Amniotic Fluid Cells, Stem Cells, and p53: Can We Stereotype p53 Functions?
Abstract
1. Introduction
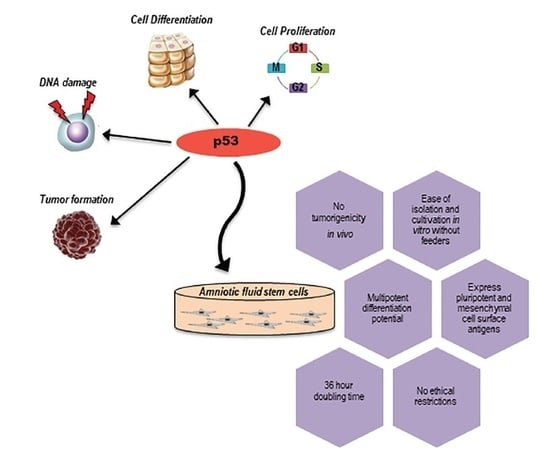
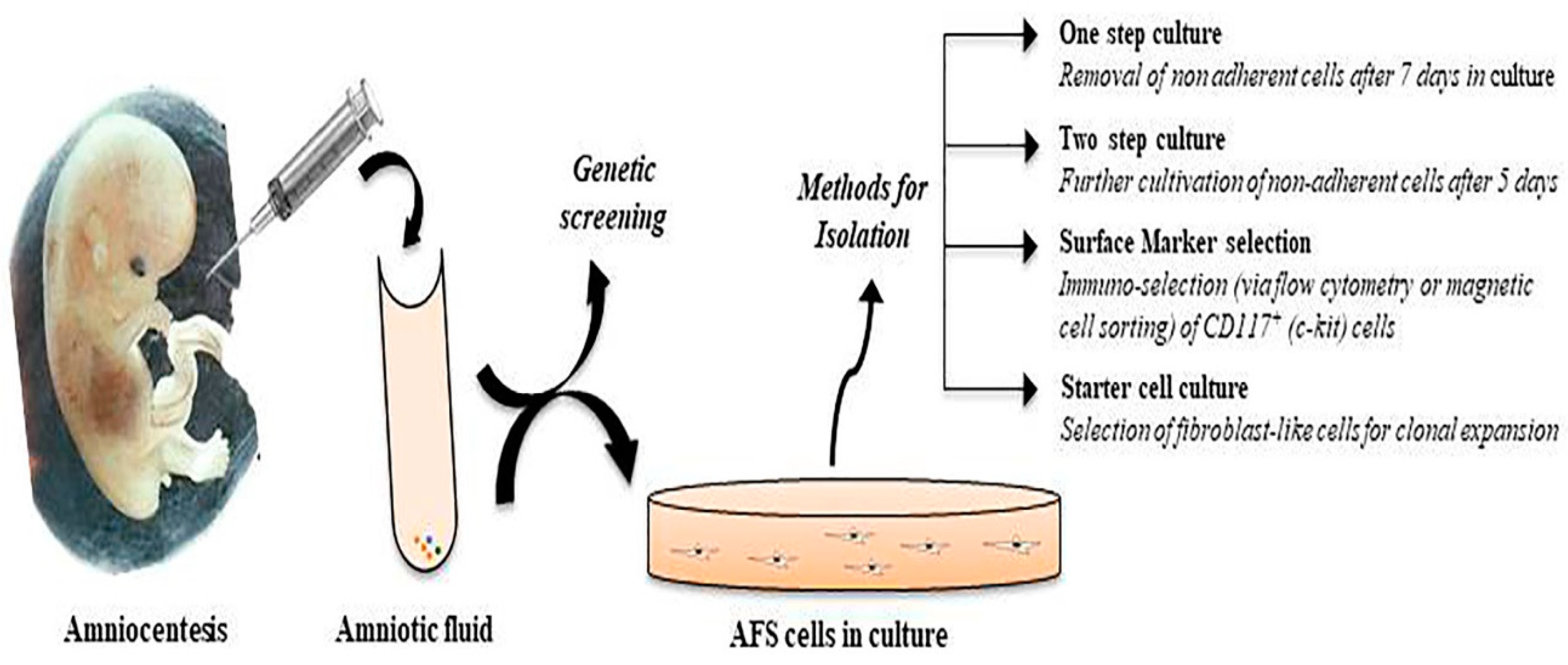
2. Phenotypic Characterization of Amniotic Fluid Stem Cells
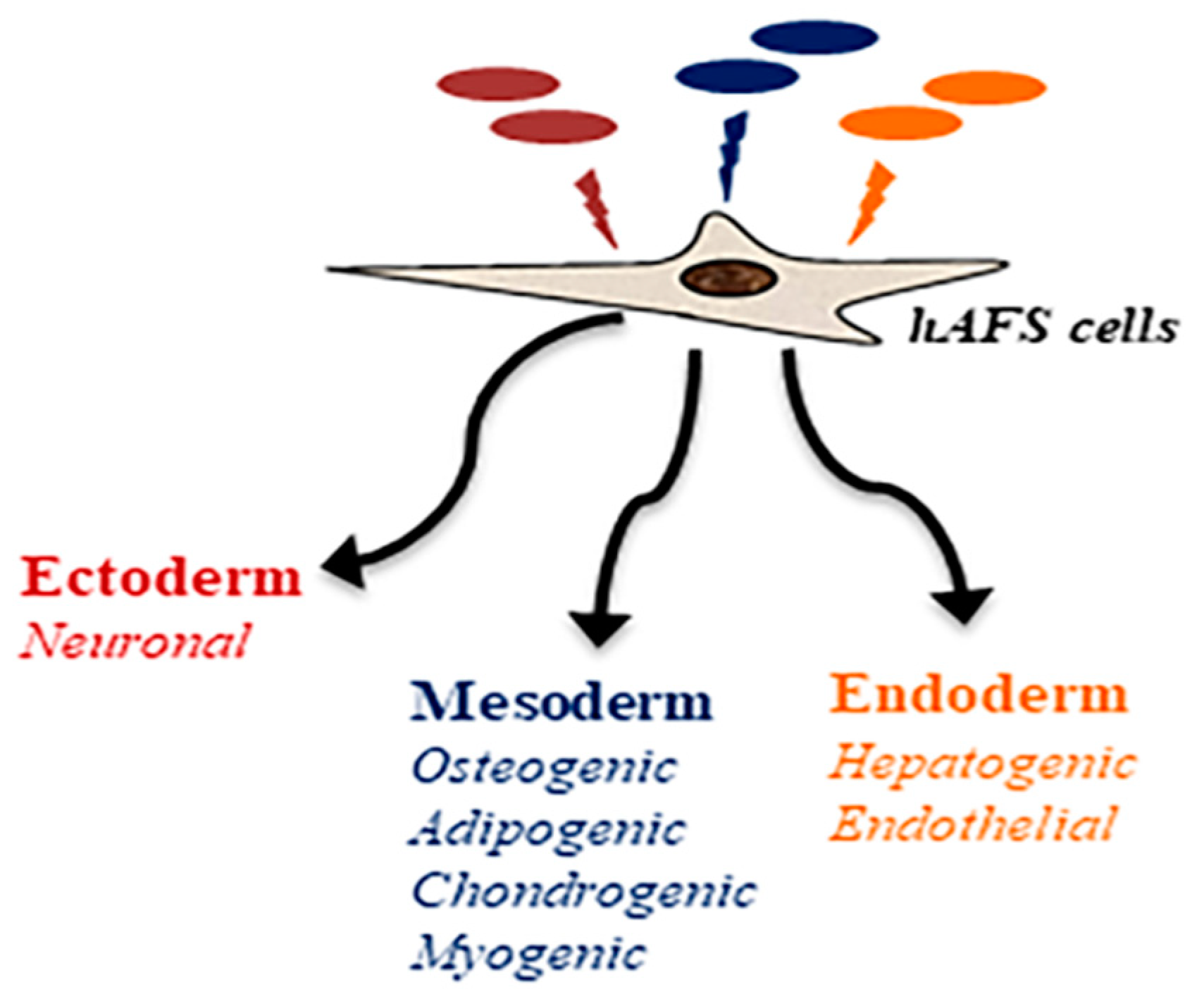
3. Differentiation Potential of Amniotic Fluid Stem Cells
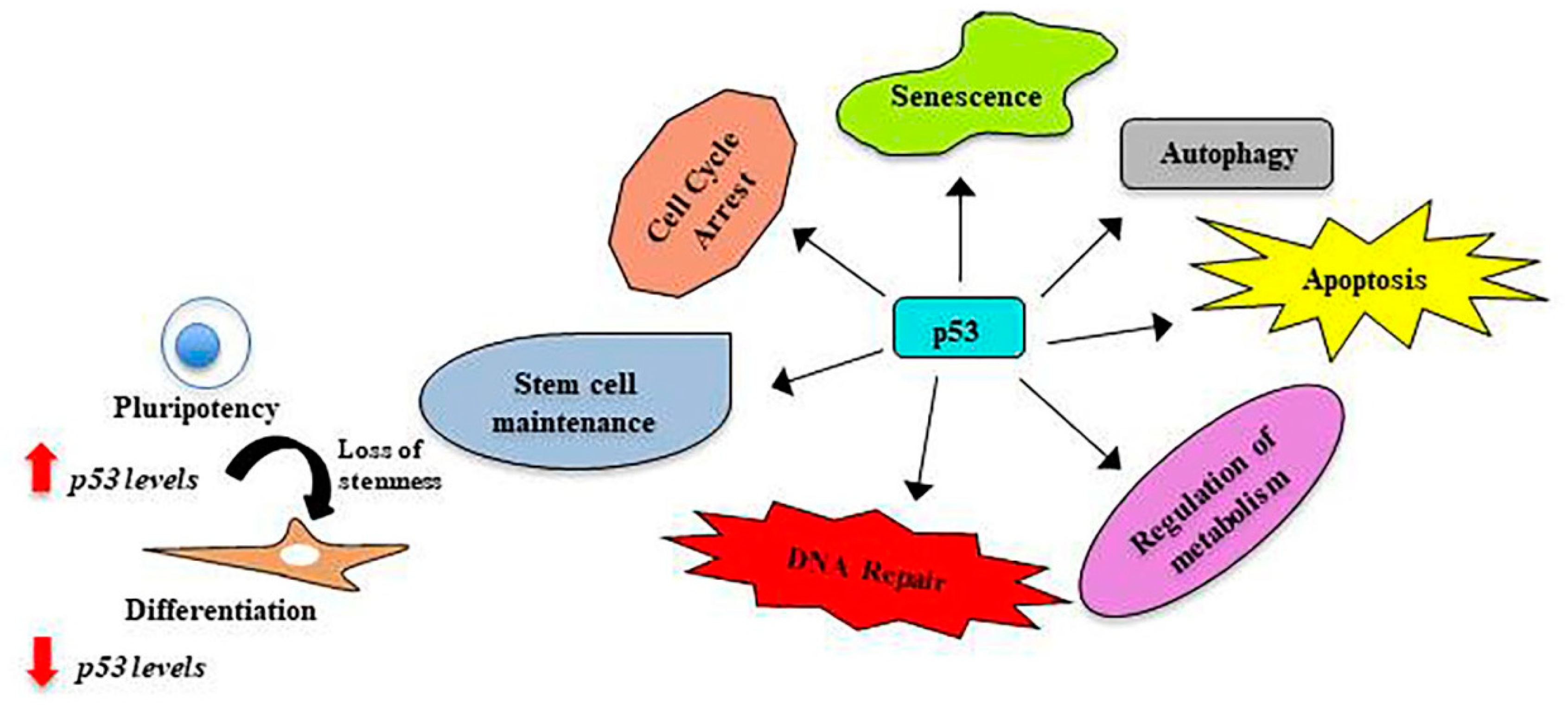
4. p53, the Guardian of the Genome
5. p53 in Stem Cells
6. p53 in Amniotic Fluid Stem Cells
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | amniotic fluid |
| bFGF | basic fibroblast growth factor |
| CD | cluster of differentiation |
| DNA | deoxyribonucleic acid |
| ES | embryonic stem |
| hAFS | human amniotic fluid stem |
| iPS | induced pluripotent stem |
| Klf4 | kruppel-like factor 4 |
| Mdm2 | mouse double minute 2 homolog |
| MEF | murine embryonic fibroblasts |
| miRNA | microRNAs |
| mRNA | messenger RNA |
| Oct4 | octamer-binding transcription factor 4 |
| RNA | ribonucleic acid |
| RING | really interesting new gene |
| Sox2 | SRY (sex determining region Y)-box 2 |
| SSEA | stage-specific embryonic antigen |
| Wnt | wingless/integrated |
References
- Mountford, J.C. Human embryonic stem cells: Origins, characteristics and potential for regenerative therapy. Transfus. Med. 2008, 18, 1–12. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Banito, A.; Gil, J. Induced pluripotent stem cells and senescence: Learning the biology to improve the technology. EMBO Rep. 2010, 11, 353–359. [Google Scholar] [CrossRef]
- Balbi, C.; Bollini, S. Fetal and perinatal stem cells in cardiac regeneration: Moving forward to the paracrine era. Placenta 2017, 59, 96–106. [Google Scholar] [CrossRef]
- Klingemann, H. Discarded stem cells with a future? Expert Opin. Biol. Ther. 2006, 6, 1251–1254. [Google Scholar] [CrossRef]
- Majore, I.; Moretti, P.; Stahl, F.; Hass, R.; Kasper, C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011, 7, 17–31. [Google Scholar] [CrossRef]
- Poloni, A.; Rosini, V.; Mondini, E.; Maurizi, G.; Mancini, S.; Discepoli, G.; Biasio, S.; Battaglini, G.; Berardinelli, E.; Serrani, F.; et al. Characterization and expansion of mesenchymal progenitor cells from first-trimester chorionic villi of human placenta. Cytotherapy 2008, 10, 690–697. [Google Scholar] [CrossRef]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef]
- Wang, H.S.; Hung, S.C.; Peng, S.T.; Huang, C.C.; Wei, H.M.; Guo, Y.J.; Fu, Y.S.; Lai, M.C.; Chen, C.C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef]
- Kaviani, A.; Perry, T.E.; Dzakovic, A.; Jennings, R.W.; Ziegler, M.M.; Fauza, D.O. The amniotic fluid as a source of cells for fetal tissue engineering. J. Pediatr. Surg. 2001, 36, 1662–1665. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Marx, C.; Oppliger, B.; Mueller, M.; Surbek, D.V.; Schoeberlein, A. Mesenchymal Stem Cells from Wharton’s Jelly and Amniotic Fluid. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 30–44. [Google Scholar] [CrossRef]
- Poloni, A.; Maurizi, G.; Babini, L.; Serrani, F.; Berardinelli, E.; Mancini, S.; Costantini, B.; Discepoli, G.; Leoni, P. Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant. 2011, 20, 643–654. [Google Scholar] [CrossRef]
- Yen, B.L.; Huang, H.I.; Chien, C.C.; Jui, H.Y.; Ko, B.S.; Yao, M.; Shun, C.T.; Yen, M.L.; Lee, M.C.; Chen, Y.C. Isolation of multipotent cells from human term placenta. Stem Cells 2005, 23, 3–9. [Google Scholar] [CrossRef]
