Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement
Abstract
1. Introduction
2. Results
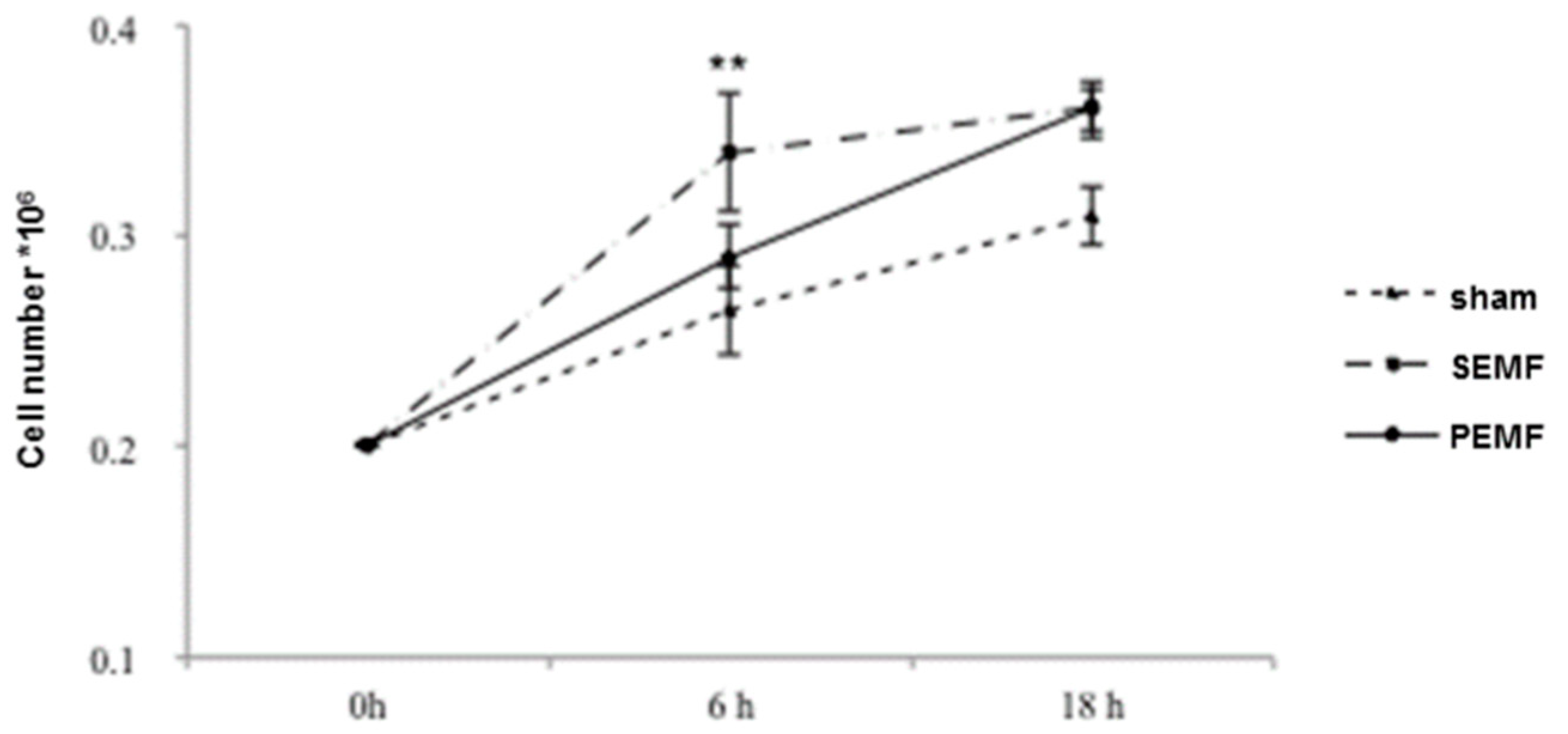
2.1. Human Gingival Fibroblast Growth Curves
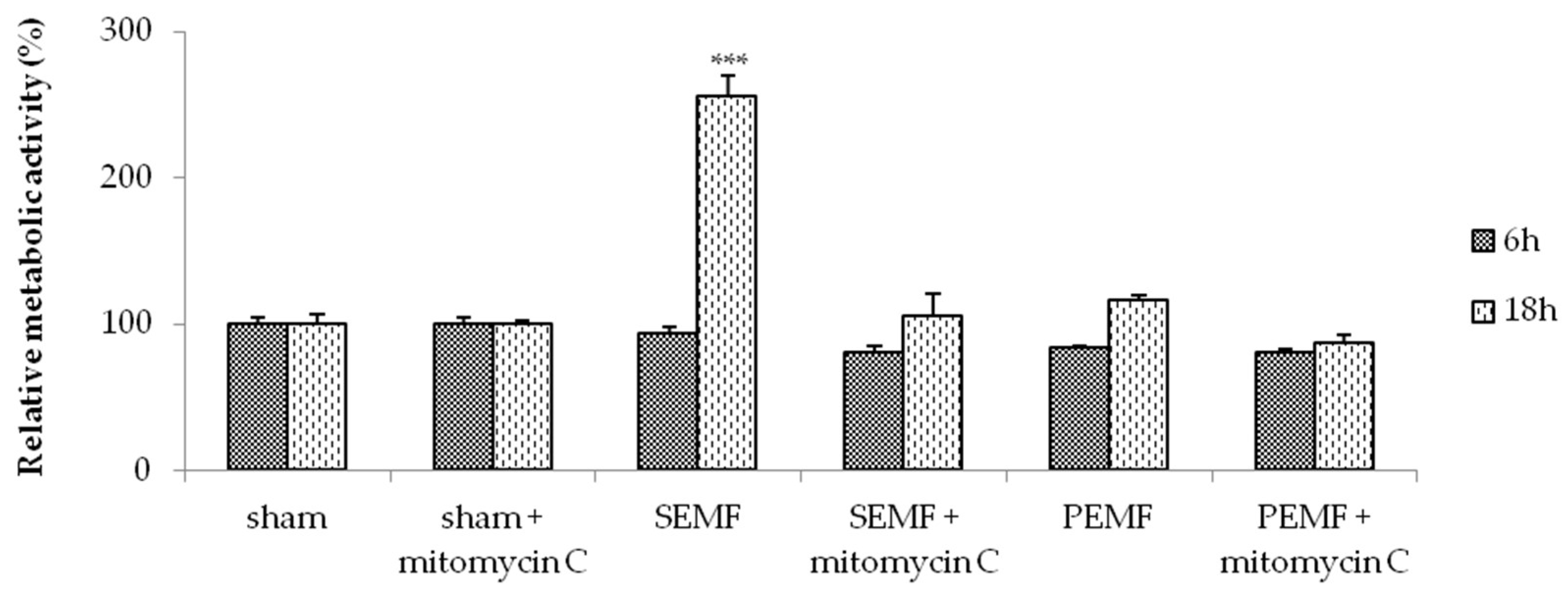
2.2. Electromagnetic Field (EMF) and Cell Metabolic Activity
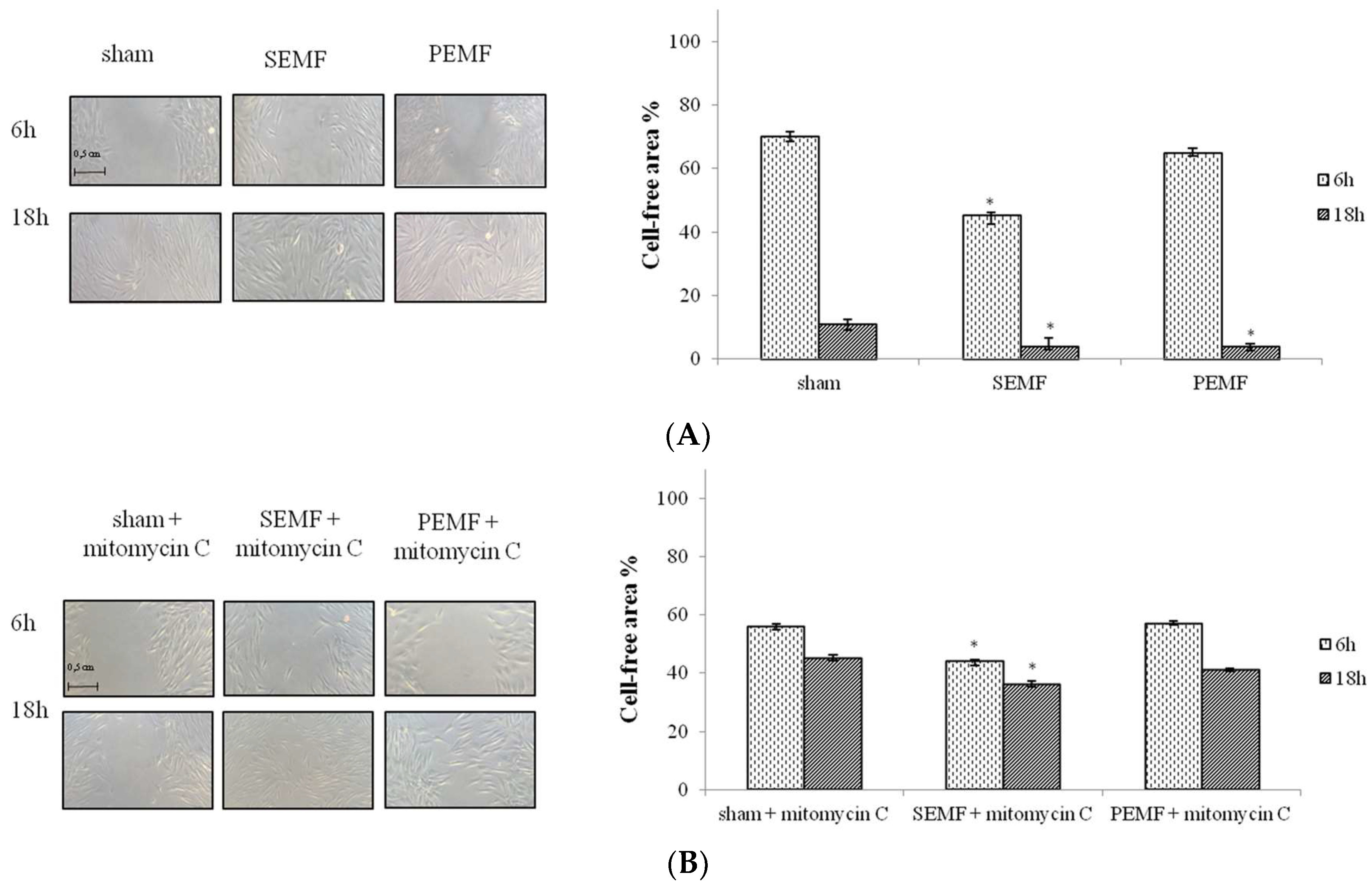
2.3. Effect of Sinusoidal Electromagnetic Field (SEMF) and Pulsed Electromagnetic Field (PEMF) on Wound Closure
2.4. Effect of EMFs on the mRNA Expression
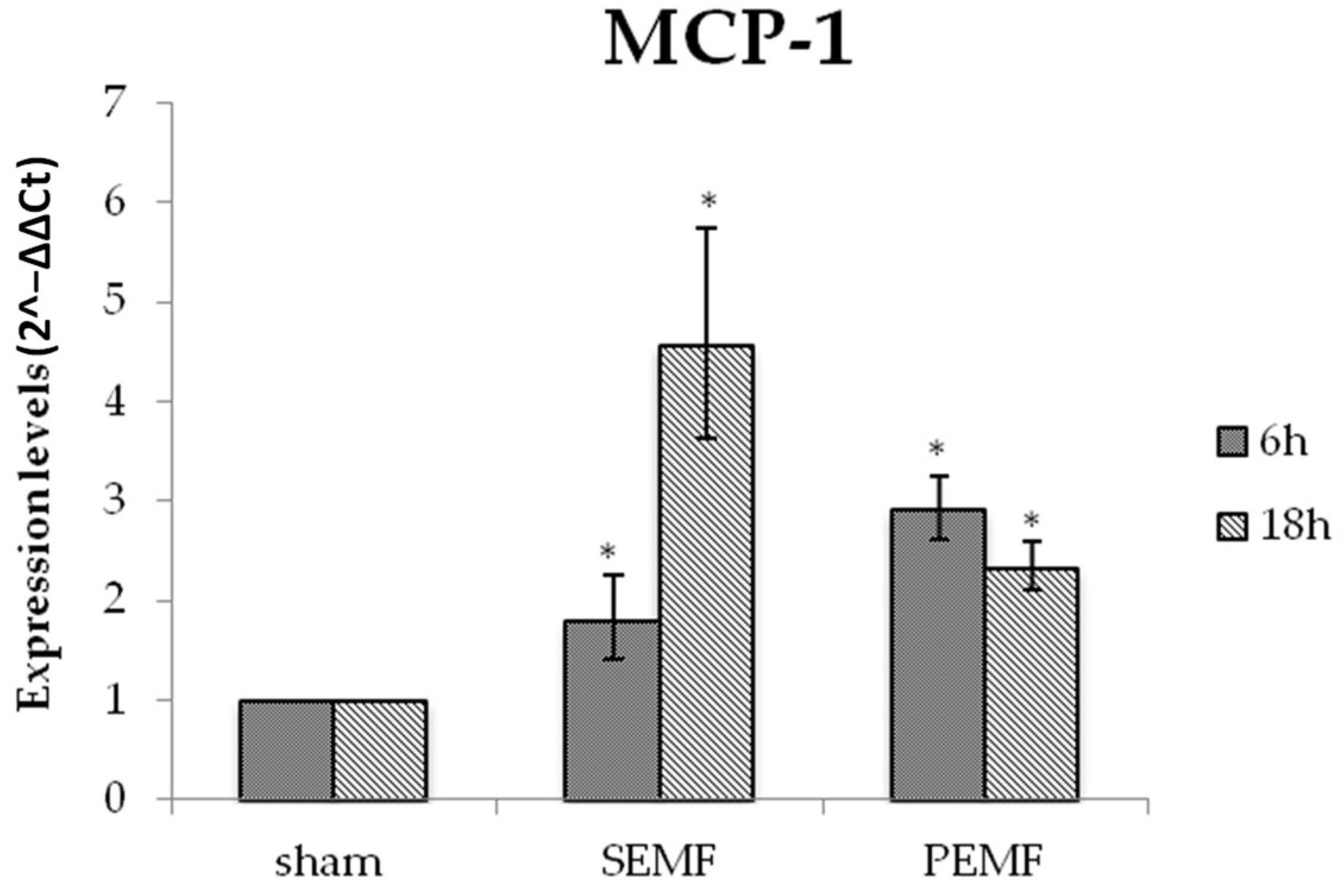
2.4.1. Effect of EMFs on Interleukin 6 (IL-6), Transforming Growth Factor Beta 1 (TGF-β), and Monocyte Chemoattractant Protein 1 (MCP-1) Gene Expression
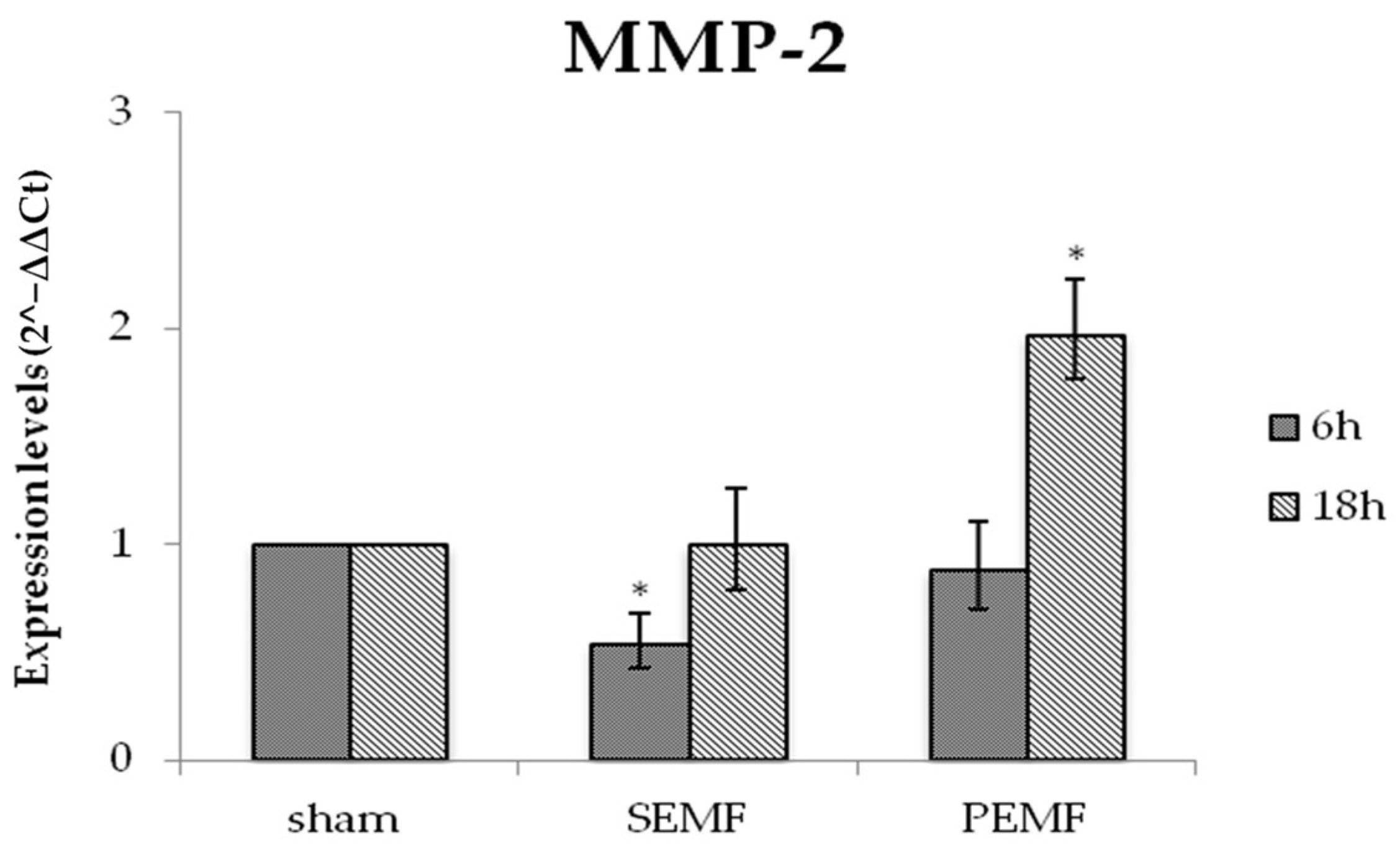
2.4.2. Effect of EMF on Metalloproteinase 2 (MMP-2) Gene Expression
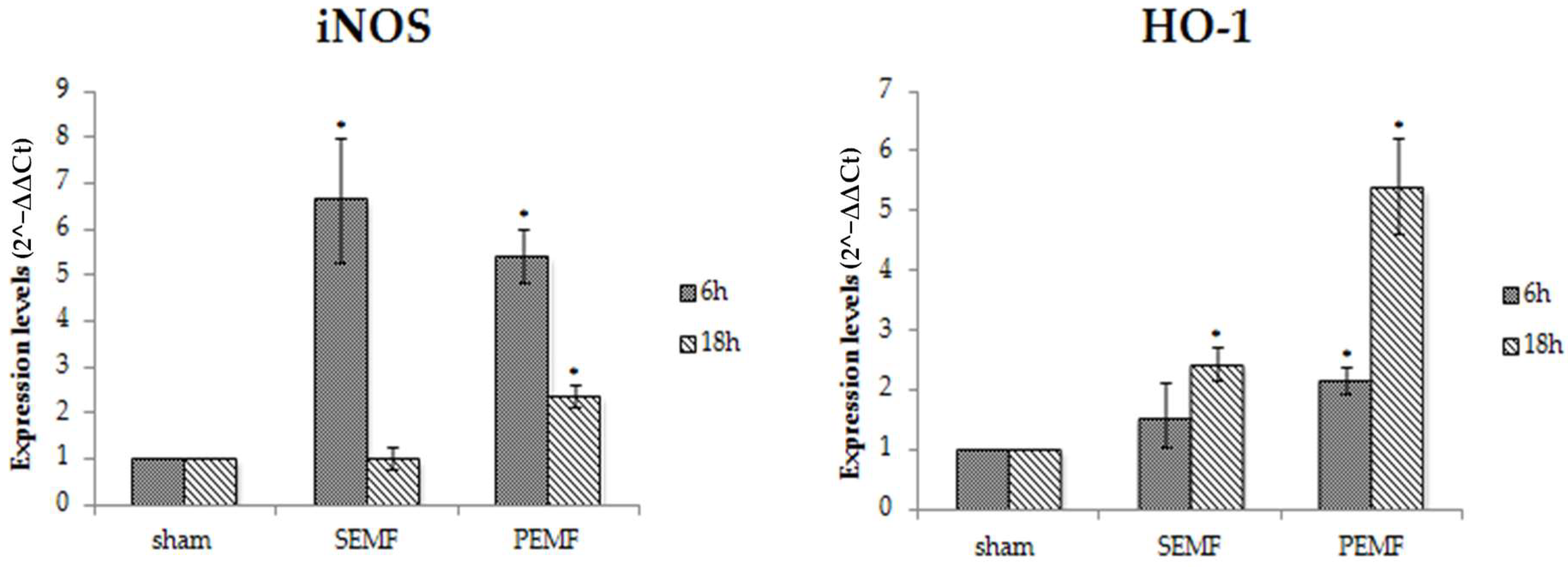
2.4.3. Effect of EMFs on Inducible Nitric Oxide Synthase (iNOS) and Heme Oxygenase 1 (HO-1) Gene Expression
3. Discussion
4. Materials and Methods
4.1. Collection of Tissue Samples
4.2. Primary Human Gingival Fibroblast Cell Culture
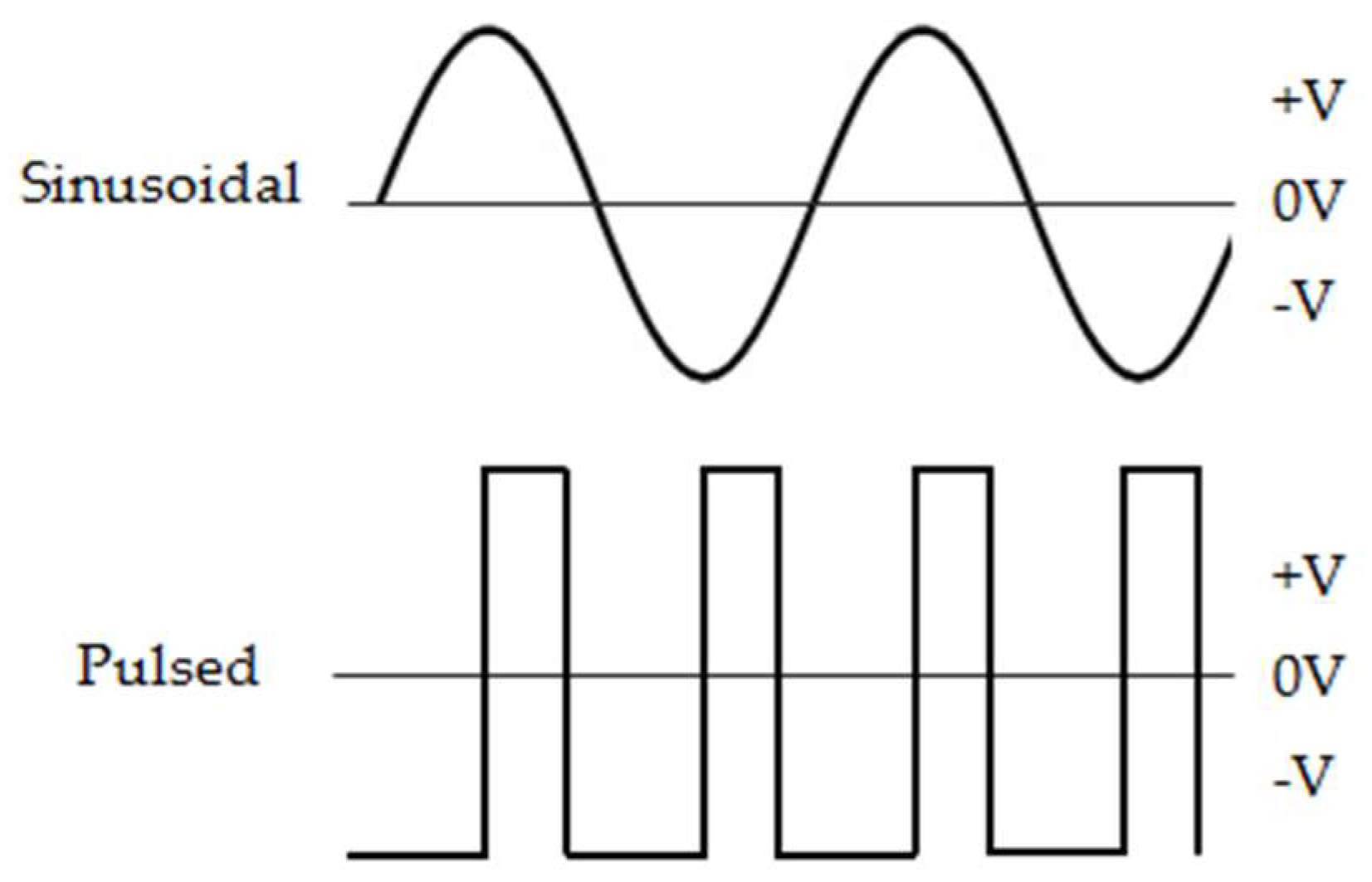
4.3. Generation and Application of Magnetic Fields
4.4. EMF Exposure Procedures
4.5. Cell Count and Viability
4.6. In Vitro Mechanical Injury Model and Wound-Healing Assay
4.7. Cell Metabolism (MTT Uptake)
