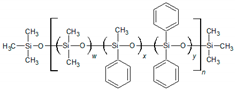
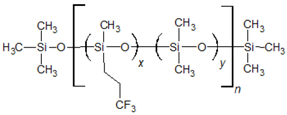
2.1. QSRR Dataset
The dataset investigated in this work consists of 29 poly(siloxane) stationary phases belonging to poly(methylphenylsiloxane), poly(methyltrifluoropropylsiloxane) and poly(cyanoalkylmethylphenylsiloxane) subgroups displayed in
Table 1 and
Table 2 together with the first five McReynolds constants X, Y, Z, U and S, taken from scientific [
25] or commercial literature [
26]. The code of a GLC commercial column is associated to each stationary phase, but it must be noted that many equivalent poly(siloxane)-based columns can be provided by different manufacturers [
20].
Poly(dimethylsiloxane) (column OV-1) is a nonpolar and low-selectivity phase that can be regarded as the basic structure of the stationary phases here investigated. Substitution of methyl groups with phenyl, trifluoropropyl and cyanoalkyl groups in variable concentration permits extending the selectivity of the poly(siloxanes) over a wide range [
25], which makes them the most versatile in GLC analytical separations.
In previous analogous investigations [
23,
24] aimed at modelling the McReynolds constants of nonpolymeric stationary phases (esters of dicarboxylic acids, for instance) the molecular descriptors were determined using a standard procedure, consisting of a preliminary geometry optimisation followed by the computation of the structural properties. Polysiloxane stationary phases, by contrast, are polymers with a high molecular weight (generally in the range of 10
3 to 10
6) [
25], and therefore a simplified geometrical model must be generated. In this work, each stationary phase was represented by an oligomer formed by 20 siloxane units ending with trimethylsiloxy groups. This choice represents a good compromise between the needs of an acceptable computation time and adequate representation of the bulk properties of the stationary phase. In variously-substituted poly(siloxanes), the different comonomers were uniformly positioned within the polysiloxane backbone. In few cases (columns OV-61, FS-328, FS-169 and NSKT-33), the requisite of having an integer number of each comonomer resulted in a slight deviation of the geometrical composition in the geometrical model compared to the nominal one.
Figure 1 displays the optimised molecular models of poly(dimethylsiloxane) (column OV-1) and 65% diphenyl-35% dimethyl poly(siloxane) (column Rtx-65). Regardless of the chemical composition, the polysiloxane backbone in the optimised structures is coiled to favour the attractive intrachain interactions. In spite of the much lower polymerisation degree and the absence of interchain interactions, the optimised geometrical models should provide a reliable representation of the reciprocal position of the siloxane substituents within the real stationary phases which governs the retention of solutes and column selectivity. The optimised structures were processed by computer package Dragon 6 which provides 4885 molecular descriptors belonging to various classes [
27]. However, to avoid including redundant variables in the QSRR dataset, the descriptors with little variance were removed, and only one descriptor was retained among groups of highly correlated ones (
r > 0.85). After this preliminary variable selection, 177 molecular descriptors were identified and stored for further analysis.
2.2. QSRR Modelling of McReynolds Constants by GA-MLR
A specific QSRR model was established for each of the five McReynolds constants by multilinear regression (MLR) coupled with genetic algorithm (GA) variable selection [
28,
29]. Before developing the QSRR models, we designed an external prediction set by selecting five columns (OV-7, OV-25, SILAR 7CP, XE-60 and FS-328) covering as far as possible the structural variability of the 29 poly(siloxanes) in terms of qualitative and quantitative composition. A preliminary GA-MLR exploration was carried out to identify the optimal complexity of the QSRRs. We observed that including six descriptors into the various QSRR models gave satisfactory results, while incorporation of a seventh descriptor produced only a negligible improvement of Q
2loo-cv. Therefore, the maximum number of descriptors to be selected by GA was set to six. Some hundreds of GA-MLR runs with different starting chromosome populations were performed for each of the five responses and the descriptors selected at least one time were collected together in a same data set that was subjected to a final GA-MLR analysis. The models with the highest Q
2loo-cv values for each of the five different responses were finally chosen. These are presented in
Table 3, while the selected molecular descriptors are listed in
Table 4.