- Fauza, D. Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 877–891. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Liu, L.; Chen, J.; Yang, W.; Gao, L.; Wang, Y. Human amniotic fluid-derived c-kit(+) and c-kit (-) stem cells: Growth characteristics and some differentiation potential capacities comparison. Cytotechnology 2012, 64, 577–589. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef]
- Polgar, K.; Adany, R.; Abel, G.; Kappelmayer, J.; Muszbek, L.; Papp, Z. Characterization of rapidly adhering amniotic fluid cells by combined immunofluorescence and phagocytosis assays. Am. J. Hum. Genet. 1989, 45, 786–792. [Google Scholar]
- Cananzi, M.; Atala, A.; De Coppi, P. Stem cells derived from amniotic fluid: New potentials in regenerative medicine. Reprod. Biomed. Online 2009, 18 (Suppl. 1), 17–27. [Google Scholar] [CrossRef]
- Pozzobon, M.; Ghionzoli, M.; De Coppi, P. ES, iPS, MSC, and AFS cells. Stem cells exploitation for Pediatric Surgery: Current research and perspective. Pediatr. Surg. Int. 2010, 26, 3–10. [Google Scholar] [CrossRef]
- Davydova, D.A.; Vorotelyak, E.A.; Smirnova, Y.A.; Zinovieva, R.D.; Romanov, Y.A.; Kabaeva, N.V.; Terskikh, V.V.; Vasiliev, A.V. Cell phenotypes in human amniotic fluid. Acta Naturae. 2009, 1, 98–103. [Google Scholar]
- Galende, E.; Karakikes, I.; Edelmann, L.; Desnick, R.J.; Kerenyi, T.; Khoueiry, G.; Lafferty, J.; McGinn, J.T.; Brodman, M.; Fuster, V.; et al. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram 2010, 12, 117–125. [Google Scholar] [CrossRef]
- Li, Q.; Fan, Y.; Sun, X.; Yu, Y. Generation of induced pluripotent stem cells from human amniotic fluid cells by reprogramming with two factors in feeder-free conditions. J. Reprod. Dev. 2013, 59, 72–77. [Google Scholar] [CrossRef]
- Velasquez-Mao, A.J.; Tsao, C.J.M.; Monroe, M.N.; Legras, X.; Bissig-Choisat, B.; Bissig, K.D.; Ruano, R.; Jacot, J.G. Differentiation of spontaneously contracting cardiomyocytes from non-virally reprogrammed human amniotic fluid stem cells. PLoS ONE 2017, 12, e0177824. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Shi, G.; Ma, Y.; Yang, Y.; Gu, J.; Yu, H.; Jin, S.; Wei, Z.; Chen, F.; et al. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum. Mol. Genet. 2009, 18, 4340–4349. [Google Scholar] [CrossRef]
- Qin, M.; Chen, R.; Li, H.; Liang, H.; Xue, Q.; Li, F.; Chen, Y.; Zhang, X. Direct Reprogramming of Human Amniotic Fluid Stem Cells by OCT4 and Application in Repairing of Cerebral Ischemia Damage. Int. J. Biol. Sci. 2016, 12, 558–568. [Google Scholar] [CrossRef]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Drews, K.; Jones, G.N.; Abdulrazzak, H.; Nowakowska, B.; Phoolchund, A.; Lay, K.; Ramasamy, T.S.; et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol. Ther. 2012, 20, 1953–1967. [Google Scholar] [CrossRef]
- Solozobova, V.; Blattner, C. p53 in stem cells. World J. Biol. Chem. 2011, 2, 202–214. [Google Scholar] [CrossRef]
