Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Comparison of Arterial Stiffness between Systemic Lupus Erythematosus (SLE) and Healthy Controls
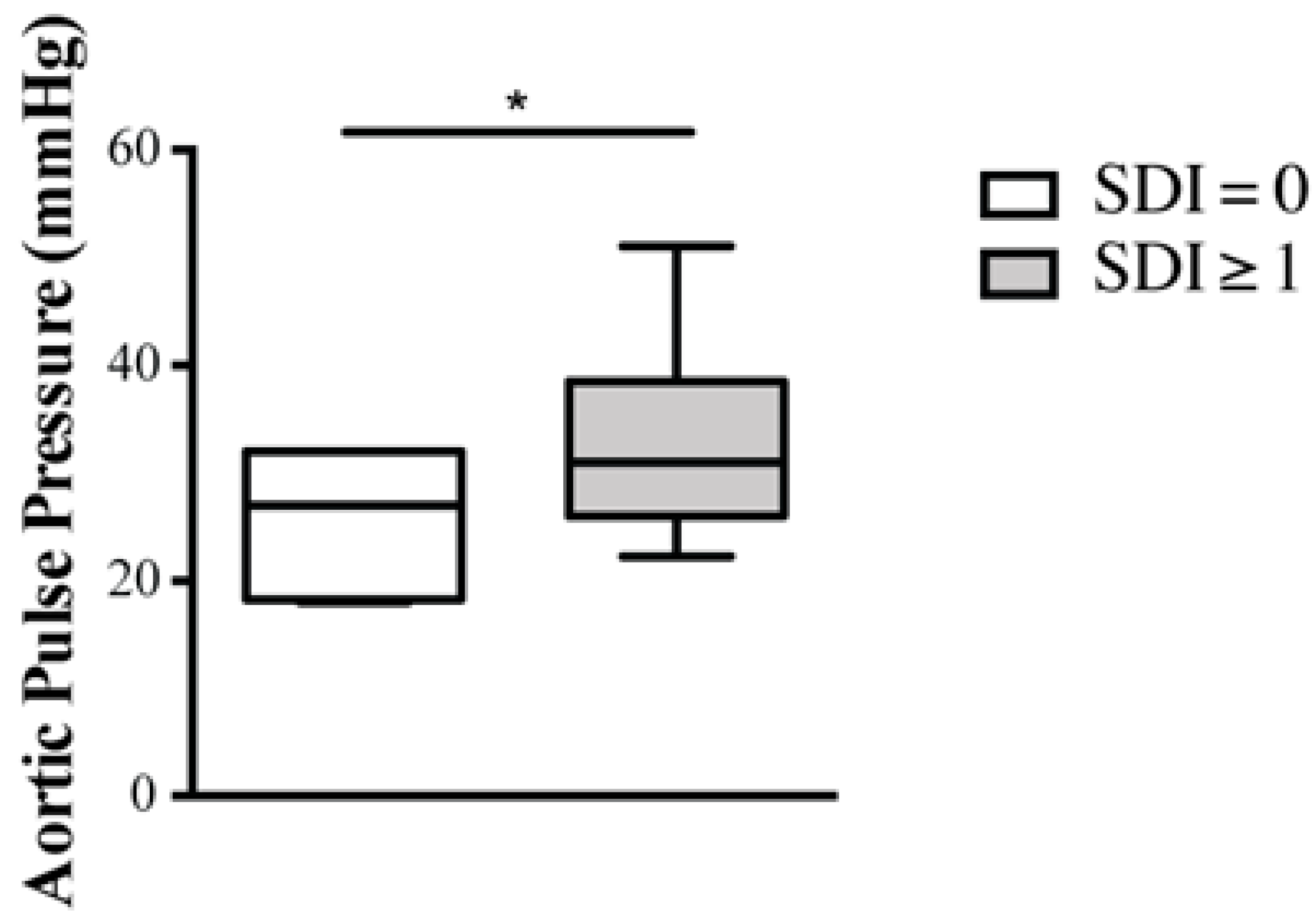
2.3. Predictors of Arterial Stiffness
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethics Statement
4.2. Clinical and Laboratory Data
4.3. Vascular Assessment
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramos-Casals, M.; Brito-Zerón, P.; Kostov, B.; Sisó-Almirall, A.; Bosch, X.; Buss, D.; Trilla, A.; Stone, J.H.; Khamashta, M.A.; Shoenfeld, Y. Google-driven search for big data in autoimmune geoepidemiology: Analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun. Rev. 2015, 14, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Lerang, K.; Gilboe, I.M.; Steinar Thelle, D.; Gran, J.T. Mortality and years of potential life loss in systemic lupus erythematosus: A population-based cohort study. Lupus 2014, 23, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, L.B.; Kaplan, M.J. Update on cardiovascular disease in lupus. Curr. Opin. Rheumatol. 2016, 28, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.E.; Roman, M.J. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am. J. Med. 2008, 121, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Urowitz, M.B.; Bookman, A.A.; Koehler, B.E.; Gordon, D.A.; Smythe, H.A.; Ogryzlo, M.A. The bimodal mortality pattern in systemic lupus erythematosus. Am. J. Med. 1976, 60, 221–225. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Stavropoulos-Kalinoglou, A.; Mikhailidis, D.P.; Toms, T.E.; Douglas, K.M.; Kitas, G.D. The rationale for comparative studies of accelerated atherosclerosis in rheumatic diseases. Curr. Vasc. Pharmacol. 2010, 8, 437–449. [Google Scholar] [CrossRef]
- Magder, L.S.; Petri, M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am. J. Epidemiol. 2012, 176, 708–719. [Google Scholar] [CrossRef]
- Fors Nieves, C.E.; Izmirly, P.M. Mortality in systemic lupus erythematosus: An updated review. Curr. Rheumatol. Rep. 2016, 18, 21. [Google Scholar] [CrossRef]
- Wu, G.C.; Liu, H.R.; Leng, R.X.; Li, X.P.; Li, X.M.; Pan, H.F.; Ye, D.Q. Subclinical atherosclerosis in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Autoimmun. Rev. 2016, 15, 22–37. [Google Scholar] [CrossRef]
- Soubrier, M.; Mathieu, S.; Dubost, J.J. Atheroma and systemic lupus erythematosus. Joint Bone Spine 2007, 74, 566–570. [Google Scholar] [CrossRef]
- Watkins, A.A.; Yasuda, K.; Wilson, G.E.; Aprahamian, T.; Xie, Y.; Maganto-Garcia, E.; Shukla, P.; Oberlander, L.; Laskow, B.; Menn-Josephy, H.; et al. IRF5 deficiency ameliorates lupus but promotes atherosclerosis in a mouse model of lupus-associated atherosclerosis. J. Immunol. 