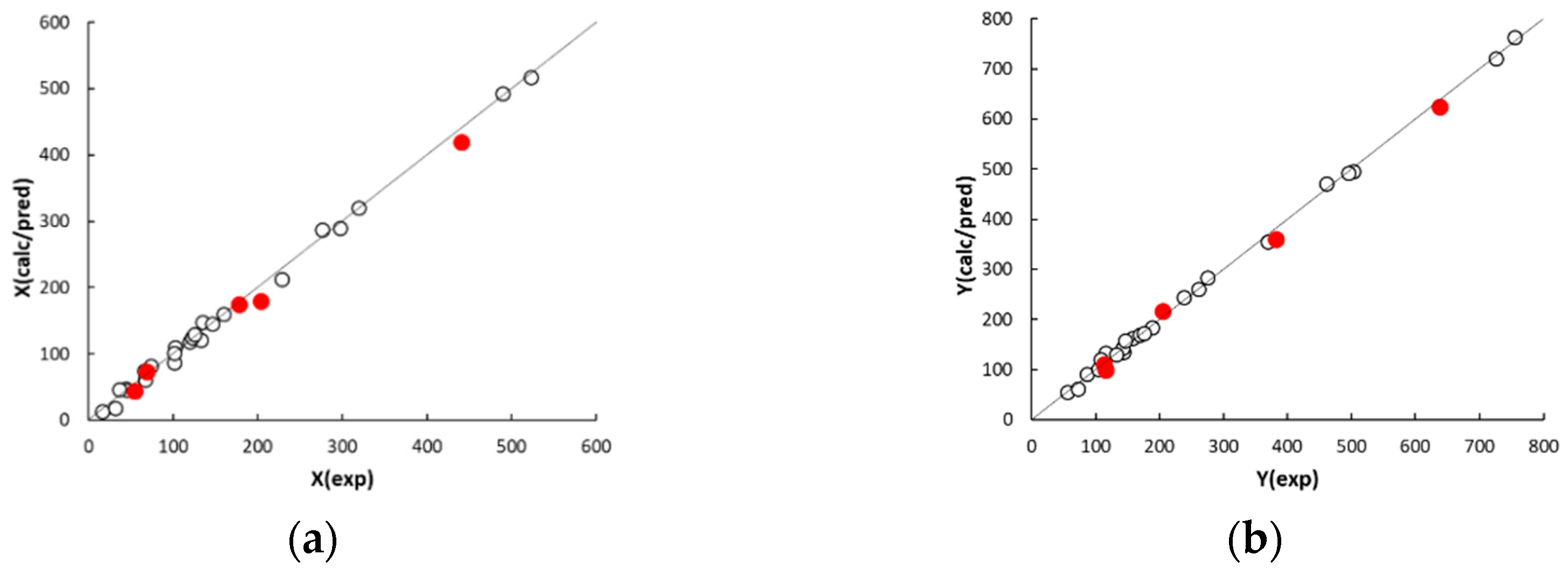
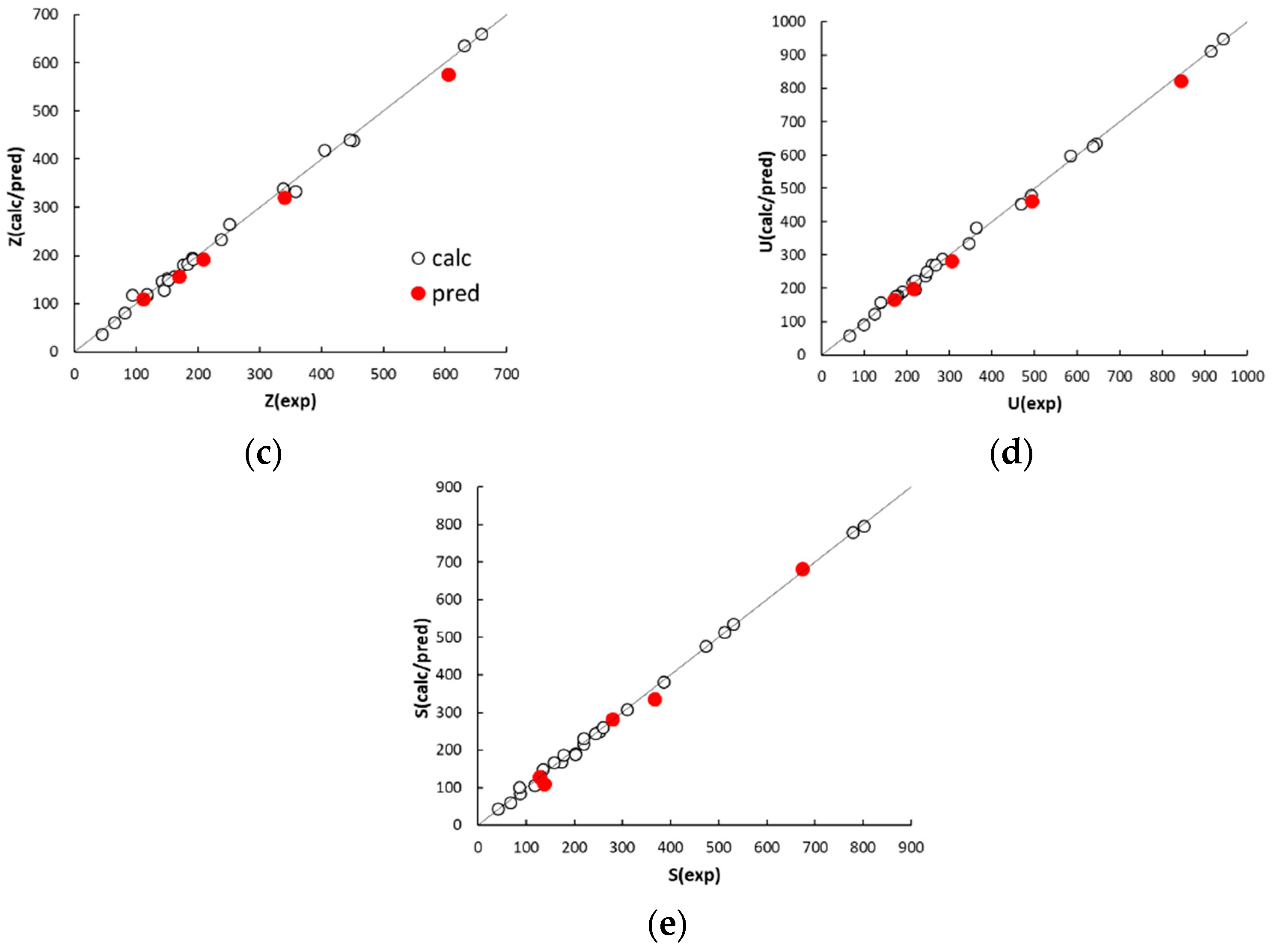
The agreement plots of the computed or predicted McReynolds constants and the experimental values (displayed in
Figure 2) reveal a distribution of both calibration and prediction data samples close to the ideal line. The observed determination coefficients of calibration and external prediction, R
2 and Q
2 (displayed in
Table 3), fall within 0.9964 to 0.9986 and 0.9867 to 0.9940, respectively, suggesting a good descriptive and predictive performance of the five QSRR models. The values of standard deviation of the error in calibration (SDEC) and prediction (SDEP) are within 9 to 12 and 15 to 21, respectively. In this regard, it must be noted that phases with McReynolds constants differing by within ±10 units generally exhibit a same separation performance [
26]. The individual residuals associated to the calibration columns (displayed in
Table A2,
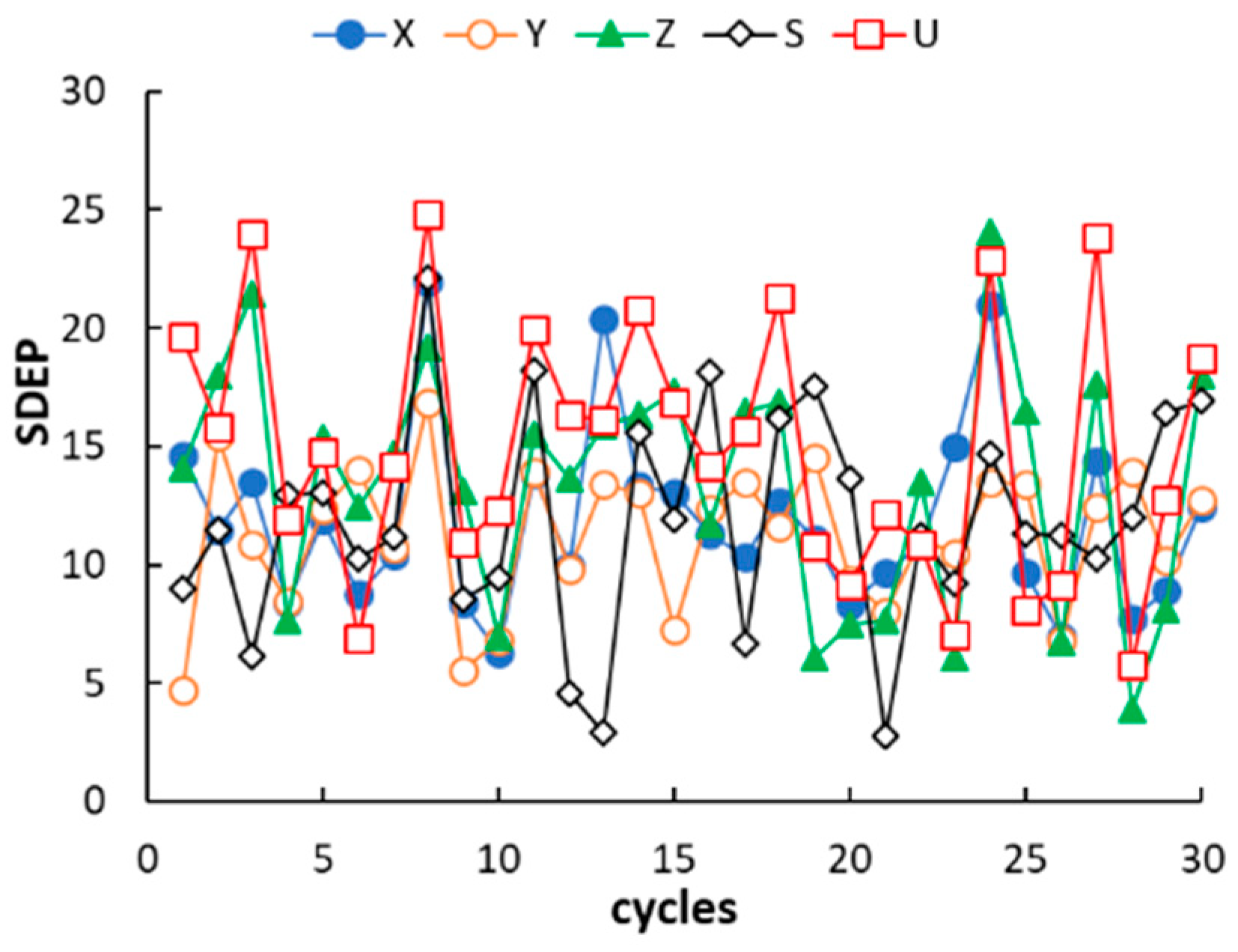
Appendix A) are randomly distributed around zero and only in a limited number of cases fall outside the ±10 range, which suggests a very good fitting. As expected, the model residuals in prediction are higher than those observed in calibration but worsening of the model performance is anyway acceptable. It must be noted that most of the residuals in prediction are positive. This trend, however, was not observed in leave-one-out cross validation, which leads to exclude the effect of systematic errors