- Klemmt, P.A.; Vafaizadeh, V.; Groner, B. The potential of amniotic fluid stem cells for cellular therapy and tissue engineering. Expert Opin. Biol. Ther. 2011, 11, 1297–1314. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lee, J.L.; Chang, Y.J.; Hwang, S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456. [Google Scholar] [CrossRef]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Antonucci, I.; Iezzi, I.; Morizio, E.; Mastrangelo, F.; Pantalone, A.; Mattioli-Belmonte, M.; Gigante, A.; Salini, V.; Calabrese, G.; Tete, S.; et al. Isolation of osteogenic progenitors from human amniotic fluid using a single step culture protocol. BMC Biotechnol. 2009, 9, 9. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Pappa, K.I.; Bitsika, V.; Zagoura, D.; Vlahou, A.; Papadaki, H.A.; Antsaklis, A.; Anagnou, N.P. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007, 16, 931–952. [Google Scholar] [CrossRef]
- Bossolasco, P.; Montemurro, T.; Cova, L.; Zangrossi, S.; Calzarossa, C.; Buiatiotis, S.; Soligo, D.; Bosari, S.; Silani, V.; Deliliers, G.L.; et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006, 16, 329–336. [Google Scholar] [CrossRef]
- Ditadi, A.; de Coppi, P.; Picone, O.; Gautreau, L.; Smati, R.; Six, E.; Bonhomme, D.; Ezine, S.; Frydman, R.; Cavazzana-Calvo, M.; et al. Human and murine amniotic fluid c-Kit+Lin- cells display hematopoietic activity. Blood 2009, 113, 3953–3960. [Google Scholar] [CrossRef]
- Sessarego, N.; Parodi, A.; Podesta, M.; Benvenuto, F.; Mogni, M.; Raviolo, V.; Lituania, M.; Kunkl, A.; Ferlazzo, G.; Bricarelli, F.D.; et al. Multipotent mesenchymal stromal cells from amniotic fluid: Solid perspectives for clinical application. Haematologica 2008, 93, 339–346. [Google Scholar] [CrossRef]
- Phermthai, T.; Odglun, Y.; Julavijitphong, S.; Titapant, V.; Chuenwattana, P.; Vantanasiri, C.; Pattanapanyasat, K. A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol. 2010, 11, 79. [Google Scholar] [CrossRef]
- Hoehn, H.; Salk, D. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol. 1982, 26, 11–34. [Google Scholar]
- Prusa, A.R.; Hengstschlager, M. Amniotic fluid cells and human stem cell research: A new connection. Med. Sci. Monit. 2002, 8, RA253–RA257. [Google Scholar]
- Antonucci, I.; Pantalone, A.; Tete, S.; Salini, V.; Borlongan, C.V.; Hess, D.; Stuppia, L. Amniotic fluid stem cells: A promising therapeutic resource for cell-based regenerative therapy. Curr. Pharm. Des. 2012, 18, 1846–1863. [Google Scholar] [CrossRef]
- Torricelli, F.; Brizzi, L.; Bernabei, P.A.; Gheri, G.; Di Lollo, S.; Nutini, L.; Lisi, E.; Di Tommaso, M.; Cariati, E. Identification of hematopoietic progenitor cells in human amniotic fluid before the 12th week of gestation. Ital. J. Anat Embryol 1993, 98, 119–126. [Google Scholar] [PubMed]
- Antonucci, I.; Stuppia, L.; Kaneko, Y.; Yu, S.; Tajiri, N.; Bae, E.C.; Chheda, S.H.; Weinbren, N.L.; Borlongan, C.V. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011, 20, 789–795. [Google Scholar] [CrossRef]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef]
- Tsai, M.S.; Hwang, S.M.; Tsai, Y.L.; Cheng, F.C.; Lee, J.L.; Chang, Y.J. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 2006, 74, 545–551. [Google Scholar] [CrossRef]
- Delo, D.M.; De Coppi, P.; Bartsch, G., Jr.; Atala, A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006, 419, 426–438. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Bitsika, V.; Zagoura, D.; Trohatou, O.; Pappa, K.I.; Makridakis, M.; Antsaklis, A.; Vlahou, A.; Anagnou, N.P. In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. J. Cell Mol. Med. 2011, 15, 1896–1913. [Google Scholar] [CrossRef]
- Zagoura, D.S.; Roubelakis, M.G.; Bitsika, V.; Trohatou, O.; Pappa, K.I.; Kapelouzou, A.; Antsaklis, A.; Anagnou, N.P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012, 61, 894–906. [Google Scholar] [CrossRef]