4.8. RNA Extraction, Retro-Transcription and Real-Time Polymerase Chain Reaction
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EMF | Electromagnetic fields |
| LF | Low frequency |
| ELF | Extremely low-frequency |
| hSSCs | Human skeletal stem cells |
| hBMSCs | Human bone marrow stromal cells |
| hGF | Human gingival fibroblasts |
| PBS | Phosphate buffered saline |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EDTA | Ethylenediaminetetraacetic acid |
| PEMF | Pulsed electromagnetic fields |
| SEMF | Sinusoidal extremely low-frequency electromagnetic field |
| Hz | Hertz |
| mT | Millitelsa |
| mV | Millivolt |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| mRNA | Mitochondrial RNA |
| cDNA | Complementary DNA |
| IL-6 | Interleukin-6 |
| TGF-β | Transforming growth factor beta 1 |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MMP-2 | Matrix metalloproteinase-2 |
| TNF-α | Tumor necrosis factor alpha |
| ROS | Reactive oxygen species |
| iNOS | Inducible nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| HO-1 | Heme oxygenase 1 |
References
- Karthick, T.; Sengottuvelu, S.; Haja Sherief, S.; Duraisami, S. A Review: Biological Effects of Magnetic Fields on Rodents. Sch. J. App. Med. Sci. 2017, 5, 1569–1580. [Google Scholar] [CrossRef]