in QSRR prediction. It follows that the partitioning properties of poly(siloxane) stationary phases can be predicted with acceptable accuracy by QSRR modelling. Apart from using the preselected external set, the generalisation ability of the QSRR models was further evaluated for different partitions of the columns between the calibration and prediction sets. Following a repeated (or Monte Carlo) validation scheme, 30 random partitions were generated with an average of 20% of columns in each prediction set. The mean SDEP value and the associated standard deviation observed for each response is given in
Table 3, while the SDEP trend over the 30 repetitions is displayed in
Figure 3. The number of external columns in repetitions ranged from two to nine; it follows that structural variability may be not adequately represented by the calibration set when a relatively high number of columns belonging to a same subgroup is transferred in the prediction set. Nevertheless, the mean and individual SDEP values in repetitions confirmed the good generalisation ability of the QSRR models observed in external prediction. The QSRRs previously developed to model the McReynolds constants of nonpolymeric (molecular) stationary phases using semiempirical quantum chemical descriptors can be considered for comparison. In a first study, regarding 25 stationary phases (phthalates, adipates, sebacates, phosphates, citrates and nitrils) [
24], all the 10 McReynolds constants were simultaneously modelled by partial least-square regression and the observed Q
2 values related to various external sets consisting of six columns ranged between 0.9736 and 0.9834. In a successive investigation [
23], the McReynolds constants of 36 nonpolymeric stationary phases were modelled by MLR, seven of the investigated columns being selected for external prediction. The SDEP values associated to the first five McReynolds were found to fall between 27 and 50. Therefore, generalisation ability of the QSRR models for the poly(siloxane) stationary phases here developed is better than that reported in literature for nonpolymeric columns, despite poly(siloxanes) are more complex structures and less sophisticated molecular descriptors were used to describe their partitioning properties.
2.3. Interpretation of the QSRR Models
The molecular descriptors selected in the QSRR modelling of the five McReynolds constants are collected in
Table 2.
Table 3 displays the regression coefficient b of each significant molecular descriptor, while its relative importance in defining the QSRR response is quantified by the standardised b value (b’). The values of the selected molecular descriptors associated to the 29 poly(siloxane) stationary phases are listed in
Table A1 (
Appendix A).
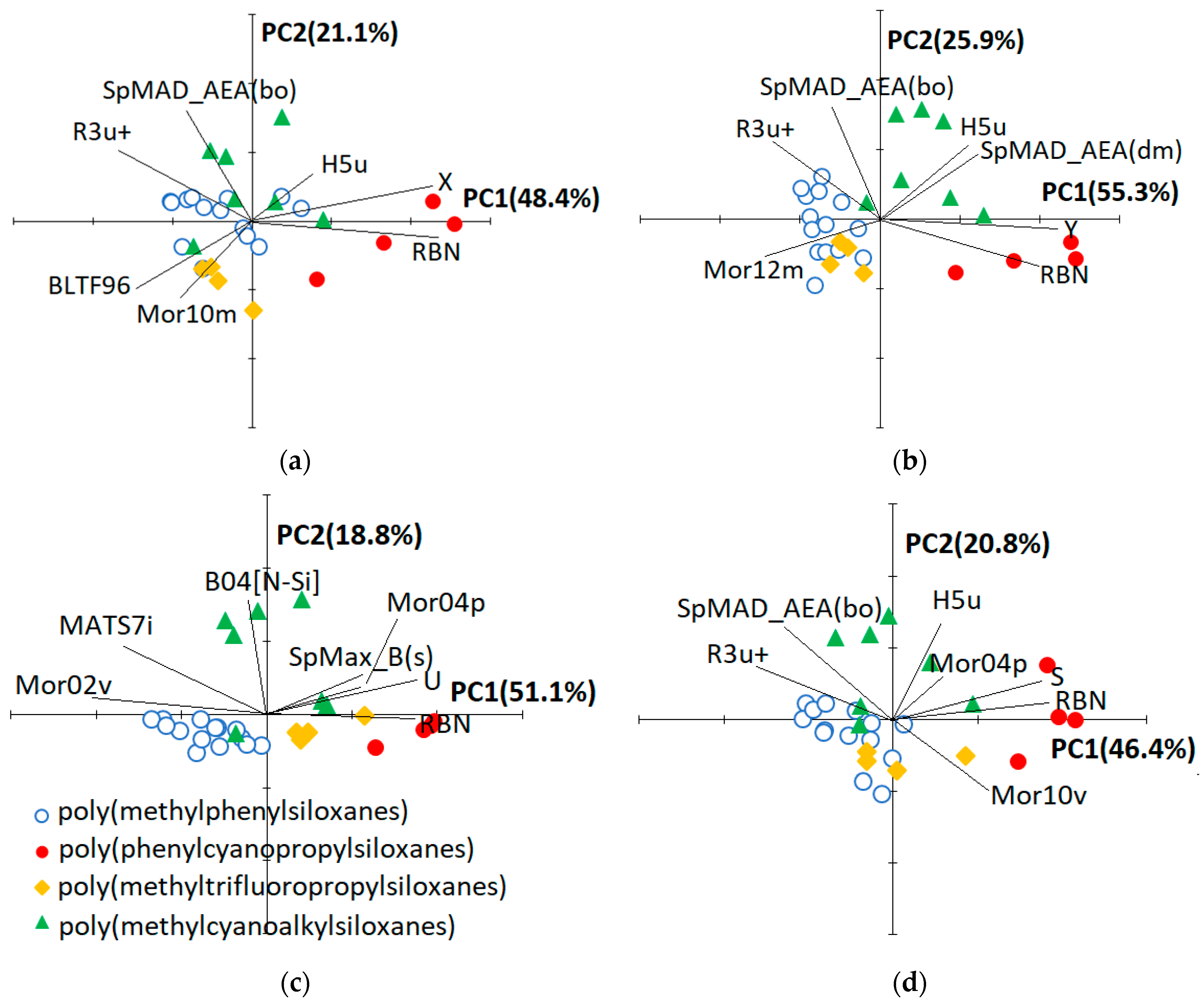
To facilitate the physical interpretation of the QSRR models, PCA was performed on the autoscaled QSRR variables (descriptors and response) and both scores (columns) and loadings (variables) were plotted in the plane of the first two principal components (