- Cananzi, M.; De Coppi, P. CD117(+) amniotic fluid stem cells: State of the art and future perspectives. Organogenesis 2012, 8, 77–88. [Google Scholar] [CrossRef]
- Prusa, A.R.; Marton, E.; Rosner, M.; Bernaschek, G.; Hengstschlager, M. Oct-4-expressing cells in human amniotic fluid: A new source for stem cell research? Hum. Reprod. 2003, 18, 1489–1493. [Google Scholar] [CrossRef]
- Di Trapani, M.; Bassi, G.; Fontana, E.; Giacomello, L.; Pozzobon, M.; Guillot, P.V.; De Coppi, P.; Krampera, M. Immune regulatory properties of CD117(pos) amniotic fluid stem cells vary according to gestational age. Stem Cells Dev. 2015, 24, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Savickiene, J.; Treigyte, G.; Baronaite, S.; Valiuliene, G.; Kaupinis, A.; Valius, M.; Arlauskiene, A.; Navakauskiene, R. Human Amniotic Fluid Mesenchymal Stem Cells from Second- and Third-Trimester Amniocentesis: Differentiation Potential, Molecular Signature, and Proteome Analysis. Stem Cells Int. 2015, 2015, 319238. [Google Scholar] [CrossRef]
- Schiavo, A.A.; Franzin, C.; Albiero, M.; Piccoli, M.; Spiro, G.; Bertin, E.; Urbani, L.; Visentin, S.; Cosmi, E.; Fadini, G.P.; et al. Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cell Res. Ther 2015, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Moschidou, D.; Mukherjee, S.; Blundell, M.P.; Jones, G.N.; Atala, A.J.; Thrasher, A.J.; Fisk, N.M.; De Coppi, P.; Guillot, P.V. Human mid-trimester amniotic fluid stem cells cultured under embryonic stem cell conditions with valproic acid acquire pluripotent characteristics. Stem Cells Dev. 2013, 22, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Carraro, G.; Perin, L.; Sedrakyan, S.; Giuliani, S.; Tiozzo, C.; Lee, J.; Turcatel, G.; De Langhe, S.P.; Driscoll, B.; Bellusci, S.; et al. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells 2008, 26, 2902–2911. [Google Scholar] [CrossRef]
- Streubel, B.; Martucci-Ivessa, G.; Fleck, T.; Bittner, R.E. In vitro transformation of amniotic cells to muscle cells—Background and outlook. Wien. Med. Wochenschr. 1996, 146, 216–217. [Google Scholar] [PubMed]
- Kim, J.; Lee, Y.; Kim, H.; Hwang, K.J.; Kwon, H.C.; Kim, S.K.; Cho, D.J.; Kang, S.G.; You, J. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007, 40, 75–90. [Google Scholar] [CrossRef]
- Prusa, A.R.; Marton, E.; Rosner, M.; Bettelheim, D.; Lubec, G.; Pollack, A.; Bernaschek, G.; Hengstschlager, M. Neurogenic cells in human amniotic fluid. Am. J. Obstet Gynecol 2004, 191, 309–314. [Google Scholar] [CrossRef]
- Kolambkar, Y.M.; Peister, A.; Soker, S.; Atala, A.; Guldberg, R.E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J. Mol. Histol 2007, 38, 405–413. [Google Scholar] [CrossRef]
- Perin, L.; Giuliani, S.; Jin, D.; Sedrakyan, S.; Carraro, G.; Habibian, R.; Warburton, D.; Atala, A.; De Filippo, R.E. Renal differentiation of amniotic fluid stem cells. Cell Prolif. 2007, 40, 936–948. [Google Scholar] [CrossRef]
- Saulnier, N.; Lattanzi, W.; Puglisi, M.A.; Pani, G.; Barba, M.; Piscaglia, A.C.; Giachelia, M.; Alfieri, S.; Neri, G.; Gasbarrini, G.; et al. Mesenchymal stromal cells multipotency and plasticity: Induction toward the hepatic lineage. Eur. Rev. Med. Pharmacol. Sci. 2009, 13 (Suppl. 1), 71–78. [Google Scholar]