2015, 194, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, P.; Tasso, M.; Lupoli, R.; Di Minno, A.; Baldassarre, D.; Tremoli, E.; Di Minno, M.N. Non-invasice assessment of arterial stiffness in patients with rheumatoid arthritis: A systematic review and meta-analysis of literature studies. Ann. Med. 2015, 47, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Palombo, C.; Kozakova, M. Arterial stiffness, atherosclerosis and cardiovascular risk: Patophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 2016, 77, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Braam, B. Assessment of arterial stiffness using applanation tonometry. Can. J. Physiol. Pharmacol. 2013, 91, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Pulse pressure, arterial stiffness and wave reflection (augmentation index) as cardiovascular risk factors in hypertension. Ther. Adv. Cardiovasc. Dis. 2008, 2, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Auer, J.; Lamm, G.; O’Rourke, M.F.; Eber, B. Arterial stiffness, central blood pressures, and wave reflections in cardiomyopathy-implications for risk stratification. J. Card. Fail 2007, 13, 353–359. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Shang, Q.; Tam, L.S.; Li, E.K.; Yip, G.W.; Yu, C.M. Increased arterial stiffness correlated with disease activity in systemic lupus erythematosus. Lupus 2008, 17, 1096–1102. [Google Scholar] [CrossRef]
- Barbulescu, A.L.; Vreju, F.; Cojocaru-Gofita, I.R.; Musetescu, A.E.; Ciurea, P.L. Impaired arterial stiffness in systemic lupus erythematosus – correlations with inflammation markers. Curr. Health Sci. J. 2012, 38, 61–65. [Google Scholar]
- Sacre, K.; Escoubet, B.; Pasquet, B.; Chauveheid, M.P.; Zennaro, M.C.; Tubach, F.; Papo, T. Increased arterial stiffness in systemic lupus erythematosus (SLE) patients at low risk for cardiovascular disease: A cross-sectional controlled study. PLoS ONE 2014, 9, e94511. [Google Scholar] [CrossRef]
- Bjarnegråd, N.; Bengtsson, C.; Brodszki, J.; Sturfelt, G.; Nived, O.; Länne, T. Increased aortic pulse wave velocity in middle aged women with systemic lupus erythematosus. Lupus 2006, 15, 644–650. [Google Scholar] [CrossRef]
- Morreale, M.; Mulè, G.; Ferrante, A.; D’Ignoto, F.; Cottone, S. Early vascular aging in normotensive patients with systemic lupus erythematosus: Comparison with young patients having hypertension. Angiology 2016, 67, 676–682. [Google Scholar] [CrossRef]
- Sabio, J.M.; Vargas-Hitos, J.A.; Martinez-Bordonado, J.; Navarrete-Navarrete, N.; Díaz-Chamorro, A.; Olvera-Porcel, C.; Zamora, M.; Jiménez-Alonso, J. Association between low 25-hydroxyvitamin D, insulin resistance and arterial stiffness in nondiabetic women with systemic lupus erythematosus. Lupus 2015, 24, 155–163. [Google Scholar] [CrossRef]
- Tziomalos, K.; Gkougkourelas, I.; Sarantopoulos, A.; Bekiari, E.; Makri, E.; Raptis, N.; Tselios, K.; Pantoura, M.; Hatzitolios, A.I.; Boura, P. Arterial stiffness and peripheral arterial disease in patients with systemic lupus erythematosus. Rheumatol. Int. 2017, 37, 293–298. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M.; Tennent, G.A.; Gallimore, J.R.; Kahan, M.C.; Bellotti, V.; Hawkins, P.N.; Myers, R.M.; Smith, M.D.; Polara, A.; et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006, 440, 1217–1221. [Google Scholar] [CrossRef]