Figure 4). Based on the three plots (not shown) reporting the variance explained by each PC, the third principal component seems to be also significant. Nevertheless, to have a simple graphical representation of the PCA results, we considered only the first two PCs, that together account for a percentage of total variance ranging between 67% and 81% (
Figure 4). The biplots associated to the QSRR model for Z and U are almost identical according to the fact that the same set of molecular descriptors was selected, and the two responses are highly correlated, therefore, PCA results referring to the first case were not reported in
Figure 4. PCA also offers a graphical tool to rank the chromatographic phases, this approach being already used to classify the columns based on various kind of empirical descriptors [
9,
21,
30,
31]. It must be noted that the plots displayed in
Figure 4 do not change appreciably if the experimental response is removed from the variable set subjected to PCA.
Figure 4 reveals that the 29 poly(siloxane) stationary phases are generally grouped according to their chemical composition, but the reciprocal position of the columns in the PC1−PC2 plane is also dependent on the QSRR response and, therefore, on the set of molecular descriptors entering the model. To explain this finding it must be reminded that the first five McReynolds constants X, Y, Z, U and S are associated to predefined compounds able to establish specific interactions [
9]: benzene (weak proton acceptor and π−π interactions), butanol (proton donor and proton acceptor interactions), 2−pentanone (proton acceptor interactions), nitropropane (dipole interactions) and pyridine (strong proton acceptor interactions), respectively. Regarding the stationary phases [
21,
25], progressive introduction of phenyl groups in poly(methylphenylsiloxanes) influences the column selectivity because of both a strong dispersion interaction and a high polarisability of the phenyl groups compared to a methyl group. Poly(trifluoropropylmethylsiloxanes) are moderately polar; their selectivity is based on the pronounced acceptor character of the 3,3,3−trifluoropropyl group that can interact with free electron pairs. Cyanoalkyl-containing polysiloxanes are the most polar stationary phases. The cyano group, attached to the siloxane backbone via two or three CH
2 groups, is dipolar and strongly electron attracting. It is therefore able to display dipole−dipole, dipole-induced dipole and charge-transfer interactions. Moreover, the unshared electron pair on the nitrile nitrogen can promote hydrogen-bonding interactions with H-donor solutes. It follows that differentiation of the columns in the subspace of the significant PCs extracted from the QSRR descriptors should reflect the ability of the related McReynolds solute to interact with the various poly(siloxane) stationary phases.