- Dogru, A.G.; Tunik, S.; Akpolat, V.; Dogru, M.; Saribas, E.E.; Kaya, F.A.; Nergiz, Y. The Effects of Pulsed and Sinusoidal Electromagnetic Fields on E-cadherin and Type IV Collagen in Gingiva: A Histopathological and Immunohistochemical. Study Adv. Clin. Exp. Med. 2013, 22, 245–252. [Google Scholar] [PubMed]
- Lohmann, C.H.; Schwartz, Z.; Liu, Y.; Li, Z.; Simon, B.J.; Sylvia, V.L.; Dean, D.D.; Bonewald, L.F.; Donahue, H.J.; Boyan, B.D. Pulsed electromagnetic field affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J. Orthop. Res. 2003, 21, 326–334. [Google Scholar] [CrossRef]
- Santini, M.T.; Rainaldi, G.; Ferrante, A.; Indovina, P.L.; Vecchia, P.; Donelli, G. Effects of a 50Hz sinusoidal magnetic field on cell adhesion molecule expression in two human osteosarcoma cell lines (MG-63 and Saos-2). Bioelectromagnetics 2003, 24, 327–338. [Google Scholar] [CrossRef]
- Diniz, P.; Shomura, K.; Soejima, K.; Ito, G. Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics 2002, 23, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.T.; Huang, J.; Ma, D.; Wang, P.K. Inhibition of gap junction intercellular communication by extremely low-frequency electromagnetic fields in osteoblast-like models is dependent on cell differentiation. J. Cell. Physiol. 2002, 190, 180–188. [Google Scholar] [CrossRef]