- Siegel, N.; Rosner, M.; Unbekandt, M.; Fuchs, C.; Slabina, N.; Dolznig, H.; Davies, J.A.; Lubec, G.; Hengstschlager, M. Contribution of human amniotic fluid stem cells to renal tissue formation depends on mTOR. Hum. Mol. Genet. 2010, 19, 3320–3331. [Google Scholar] [CrossRef]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. 2013, 9, 642–654. [Google Scholar] [CrossRef]
- Gholizadeh-Ghaleh Aziz, S.; Pashaei-Asl, F.; Fardyazar, Z.; Pashaiasl, M. Isolation, Characterization, Cryopreservation of Human Amniotic Stem Cells and Differentiation to Osteogenic and Adipogenic Cells. PLoS ONE 2016, 11, e0158281. [Google Scholar] [CrossRef]
- Guan, X.; Delo, D.M.; Atala, A.; Soker, S. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J. Tissue Eng. Regen. Med. 2011, 5, 220–228. [Google Scholar] [CrossRef]
- Bollini, S.; Pozzobon, M.; Nobles, M.; Riegler, J.; Dong, X.; Piccoli, M.; Chiavegato, A.; Price, A.N.; Ghionzoli, M.; Cheung, K.K.; et al. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev 2011, 7, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Yang, Y.S.; Chou, C.J.; Lin, K.Y.; Wu, S.C. Differentiation of Enhanced Green Fluorescent Protein-Labeled Mouse Amniotic Fluid-Derived Stem Cells Int.o Cardiomyocyte-Like Beating Cells. Acta Cardiol Sin. 2015, 31, 209–214. [Google Scholar] [PubMed]
- Di Baldassarre, A.; D’Amico, M.A.; Izzicupo, P.; Gaggi, G.; Guarnieri, S.; Mariggio, M.A.; Antonucci, I.; Corneo, B.; Sirabella, D.; Stuppia, L.; et al. Cardiomyocytes Derived from Human (Cardiopoietic)Amniotic Fluids. Sci. Rep. 2018, 8, 12028. [Google Scholar] [CrossRef]
- Valli, A.; Rosner, M.; Fuchs, C.; Siegel, N.; Bishop, C.E.; Dolznig, H.; Madel, U.; Feichtinger, W.; Atala, A.; Hengstschlager, M. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene 2010, 29, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, I.; Di Pietro, R.; Alfonsi, M.; Centurione, M.A.; Centurione, L.; Sancilio, S.; Pelagatti, F.; D’Amico, M.A.; Di Baldassarre, A.; Piattelli, A.; et al. Human second trimester amniotic fluid cells are able to create embryoid body-like structures in vitro and to show typical expression profiles of embryonic and primordial germ cells. Cell Transplant. 2014, 23, 1501–1515. [Google Scholar] [CrossRef]
- Yu, X.; Wang, N.; Qiang, R.; Wan, Q.; Qin, M.; Chen, S.; Wang, H. Human amniotic fluid stem cells possess the potential to differentiate into primordial follicle oocytes in vitro. Biol. Reprod. 2014, 90, 73. [Google Scholar] [CrossRef]
- Moll, U.M.; Wolff, S.; Speidel, D.; Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005, 17, 631–636. [Google Scholar] [CrossRef]
- Maki, C.G.; Huibregtse, J.M.; Howley, P.M. In vivo ubiquitination and proteasome-mediated degradation of p53(1). Cancer Res. 1996, 56, 2649–2654. [Google Scholar]
- Kubbutat, M.H.; Vousden, K.H. Proteolytic cleavage of human p53 by calpain: A potential regulator of protein stability. Mol. Cell Biol. 1997, 17, 460–468. [Google Scholar] [CrossRef]
- Boehme, K.A.; Blattner, C. Regulation of p53—Insights into a complex process. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.P.; Gu, W. Modes of p53 regulation. Cell 2009, 137, 609–622. [Google Scholar] [CrossRef]
- Oren, M. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 1999, 274, 36031–36034. [Google Scholar] [CrossRef]