The number of rotatable bonds (RBN) is the most influent molecular descriptor regardless of the QSRR response, according to the higher b’ value of this variable in all the generated models (
Table 3). The contribution of the various siloxane substituents to RBN follows the order methyl < phenyl < cyanoethyl < trifluoropropyl = cyanopropyl that closely reflects the polarity order for these groups. B04[N−Si] (presence/absence of N−Si at topological distance 4) is the second most important descriptor in QSRR models for Z and U (
Table 3); its value is 1 for cyanoethyl-containing poly(siloxanes) (NPS−100, NSKI−25, NSKT−33 and XE−60) and 0 for all the other phases (
Table A1). The second most important descriptor in QSRR models for X, Y and S is SpMAD_AEA(bo) (spectral mean absolute deviation from augmented edge adjacency matrix weighted by bond order) that partially duplicates structural information of B04[N−Si], according to the moderate correlation between these two quantities (
r = 0.79,
Table A3); the other descriptors entering the various models seem to describe minor structural effects, according to their relatively low b’ values.
About the ability of the selected molecular descriptors for the classification of the poly(siloxane) stationary phases,
Figure 4 reveals that poly(phenylcyanopropylsiloxanes)(SILAR 5CP, SILAR 7CP, SILAR 9 CP and SILAR 10 CP) are well separated from the others along PC1, regardless of the QSRR response which is almost colinear with PC1 itself. These four stationary phases are also differentiated to each other along PC1 according to the increasing ratio of cyanopropyl to phenyl substituents when the QSRR response is X (
Figure 4a) or Y (
Figure 4b). This finding can be explained by the ability of the related prototype solutes benzene and butanol to strongly interact with cyano groups by means of dipole-induced dipole and H bonding interactions, respectively. Poly(methylcyanoalkilsiloxane) stationary phases, on the other hand, are always separated from the others along a direction approximately parallel to PC2 and the most external columns are those containing cyanoethyl groups (NPS−100, NSKI−25, NSKT−33 and XE−60), while poly(methylcyanopropylsiloxanes) are closer to the origin of the PC1−PC2 graph. It follows that poly(methylcyanoethylsiloxanes) and poly(methylcyanopropylsiloxanes) can be discriminated by the selected molecular descriptors (B04[N−Si] or SpMAD_AEA(bo) in particular, as previously discussed). The reciprocal position of poly(methylphenylsiloxane), poly(methyltrifluoropropylsiloxane) and poly(methylcyanopropylsiloxane) columns along PC1, that, as previously discussed, is almost colinear with the QSRR response, is moderately influenced by the kind of solute. These three subgroups are poorly separated when the prototype solute is benzene (X,
Figure 4a) or pyridine (S,
Figure 4d), which may be attributed to the ability of these two aromatic solutes to establish both π−π and dipole-induced dipole interactions. By contrast, poly(methylcyanoalkylsiloxanes) retain butanol (Y,
Figure 4b) more than both poly(methyphenylsiloxanes) and poly(methyltrifluoropropylsiloxanes), which indicates a relevant role of H bonding interactions between the alcoholic group of this prototype solute and the cyano groups of the stationary phase. The difference between the behaviour of poly(methylcyanoalkylsiloxanes) and poly(methyltrifluoropropylsiloxanes) is almost negligible when U (
Figure 4c) or Z is the QSRR response, but the two prototype solutes 2-pentanone and nitropropane are less retained by the poly(methylphenylsiloxane) stationary phases. This pattern can be explained by the dominant role of the dipole−dipole interactions between each of the two solutes and the stationary phases. In summary, the theoretical molecular descriptors entering the QSRR models, apart from allowing the accurate prediction of the McReynolds constants, can be used to classify the stationary phases by means of PCA.