- Geng, D.Y.; Li, C.H.; Wan, X.W.; Xu, G.Z. Biochemical kinetics of cell proliferation regulated by extremely low frequency electro- magnetic field. Bio-Med. Mater. Eng. 2014, 24, 1391–1397. [Google Scholar] [CrossRef]
- De Girolamo, L.; Stanco, D.; Galliera, E.; Viganò, M.; Colombini, A.; Setti, S.; Vianello, E.; Corsi Romanelli, M.M.; Sansone, V. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells. Cell Biochem. Biophys. 2013, 66, 697–708. [Google Scholar] [CrossRef]
- Pesce, M.; Patruno, A.; Speranza, L.; Reale, M. Extremely low frequency electromagnetic field and wound healing: Implication of cytokines as biological mediators. Eur. Cytokine Netw. 2013, 24, 1–10. [Google Scholar] [CrossRef]
- Sunkari, V.G.; Aranovitch, B.; Portwood, N.; Nikoshkov, A. Effects of a low-intensity electromagnetic field on fibroblast migration and proliferation. Electromagn. Biol. Med. 2011, 30, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Shupak, N.M.; Prato, F.S.; Thomas, A.W. Therapeutic Uses of Pulsed Magnetic-Field Exposure: A Review. Brows. J. Mag. URSI Radio Sci. Bull. 2003, 2003, 307. [Google Scholar] [CrossRef]
- D’Angelo, C.; Costantini, E.; Kamal, M.A.; Reale, M. Experimental model for ELF-EMF exposure: Concern for human health. Saudi J. Biol. Sci. 2015, 22, 75–84. [Google Scholar] [CrossRef]