- Shvarts, A.; Steegenga, W.T.; Riteco, N.; van Laar, T.; Dekker, P.; Bazuine, M.; van Ham, R.C.; van der Houven van Oordt, W.; Hateboer, G.; van der Eb, A.J.; et al. MDMX: A novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996, 15, 5349–5357. [Google Scholar] [CrossRef]
- Wade, M.; Wang, Y.V.; Wahl, G.M. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010, 20, 299–309. [Google Scholar] [CrossRef]
- Wang, L.; He, G.; Zhang, P.; Wang, X.; Jiang, M.; Yu, L. Interplay between MDM2, MDMX, Pirh2 and COP1: The negative regulators of p53. Mol. Biol Rep. 2011, 38, 229–236. [Google Scholar] [CrossRef]
- Sane, S.; Rezvani, K. Essential Roles of E3 Ubiquitin Ligases in p53 Regulation. Int. J. Mol. Sci. 2017, 18, 442. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, J.C.; Fernandes, K.; Murray-Zmijewski, F.; Liu, G.; Diot, A.; Xirodimas, D.P.; Saville, M.K.; Lane, D.P. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005, 19, 2122–2137. [Google Scholar] [CrossRef] [PubMed]
- Murray-Zmijewski, F.; Lane, D.P.; Bourdon, J.C. p53/p63/p73 isoforms: An orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006, 13, 962–972. [Google Scholar] [CrossRef]
- Matlashewski, G.; Lamb, P.; Pim, D.; Peacock, J.; Crawford, L.; Benchimol, S. Isolation and characterization of a human p53 cDNA clone: Expression of the human p53 gene. EMBO J. 1984, 3, 3257–3262. [Google Scholar] [CrossRef]
- Wolf, D.; Harris, N.; Goldfinger, N.; Rotter, V. Isolation of a full-length mouse cDNA clone coding for an immunologically distinct p53 molecule. Mol. Cell Biol. 1985, 5, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.P.; Bourdon, J.C. p53 Isoforms: An Intracellular Microprocessor? Genes Cancer 2011, 2, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Kastan, M.B. The complexity of p53 modulation: Emerging patterns from divergent signals. Genes Dev. 1998, 12, 2973–2983. [Google Scholar] [CrossRef]
- Solozobova, V.; Rolletschek, A.; Blattner, C. Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage. BMC Cell Biol. 2009, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Lin, T.; Chao, C.; Saito, S.; Mazur, S.J.; Murphy, M.E.; Appella, E.; Xu, Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol 2005, 7, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Solozobova, V.; Zhang, P.; Armant, O.; Kuehl, B.; Brenner-Weiss, G.; Blattner, C. p53 is active in murine stem cells and alters the transcriptome in a manner that is reminiscent of mutant p53. Cell Death Dis. 2015, 6, e1662. [Google Scholar] [CrossRef]
- Olivos, D.J.; Mayo, L.D. Emerging Non-Canonical Functions and Regulation by p53: p53 and Stemness. Int. J. Mol. Sci. 2016, 17, 1982. [Google Scholar] [CrossRef]
- Mora, P.T.; Chandrasekaran, K.; McFarland, V.W. An embryo protein induced by SV40 virus transformation of mouse cells. Nature 1980, 288, 722–724. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; McFarland, V.W.; Simmons, D.T.; Dziadek, M.; Gurney, E.G.; Mora, P.T. Quantitation and characterization of a species-specific and embryo stage-dependent 55-kilodalton phosphoprotein also present in cells transformed by simian virus 40. Proc. Natl. Acad. Sci. USA 1981, 78, 6953–6957. [Google Scholar] [CrossRef]
- Rogel, A.; Popliker, M.; Webb, C.G.; Oren, M. p53 cellular tumor antigen: Analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol. Cell Biol. 1985, 5, 2851–2855. [Google Scholar] [CrossRef]
- Sabapathy, K.; Klemm, M.; Jaenisch, R.; Wagner, E.F. Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J. 1997, 16, 6217–6229. [Google Scholar] [CrossRef]
