinicoecon. Outcomes. Res. 2014, 6, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Miki, T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 2003, 81, 133–176. [Google Scholar] [CrossRef]
- Qian, L.P.; Shen, S.R.; Chen, J.J.; Ji, L.L.; Cao, S. Peripheral KATP activation inhibits pain sensitization induced by skin/muscle incision and retraction via the nuclear factor-κB/c-Jun N-terminal kinase signaling pathway. Mol. Med. Rep. 2016, 14, 2632–2638. [Google Scholar] [CrossRef][Green Version]
- Barreras-Espinoza, I.; Soto-Zambrano, J.A.; Serafín-Higuera, N.; Zapata-Morales, R.; Alonso-Castro, Á.; Bologna-Molina, R.; Granados-Soto, V.; Isiordia-Espinoza, M.A. The Antinociceptive Effect of a Tapentadol-Ketorolac Combination in a Mouse Model of Trigeminal Pain is Mediated by Opioid Receptors and ATP-Sensitive K. Drug Dev. Res. 2017, 78, 63–70. [Google Scholar] [CrossRef]
- Koh, W.U.; Shin, J.W.; Bang, J.Y.; Kim, S.G.; Song, J.G. The Antiallodynic Effects of Nefopam Are Mediated by the Adenosine Triphosphate-Sensitive Potassium Channel in a Neuropathic Pain Model. Anesth. Analg. 2016, 123, 762–770. [Google Scholar] [CrossRef]
- Kawano, T.; Zoga, V.; McCallum, J.B.; Wu, H.E.; Gemes, G.; Liang, M.Y.; Abram, S.; Kwok, W.M.; Hogan, Q.H.; Sarantopoulos, C.D. ATP-sensitive potassium currents in rat primary afferent neurons: Biophysical, pharmacological properties, and alterations by painful nerve injury. Neuroscience 2009, 162, 431–443. [Google Scholar] [CrossRef]
- Kawano, T.; Zoga, V.; Kimura, M.; Liang, M.Y.; Wu, H.E.; Gemes, G.; McCallum, J.B.; Kwok, W.M.; Hogan, Q.H.; Sarantopoulos, C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol. Pain 2009, 5, 12. [Google Scholar] [CrossRef]
- Kawano, T.; Zoga, V.; Gemes, G.; McCallum, J.B.; Wu, H.E.; Pravdic, D.; Liang, M.Y.; Kwok, W.M.; Hogan, Q.; Sarantopoulos, C. Suppressed Ca2+/CaM/CaMKII-dependent KATP channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc. Natl. Acad. Sci. USA 2009, 106, 8725–8730. [Google Scholar] [CrossRef]
- Zoga, V.; Kawano, T.; Liang, M.Y.; Bienengraeber, M.; Weihrauch, D.; McCallum, B.; Gemes, G.; Hogan, Q.; Sarantopoulos, C. KATP channel subunits in rat dorsal root ganglia: Alterations by painful axotomy. Mol. Pain 2010, 6, 6. [Google Scholar] [CrossRef]
- Chi, X.X.; Jiang, X.; Nicol, G.D. ATP-sensitive potassium currents reduce the PGE2-mediated enhancement of excitability in adult rat sensory neurons. Brain Res. 2007, 1145, 28–40. [Google Scholar] [CrossRef]
- Shen, S.; Cao, S.; Huang, S.; Chen, J. Effect of adenosine triphosphate-sensitive potassium activation on peripheral and central pain sensitization. J. Surg. Res. 2015, 195, 481–487. [Google Scholar] [CrossRef]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Bjork, J.; Entenmann, N.; Jurgenson, T.; Luu, W. Modulation of KATP channel activity in the peripheral nervous system reduces mechanical hyperalgesia after nerve injury. J. Pain 2017, 18, S15. [Google Scholar] [CrossRef]
- Seghers, V.; Nakazaki, M.; DeMayo, F.; Aguilar-Bryan, L.; Bryan, J. Sur1 knockout mice. A model for KATP channel-independent regulation of insulin secretion. J. Biol. Chem. 2000, 275, 9270–9277. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.F.; Liu, W.T.; Liu, Y.P.; Huang, Z.J.; Zhang, Y.K.; Song, X.J. Reopening of ATP-sensitive potassium channels reduces neuropathic pain and regulates astroglial gap junctions in the rat spinal cord. Pain 2011, 152, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.; Baron, R.; Tölle, T.R.; Binder, A.; Birbaumer, N.; Birklein, F.; Gierthmühlen, J.; Flor, H.; Geber, C.; Huge, V.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010, 150, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Lahmann, C.; Clark, R.H.; Iberl, M.; Ashcroft, F.M. A mutation causing increased KATP channel activity leads to reduced anxiety in mice. Physiol. Behav. 2014, 129, 79–84. [Google Scholar] [CrossRef]