- Gobba, F.; Bargellini, A.; Scaringi, M.; Bravo, G.; Borella, P. Extremely low frequency-magnetic fields (ELF-EMF) occupational exposure and natural killer activity in peripheral blood lymphocytes. Sci. Total Env. 2009, 407, 1218–1223. [Google Scholar] [CrossRef]
- Duhaini, I. The effects of electromagnetic fields on human health. Phys. Med. 2016, 32, 3213. [Google Scholar] [CrossRef]
- Rageh, M.M.; El-Gebaly, R.H.; El-Bialy, N.S. Assessment of Genotoxic and Cytotoxic Hazards in Brain and Bone Marrow Cells of Newborn Rats Exposed to Extremely Low-Frequency Magnetic Field. J. Biomed. Biotech. 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Zuo, X.; Zhou, L.; Aoki, E.; Okamula, A.; Watanebe, M.; Wang, H.; Wu, Q.; Lu, H.; Tuncel, H.; et al. Effects of extremely low-frequency electromagnetic fields (ELF-EMF) exposure on B6C3F1 mice. Environ. Health Prev. Med. 2015, 20, 287–293. [Google Scholar] [CrossRef]
- Ross, C.L.; Almeida-Porada, M.S.G.; Porada, C.D.; Brink, P.; Christ, G.J.; Harrison, B.S. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015, 15, 96–108. [Google Scholar] [CrossRef]
- Saliev, T.; Mustapova, Z.; Kulsharova, G.; Bulanin, D.; Mikhalovsky, S. Therapeutic potential of electromagnetic fields for tissue engineering and wound healing. Cell Prolif. 2014, 47, 485–493. [Google Scholar] [CrossRef] [PubMed]