- Verma, S.; Kuliszewski, M.A.; Li, S.H.; Szmitko, P.E.; Zucco, L.; Wang, C.H.; Badiwala, M.V.; Mickle, D.A.; Weisel, R.D.; Fedak, P.W.; et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: Further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation 2004, 109, 2058–2067. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Somers, E.C.; Brook, R.D.; Kehrer, C.; Pfenninger, D.; Lewis, E.; Chakrabarti, A.; Richardson, B.C.; Shelden, E.; McCune, W.J.; et al. Endothelial cell apoptosis in systemic lupus erythematosus: A common pathway for abnormal vascular function and thrombosis propensity. Blood 2004, 103, 3677–3683. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.F.; Bonfà, E. Dyslipoproteinemia in systemic lupus erythematosus: Influence of disease, activity, and anticardiolipin antibodies. Lupus 1997, 6, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.F.; Thacker, S.; Mehta, H.; Somers, E.C.; Dodick, T.; Barrat, F.J.; McCune, W.J.; Kaplan, M.J. Interferon-alpha promotes abnormal vasculogenesis in lupus: A potential pathway for premature atherosclerosis. Blood 2007, 110, 2907–2915. [Google Scholar] [CrossRef]
- Mohan, S.; Barsalou, J.; Bradley, T.J.; Slorach, C.; Reynolds, J.A.; Hasni, S.; Thompson, B.; Ng, L.; Levy, D.; Silverman, E.; et al. Endothelial progenitor cell phenotype and function are impaired in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. 2015, 67, 2257–2262. [Google Scholar] [CrossRef]
- Manzi, S.; Meilahn, E.N.; Rairie, J.E.; Conte, C.G.; Medsger, T.A., Jr.; Jansen-McWilliams, L.; D’Agostino, R.B.; Kuller, L.H. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham Study. Am. J. Epidemiol. 1997, 145, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Nikpour, M.; Urowitz, M.B.; Ibañez, D.; Harvey, P.J.; Gladman, D.D. Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: A prospective proof-of-concept cohort study. Arthritis Res. Ther. 2011, 13, R156. [Google Scholar] [CrossRef] [PubMed]
- Rahman, P.; Aguero, S.; Gladman, D.D.; Hallett, D.; Urowitz, M.B. Vascular events in hypertensive patients with systemic lupus erythematosus. Lupus 2000, 9, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Eräranta, A.; Tikkakoski, A.J.; Koskela, J.; Hautaniemi, E.J.; Kähönen, M.; Mustonen, J.; Pörsti, I. LDL cholesterol is associated with systemic vascular resistance and wave reflection in subjects naive to cardiovascular drugs. Blood Press 2019, 28, 4–14. [Google Scholar] [CrossRef]
- Selzer, F.; Sutton-Tyrrell, K.; Fitzgerald, S.; Tracy, R.; Kuller, L.; Manzi, S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 2001, 37, 1075–1082. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, B.H. Aging and arterial stiffness. Circ. J. 2010, 74, 2257–2262. [Google Scholar] [CrossRef]
- Raji, L.; Gonzalez-Ochoa, A.M. Vascular compliance in blood pressure. Curr. Opin. Nephrol. Hypertens. 2011, 20, 457–464. [Google Scholar] [CrossRef]