- Wellnitz, S.A.; Lesniak, D.R.; Gerling, G.J.; Lumpkin, E.A. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J. Neurophysiol. 2010, 103, 3378–3388. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Fraccarollo, D.; Pförtsch, S.; Flierl, U.; Vogt, C.; Pfrang, J.; Kobsar, A.; Renné, T.; Eigenthaler, M.; Ertl, G.; et al. Improvement of vascular function by acute and chronic treatment with the PDE-5 inhibitor sildenafil in experimental diabetes mellitus. Br. J. Pharmacol. 2008, 153, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.S.; Singh, V.P.; Singh, S.; Kulkarni, S.K. Modulatory effect of the PDE-5 inhibitor sildenafil in diabetic neuropathy. Pharmacology 2004, 72, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Patil, C.S.; Singh, A.; Kulkarni, S.K. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001, 909, 170–178. [Google Scholar] [CrossRef]
- Shimomura, K.; Tusa, M.; Iberl, M.; Brereton, M.F.; Kaizik, S.; Proks, P.; Lahmann, C.; Yaluri, N.; Modi, S.; Huopio, H.; et al. A mouse model of human hyperinsulinism produced by the E1506K mutation in the sulphonylurea receptor SUR1. Diabetes 2013, 62, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Kwon, M.S.; Ivanov, A.; Gerzanich, V.; Simard, J.M. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J. Biol. Chem. 2013, 288, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Kwon, M.S.; Woo, S.K.; Tsymbalyuk, O.; Vennekens, R.; Gerzanich, V.; Simard, J.M. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 2018, 66, 108–125. [Google Scholar] [CrossRef]
- Tosun, C.; Kurland, D.B.; Mehta, R.; Castellani, R.J.; deJong, J.L.; Kwon, M.S.; Woo, S.K.; Gerzanich, V.; Simard, J.M. Inhibition of the Sur1-Trpm4 channel reduces neuroinflammation and cognitive impairment in subarachnoid hemorrhage. Stroke 2013, 44, 3522–3528. [Google Scholar] [CrossRef]
- Mehta, R.I.; Tosun, C.; Ivanova, S.; Tsymbalyuk, N.; Famakin, B.M.; Kwon, M.S.; Castellani, R.J.; Gerzanich, V.; Simard, J.M. Sur1-Trpm4 Cation Channel Expression in Human Cerebral Infarcts. J. Neuropathol. Exp. Neurol. 2015, 74, 835–849. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Zhang, H. Activation of ATP-sensitive potassium channels antagonize nociceptive behavior and hyperexcitability of DRG neurons from rats. Mol. Pain 2011, 7, 35. [Google Scholar] [CrossRef]
- Niu, K.; Saloman, J.L.; Zhang, Y.; Ro, J.Y. Sex differences in the contribution of ATP-sensitive K+ channels in trigeminal ganglia under an acute muscle pain condition. Neuroscience 2011, 180, 344–352. [Google Scholar] [CrossRef]
- Afify, E.A.; Khedr, M.M.; Omar, A.G.; Nasser, S.A. The involvement of KATP channels in morphine-induced antinociception and hepatic oxidative stress in acute and inflammatory pain in rats. Fundam. Clin. Pharmacol. 2013, 27, 623–631. [Google Scholar] [CrossRef]
- Thomzig, A.; Wenzel, M.; Karschin, C.; Eaton, M.J.; Skatchkov, S.N.; Karschin, A.; Veh, R.W. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol. Cell Neurosci. 2001, 18, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Vukovic, J.; Rodríguez, M.J.; Bartlett, P.F. Blockade of microglial KATP -channel abrogates suppression of inflammatory-mediated inhibition of neural precursor cells. Glia 2014, 62, 247–258. [Google Scholar] [CrossRef]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.; Moldovan, S.; Seghers, V.; Wang, X.P.; DeMayo, F.J.; Brunicardi, F.C. Sulfonylurea receptor knockout causes glucose intolerance in mice that is not alleviated by concomitant somatostatin subtype receptor 5 knockout. Ann. Surg. 