- Solozobova, V.; Blattner, C. Regulation of p53 in embryonic stem cells. Exp. Cell Res. 2010, 316, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Kwon, S.; Jun, E.K.; Kim, A.; Whang, K.Y.; Kim, H.; Oh, S.; Yoon, B.S.; You, S. Nanog-induced dedifferentiation of p53-deficient mouse astrocytes into brain cancer stem-like cells. Biochem. Biophys. Res. Commun. 2011, 412, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Yu, T.; Qing, T.; Liu, Y.; Zhao, Y.; Cai, J.; Li, J.; Song, Z.; Qu, X.; Zhou, P.; et al. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J. Biol. Chem. 2007, 282, 5842–5852. [Google Scholar] [CrossRef]
- Hong, H.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Kanagawa, O.; Nakagawa, M.; Okita, K.; Yamanaka, S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 2009, 460, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chung, S.K.; Xu, Y. Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell 2010, 6, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Allton, K.; Iacovino, M.; Mahen, E.; Milczarek, R.J.; Zwaka, T.P.; Kyba, M.; Barton, M.C. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 2012, 10, e1001268. [Google Scholar] [CrossRef]
- Maimets, T.; Neganova, I.; Armstrong, L.; Lako, M. Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells. Oncogene 2008, 27, 5277–5287. [Google Scholar] [CrossRef]
- Kelly, P.N.; Dakic, A.; Adams, J.M.; Nutt, S.L.; Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 2007, 317, 337. [Google Scholar] [CrossRef]
- Boiko, A.D.; Porteous, S.; Razorenova, O.V.; Krivokrysenko, V.I.; Williams, B.R.; Gudkov, A.V. A systematic search for downstream mediators of tumor suppressor function of p53 reveals a major role of BTG2 in suppression of Ras-induced transformation. Genes Dev. 2006, 20, 236–252. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Chung, S.K.; Xu, Z.; Xu, Y. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells 2014, 32, 157–165. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Y.; Nowotschin, S.; Kim, S.Y.; Li, Q.V.; Soh, C.L.; Su, J.; Zhang, C.; Shu, W.; Xi, Q.; et al. The p53 Family Coordinates Wnt and Nodal Inputs in Mesendodermal Differentiation of Embryonic Stem Cells. Cell Stem Cell 2017, 20, 70–86. [Google Scholar] [CrossRef]
- Meletis, K.; Wirta, V.; Hede, S.M.; Nister, M.; Lundeberg, J.; Frisen, J. p53 suppresses the self-renewal of adult neural stem cells. Development 2006, 133, 363–369. [Google Scholar] [CrossRef]
- Liu, Y.; Elf, S.E.; Miyata, Y.; Sashida, G.; Liu, Y.; Huang, G.; Di Giandomenico, S.; Lee, J.M.; Deblasio, A.; Menendez, S.; et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 2009, 4, 37–48. [Google Scholar] [CrossRef]
- He, Y.; de Castro, L.F.; Shin, M.H.; Dubois, W.; Yang, H.H.; Jiang, S.; Mishra, P.J.; Ren, L.; Gou, H.; Lal, A.; et al. p53 loss increases the osteogenic differentiation of bone marrow stromal cells. Stem Cells 2015, 33, 1304–1319. [Google Scholar] [CrossRef]
- Rodrigues, M.; Antonucci, I.; Elabd, S.; Kancherla, S.; Marchisio, M.; Blattner, C.; Stuppia, L. p53 Is Active in Human Amniotic Fluid Stem Cells. Stem Cells Dev. 2018, 27, 1507–1517. [Google Scholar] [CrossRef]