- George, I.; Geddis, M.S.; Lill, Z.; Lin, H.; Gomez, T.; Blank, M.; Oz, M.C.; Goodman, R. Myocardial function improved by electromagnetic fields induction of stress protein hsp70. J. Cell. Physiol. 2008, 216, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.L.; Farrell, J.M.; Litovitz, T.A. A simple experiment to study electromagnetic field effects: Protection induced by short-term exposures to 60Hz magnetic fields. Bioelectromagnetics 1998, 19, 498–500. [Google Scholar] [CrossRef]
- Ciombor, D.M.; Lester, G.; Aaron, R.K.; Neame, P.; Caterson, B. Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J. Orthop. Res. 2002, 20, 40–50. [Google Scholar] [CrossRef]
- Cichoń, N.; Rzeźnicka, P.; Bijak, M.; Miller, E.; Miller, S.; Saluk, J. Extremely low frequency electromagnetic field reduces oxidative stress during the rehabilitation of post-acute stroke patients. Adv. Clin. Exp. Med. 2018, 27, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Q.; He, H.C.; He, C.Q.; Chen, J.; Yang, L. Clinical update of pulsed electromagnetic fields on osteoporosis. Chin. Med. J. 2008, 121, 2095–2099. [Google Scholar] [CrossRef]
- Bassett, C.A.L. Bioeletromagnetics in the service of medicine. Adv. Chem. 1995, 250, 261–276. [Google Scholar] [CrossRef]