2002, 235, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.L.; Savelieva, K.V.; Vogel, P.; Baker, K.B.; Mason, S.; Lanthorn, T.H.; Rajan, I. Ligation of mouse L4 and L5 spinal nerves produces robust allodynia without major motor function deficit. Behav. Brain Res. 2015, 276, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Mohammad, H.K.; Ali, R.; Peper, B.; Wilson, S.P.; Raja, S.N.; Ringkamp, M.; Sweitzer, S. Overexpression of µ-Opioid Receptors in Peripheral Afferents, but Not in Combination with Enkephalin, Decreases Neuropathic Pain Behavior and Enhances Opioid Analgesia in Mouse. Anesthesiology 2018, 128, 967–983. [Google Scholar] [CrossRef]
- Martinov, T.; Mack, M.; Sykes, A.; Chatterjea, D. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J. Vis. Exp. 2013, e51212. [Google Scholar] [CrossRef]
- Klein, A.H.; Sawyer, C.M.; Carstens, M.I.; Tsagareli, M.G.; Tsiklauri, N.; Carstens, E. Topical application of L-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav. Brain Res. 2010, 212, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Röyttä, M.; Wei, H.; Pertovaara, A. Spinal nerve ligation-induced neuropathy in the rat: Sensory disorders and correlation between histology of the peripheral nerves. Pain 1999, 80, 161–170. [Google Scholar] [CrossRef]
- Hirai, T.; Enomoto, M.; Kaburagi, H.; Sotome, S.; Yoshida-Tanaka, K.; Ukegawa, M.; Kuwahara, H.; Yamamoto, M.; Tajiri, M.; Miyata, H.; et al. Intrathecal AAV serotype 9-mediated delivery of shRNA against TRPV1 attenuates thermal hyperalgesia in a mouse model of peripheral nerve injury. Mol. Ther. 2014, 22, 409–419. [Google Scholar] [CrossRef]
- Kiso, T.; Watabiki, T.; Tsukamoto, M.; Okabe, M.; Kagami, M.; Nishimura, K.; Aoki, T.; Matsuoka, N. Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience 2008, 153, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Crispim Junior, C.F.; Pederiva, C.N.; Bose, R.C.; Garcia, V.A.; Lino-de-Oliveira, C.; Marino-Neto, J. ETHOWATCHER: Validation of a tool for behavioral and video-tracking analysis in laboratory animals. Comput. Biol. Med. 2012, 42, 257–264. [Google Scholar] [CrossRef]
- Maia, J.L.; Lima-Júnior, R.C.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: Possible mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef]
- Rodrigues, A.R.A.; Duarte, I.D.G. The peripheral antinociceptive effect induced by morphine is associated with ATP-sensitive K+ channels. Br. J. Pharmacol. 2000, 129, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.I.; Torres-López, J.E.; Castañeda-Hernández, G.; Rosas, R.; Vidal-Cantú, G.C.; Granados-Soto, V. Pharmacological evidence for the activation of K+ channels by diclofenac. Eur. J. Pharmacol. 2002, 438, 85–91. [Google Scholar] [CrossRef]
- Alves, D.P.; Soares, A.C.; Francischi, J.N.; Castro, M.S.; Perez, A.C.; Duarte, I.D. Additive antinociceptive effect of the combination of diazoxide, an activator of ATP-sensitive K+ channels, and sodium nitroprusside and dibutyryl-cGMP. Eur. J. Pharmacol. 2004, 489, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.P.; Tatsuo, M.A.; Leite, R.; Duarte, I.D. Diclofenac-induced peripheral antinociception is associated with ATP-sensitive K+ channels activation. Life Sci. 2004, 74, 2577–2591. [Google Scholar] [CrossRef]
- Klein, A.H.; Sawyer, C.M.; Zanotto, K.L.; Ivanov, M.A.; Cheung, S.; Carstens, M.I.; Furrer, S.; Simons, C.T.; Slack, J.P.; Carstens, E. A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. J. Neurophysiol. 2011, 105, 1701–1710. [Google Scholar] [CrossRef]
- Fairbanks, C.A. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv. Drug Deliv. Rev. 2003, 55, 1007–1041. [Google Scholar] [CrossRef]