- Phermthai, T.; Pokathikorn, P.; Wichitwiengrat, S.; Thongbopit, S.; Tungprasertpol, K.; Julavijitphong, S. P53 Mutation and Epigenetic Imprinted IGF2/H19 Gene Analysis in Mesenchymal Stem Cells Derived from Amniotic Fluid, Amnion, Endometrium, and Wharton’s Jelly. Stem Cells Dev. 2017, 26, 1344–1354. [Google Scholar] [CrossRef]
- Miranda-Sayago, J.M.; Fernandez-Arcas, N.; Reyes-Engel, A.; Benito, C.; Narbona, I.; Alonso, A. Changes in CDKN2D, TP53, and miR125a expression: Potential role in the evaluation of human amniotic fluid-derived mesenchymal stromal cell fitness. Genes Cells 2012, 17, 673–687. [Google Scholar] [CrossRef]
- Savickiene, J.; Baronaite, S.; Zentelyte, A.; Treigyte, G.; Navakauskiene, R. Senescence-Associated Molecular and Epigenetic Alterations in Mesenchymal Stem Cell Cultures from Amniotic Fluid of Normal and Fetus-Affected Pregnancy. Stem Cells Int. 2016, 2016, 2019498. [Google Scholar] [CrossRef]
- Thangnipon, W.; Puangmalai, N.; Suwanna, N.; Soi-Ampornkul, R.; Phonchai, R.; Kotchabhakdi, N.; Mukda, S.; Phermthai, T.; Julavijitphong, S.; Tuchinda, P.; et al. Potential role of N-benzylcinnamide in inducing neuronal differentiation from human amniotic fluid mesenchymal stem cells. Neurosci. Lett. 2016, 610, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gasiuniene, M.; Zubova, A.; Utkus, A.; Navakauskiene, R. Epigenetic and metabolic alterations in human amniotic fluid stem cells induced to cardiomyogenic differentiation by DNA methyltransferases and p53 inhibitors. J. Cell Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
| Features | Human ES Cells | Human AFS Cells |
|---|---|---|
| Source of cells | Inner cell mass of a blastocyst stage embryo | Amniotic fluid from second or third trimester of pregnancy |
| Plasticity | Pluripotent | Multipotent |
| Ease of cultivation | Requirement of MEF for feeders and the presence of bFGF | Cultivation without feeders but in presence of bFGF |
| Doubling time | 24 to 96 h | Approximate 36 h |
| Marker expression: Pluripotency | Oct4, Nanog, Sox-2, Klf4 | Oct4, Nanog (low), Sox2 (low), Klf4 (low) |
| Cell Surface Antigens Mesenchymal | SSEA1, SSEA3, SSEA4, Tra-1-60, Tra-1-81 | SSEA4, CD-117 (c-kit), CD90, CD105, CD73, CD13, CD29, CD44, CD146 |
| In vitro differentiation potential | All three germ layers (under specific culture conditions) | All three germ layers (under specific culture conditions) |
| Ability to form embryoid body | Yes | Yes |
| Tumorigenic in vivo | Yes | No |
| Ethical concerns | Yes | No |
| Legal restrictions | Yes | No |
| Therapeutic animal model testing | Yes | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.; Blattner, C.; Stuppia, L. Amniotic Fluid Cells, Stem Cells, and p53: Can We Stereotype p53 Functions? Int. J. Mol. Sci. 2019, 20, 2236. https://doi.org/10.3390/ijms20092236
Rodrigues M, Blattner C, Stuppia L. Amniotic Fluid Cells, Stem Cells, and p53: Can We Stereotype p53 Functions? International Journal of Molecular Sciences. 2019; 20(9):2236. https://doi.org/10.3390/ijms20092236
Chicago/Turabian StyleRodrigues, Melissa, Christine Blattner, and Liborio Stuppia. 2019. "Amniotic Fluid Cells, Stem Cells, and p53: Can We Stereotype p53 Functions?" International Journal of Molecular Sciences 20, no. 9: 2236. https://doi.org/10.3390/ijms20092236
APA StyleRodrigues, M., Blattner, C., & Stuppia, L. (2019). Amniotic Fluid Cells, Stem Cells, and p53: Can We Stereotype p53 Functions? International Journal of Molecular Sciences, 20(9), 2236. https://doi.org/10.3390/ijms20092236