- Ito, H.; Bassett, C.A. Effect of weak, pulsing electromagnetic fields on neural regeneration in the rat. Clin. Orthop. Relat. Res. 1983, 181, 283–290. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Bloise, N.; Mantelli, M.; Gastalki, G.; Gassina, L.; De Aneglis, M.G.C.; Ferrar, D.; Imbriani, M.; Visai, L. A comparative analysis of the in vitro effects of pulsed electro- magnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineages. BioResearch 2013, 2013, 283–294. [Google Scholar] [CrossRef]
- Stiller, M.J.; Pak, G.H.; Shupack, J.L.; Thaler, S.; Kenny, C.; Jondreau, L. A portable pulsed electromagnetic-field (PEMF) device to enhance healing of recalcitrant venous ulcers–a double-blind, placebo-controlled clinical-trial. Br. J. Derm. 1992, 127, 147–154. [Google Scholar] [CrossRef]
- Wei, Y.; Xiaolin, H.; Tao, S. Effects of extremely low-frequency-pulsed electromagnetic field on different-derived osteoblast-like cells. Electromagn. Biol. Med. 2008, 27, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, J.; Yang, Y.; Tang, X.; Zhao, D.; Zhao, W.; Wu, H. Effect of 1mT sinusoidal electromagnetic fields on proliferation and osteogenic differentiation of rat bone marrow mesenchymal stromal cells. Bioelectromagnetics 2013, 34, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dong, L.; Chen, Y.; Qi, H.; Xiao, D. Effects of sinusoidal magnetic field observed on cell proliferation, ion concentration, and osmolarity in two human cancer cell lines. Electromagn. Biol. Med. 2006, 25, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Dovi, J.V.; He, L.K.; Di Pietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 2003, 73, 448–455. [Google Scholar] [CrossRef]