- Hylden, J.L.; Wilcox, G.L. Intrathecal morphine in mice: A new technique. Eur. J. Pharmacol. 1980, 67, 313–316. [Google Scholar] [CrossRef]
- Hirai, T.; Enomoto, M.; Machida, A.; Yamamoto, M.; Kuwahara, H.; Tajiri, M.; Hirai, Y.; Sotome, S.; Mizusawa, H.; Shinomiya, K.; et al. Intrathecal shRNA-AAV9 inhibits target protein expression in the spinal cord and dorsal root ganglia of adult mice. Hum. Gene Ther. Methods 2012, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pflepsen, K.R.; Peterson, C.D.; Kitto, K.F.; Vulchanova, L.; Wilcox, G.L.; Fairbanks, C.A. Detailed Method for Intrathecal Delivery of Gene Therapeutics by Direct Lumbar Puncture in Mice. Methods Mol. Biol. 2019, 1937, 305–312. [Google Scholar] [CrossRef]
- Vulchanova, L.; Schuster, D.J.; Belur, L.R.; Riedl, M.S.; Podetz-Pedersen, K.M.; Kitto, K.F.; Wilcox, G.L.; McIvor, R.S.; Fairbanks, C.A. Differential adeno-associated virus mediated gene transfer to sensory neurons following intrathecal delivery by direct lumbar puncture. Mol. Pain 2010, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.J.; Dykstra, J.A.; Riedl, M.S.; Kitto, K.F.; Honda, C.N.; McIvor, R.S.; Fairbanks, C.A.; Vulchanova, L. Visualization of spinal afferent innervation in the mouse colon by AAV8-mediated GFP expression. Neurogastroenterol. Motil. 2013, 25, e89–e100. [Google Scholar] [CrossRef] [PubMed]
- Ringkamp, M.; Tal, M.; Hartke, T.V.; Wooten, M.; McKelvy, A.; Turnquist, B.P.; Guan, Y.; Meyer, R.A.; Raja, S.N. Local loperamide injection reduces mechanosensitivity of rat cutaneous, nociceptive C-fibers. PLoS ONE 2012, 7, e42105. [Google Scholar] [CrossRef]
- Zimmermann, K.; Hein, A.; Hager, U.; Kaczmarek, J.S.; Turnquist, B.P.; Clapham, D.E.; Reeh, P.W. Phenotyping sensory nerve endings in vitro in the mouse. Nat. Protoc. 2009, 4, 174–196. [Google Scholar] [CrossRef]
- Koerber, H.R.; McIlwrath, S.L.; Lawson, J.J.; Malin, S.A.; Anderson, C.E.; Jankowski, M.P.; Davis, B.M. Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TPV1 containing C-heat fibers. Mol. Pain 2010, 6, 58. [Google Scholar] [CrossRef]
| Gene | Accession Number | Protein | Forward | Reverse |
|---|---|---|---|---|
| Abcc8 | NM_011510.3 | SUR1 | CCAACACGAGCCTTGAACTT | AGGTTGTTGGTGGAGGTCAG |
| Abcc9 | NM_011511.2 | SUR2 | TGTAGGCCAAGTGGGTTGTG | TCTGCTTCGGGTTGCTTCAA |
| Kcnj11 iso1 | NM_010602.3 | Kir6.2 isoform 1 | CGCCCACAAGAACATTCGAG | GCAGAGTGTGTGGCCATTTG |
| Kcnj11 iso2 | NM_001204411.1 | Kir6.2 isoform 2 | ACCACGTCATCGACTCCAAC | TGGTTTCTACCACGCCTTCC |
| Kcnj8 | NM_008428.5 | Kir6.1 | GGCACCATGGAGAAGAGTGG | CAAAACCGTGATGGCCAGAG |
| Rn18s | NR_003278.3 | 18s | CGCCGCTAGAGGTGAAATTCTT | CAGTCGGCATCGTTTATGGTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu, W.; Bjork, J.; Salo, E.; Entenmann, N.; Jurgenson, T.; Fisher, C.; Klein, A.H. Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice. Int. J. Mol. Sci. 2019, 20, 2251. https://doi.org/10.3390/ijms20092251
Luu W, Bjork J, Salo E, Entenmann N, Jurgenson T, Fisher C, Klein AH. Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice. International Journal of Molecular Sciences. 2019; 20(9):2251. https://doi.org/10.3390/ijms20092251
Chicago/Turabian StyleLuu, Wing, James Bjork, Erin Salo, Nicole Entenmann, Taylor Jurgenson, Cole Fisher, and Amanda H. Klein. 2019. "Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice" International Journal of Molecular Sciences 20, no. 9: 2251. https://doi.org/10.3390/ijms20092251
APA StyleLuu, W., Bjork, J., Salo, E., Entenmann, N., Jurgenson, T., Fisher, C., & Klein, A. H. (2019). Modulation of SUR1 KATP Channel Subunit Activity in the Peripheral Nervous System Reduces Mechanical Hyperalgesia after Nerve Injury in Mice. International Journal of Molecular Sciences, 20(9), 2251. https://doi.org/10.3390/ijms20092251