- Asmar, R.; Benetos, A.; London, G.; Hugue, C.; Weiss, Y.; Topouchian, J.; Laloux, B.; Safar, M. Aortic distensibility in normotensive, untreated and treated hypertensive patients. Blood Press 1995, 4, 48–54. [Google Scholar] [CrossRef]
- Taquet, A.; Bonithon-Kopp, C.; Simon, A.; Levenson, J.; Scarabin, Y.; Malmejac, A.; Ducimetiere, P.; Guize, L. Relations of cardiovascular risk factors to aortic pulse wave velocity in asymptomatic middle-aged woman. Eur. J. Epidemiol. 1993, 9, 298–306. [Google Scholar] [CrossRef]
- Manger, K.; Kusus, M.; Forster, C.; Ropers, D.; Daniel, W.; Kalden, J.; Achenbach, S.; Manger, B. Factors associated with coronary artery calcification in young female patients with, S.L.E. Ann. Rheum. Dis. 2003, 62, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Bruce, I.N. Therapy insight: Systemic lupus erythematosus as a risk factor for cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Pucci, G.; Bocci, E.B.; Anastasio, F.; Crapa, M.; Sanesi, L.; Gerli, R.; Schillaci, G. Arterial stiffness and disease-related organ damage in systemic lupus erythematosus. J. Hypertens. 2015, 33, e24. [Google Scholar] [CrossRef]
- Bulkley, B.H.; Roberts, W.C. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy. A study of 36 necropsy patients. Am. J. Med. 1975, 58, 243–264. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Dhillon, V.B.; Binder, A.; Forte, C.A.; Knight, B.C.; Betteridge, D.J.; Isenberg, D.A. Fasting lipids and anticardiolipin antibodies as risk factors for vascular disease in systemic lupus erythematosus. Ann. Rheum. Dis. 1992, 51, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.F.; Bonfà, E. Long term beneficial effect of chloroquine diphosphate on lipoprotein profile in lupus patients with and without steroid therapy. J. Rheumatol. 2001, 28, 780–785. [Google Scholar] [PubMed]
- Husmann, M.; Jacomella, V.; Thalhammer, C.; Amann-Vesti, B.R. Markers of arterial stiffness in peripheral arterial disease. Vasa 2015, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, F.; Gatto, M.; Larosa, M.; Iaccarino, L.; Punzi, L.; Doria, A. Cardiovascular risk factors, burden of disease and preventive strategies in patients with systemic lupus erythematosus: A literature review. Expert Opin. Drug Saf. 2015, 14, 1373–1385. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | SLE Patients, n = 43 | Controls, n = 43 | p |
|---|---|---|---|
| Age, years | 41 (11) | 39.0 (12) | 0.18 |
| Males, n (%) | 4 (9.3%) | 4 (9.3%) | 0.10 |
| Systolic blood pressure, mmHg | 120 (20) | 118 (15) | 0.334 |
| Diastolic blood pressure, mmHg | 80 (15) | 8 (10) | 0.225 |
| Body mass index, kg/m2 | 23.2 (6.0) | 23.0 (6) | 0.720 |
| Hypertension, n (%) | 7 (16.3%) | 6 (13.9%) | 0.422 |
| Type 2 diabetes mellitus, n (%) | 1 (2.5%) | 0 | 0.225 |
| Smoking habit, n (%) | 21 (48.8%) | 18 (41.9%) | 0.633 |
| Disease duration, years | 14.0 (18) | ||
| Duration of steroid therapy, years | 10 (13) | ||
| Mean daily dose of prednisone, mg | 7.5 (8) | ||
| Diagnosis of nephritis, n (%) | 9 (22.5%) | ||
| Mean SLEDAI score | 8.0 (10) | ||
| Mean SDI score | 2 (2) | ||
| ANA | 37 (86%) | ||
| anti-dsDNA | 19 (44.2%) | ||
| ENA | 25 (58.1%) | ||
| Low complement levels (C3 or C4) | 21 (48.8%) | ||
| Total cholesterol, mg/dL | 174 (75) | ||
| LDL cholesterol, mg/dL | 112 (53) | ||
| HDL cholesterol, mg/dL | 52 (17) | ||
| Homocysteine, µmol/dL | 14 (7) | ||
| Creatinine, mg/dL | 0.7 (0.2) | ||
| Estimated GFR, mL/min/1.73 m2 | 109 (23.5) | ||
| Proteinuria, mg/day | 345 (90) | ||
| Uric acid, mg/dL | 4.8 (1.5) | ||
| Glucose, mg/dL | 77 (7) | ||
| Lymphocytes, n/µL | 1800 (1000) | ||
| C-reactive protein, mg/L | 0.33 (0.48) | ||
| Erythrocyte sedimentation rate, mm/h | 18 (25) | ||
| Fibrinogen, mg/dL | 282 (85) |
| Clinical and Laboratory Parameters | Linear Correlation Coefficient (r) | 95% C.I. | p |
|---|---|---|---|
| Univariate Analysis for AoPP | |||
| Age | 0.40 | 0.21 to 0.62 | 0.02 |
| Systolic blood pressure | 0.66 | 0.44 to 0.80 | < 0.001 |
| C-reactive protein | 0.37 | 0.07 to 0.61 | 0.03 |
| Erythrosedimentation rate | 0.34 | 0.10 to 0.53 | 0.05 |
| Daily dose of prednisone | 0.33 | 0.15 to 0.53 | 0.05 |
| SDI score | 0.30 | 0.12 to 0.44 | 0.05 |
| Univariate Analysis for AIx@75 | |||
| Age | 0.57 | 0.19 to 0.82 | < 0.001 |
| Lymphocytes | −0.44 | −0.50 to −0.12 | < 0.001 |
| Univariate Analysis for AIx | |||
| Age | 0.59 | 0.21 to 0.85 | < 0.001 |
| Lymphocytes | −0.34 | −0.55 to −0.15 | 0.05 |
| Creatinine | −0.37 | −0.62 to −0.12 | 0.04 |
| Clinical and Laboratory Parameters | Coefficient of Determination (r2) | 95 % C.I. | p |
|---|---|---|---|
| Multiregression Analysis for AoPP | |||
| Age | 0.53 | 0.20 to 0.89 | 0.01 |
| Systolic blood pressure | 0.29 | 0.12 to 0.45 | 0.001 |
| Erythrosedimentation rate | 0.29 | 0.09 to -0.49 | 0.005 |
| Multiregression Analysis for AIx@75 | |||
| Age | 0.80 | 0.33 to 1.28 | < 0.001 |
| Multiregression Analysis for AIx | |||
| Age | 0.95 | 0.48 to 1.43 | < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2019, 20, 2154. https://doi.org/10.3390/ijms20092154
Mercurio V, Lobasso A, Barbieri L, Parrella P, Ciervo D, Liccardo B, Bonaduce D, Tocchetti CG, De Paulis A, Rossi FW. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. International Journal of Molecular Sciences. 2019; 20(9):2154. https://doi.org/10.3390/ijms20092154
Chicago/Turabian StyleMercurio, Valentina, Antonio Lobasso, Letizia Barbieri, Paolo Parrella, Deasy Ciervo, Bianca Liccardo, Domenico Bonaduce, Carlo G. Tocchetti, Amato De Paulis, and Francesca W. Rossi. 2019. "Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients" International Journal of Molecular Sciences 20, no. 9: 2154. https://doi.org/10.3390/ijms20092154
APA StyleMercurio, V., Lobasso, A., Barbieri, L., Parrella, P., Ciervo, D., Liccardo, B., Bonaduce, D., Tocchetti, C. G., De Paulis, A., & Rossi, F. W. (2019). Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. International Journal of Molecular Sciences, 20(9), 2154. https://doi.org/10.3390/ijms20092154