- Boink, M.A.; van den Broek, L.J.; Roffel, S.; Nazmi, K.; Bolscher, J.G.; Gefen, A.; Veerman, E.C.; Gibbs, S. Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repai. Regen. 2016, 24, 100–109. [Google Scholar] [CrossRef]
- Smith, P.C.; Cáceres, M.; Martínez, C.; Oyarzún, A.; Martínez, J. Gingival Wound Healing: An Essential Response Disturbed by Aging? J. Dent. Res. 2015, 94, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Gurtner, G.C.; Longaker, M.T. Wound healing: A paradigm for regeneration. Mayo Clin. Proc. 2013, 88, 1022–1031. [Google Scholar] [CrossRef]
- Larjava, H.; Wiebe, C.; Gallant-Behm, C.; Hart, D.A.; Heino, J.; Hakkinen, L. Exploring scarless healing of oral soft tissues. J. Can. Dent. Assoc 2011, 77, b18. [Google Scholar] [PubMed]
- Discepoli, N.; Vignoletti, F.; Laino, L.; de Sanctis, M.; Muñoz, F.; Sanz, M. Early healing of the alveolar process after tooth extraction: An experimental study in the beagle dog. J. Clin. Periodontol. 2013, 40, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Eriksson, B.; Nylén, O.; Ridell, A. Delayed healing of fractures of the mandibular body. Int. J. Oral. Maxillofac. Surg. 1987, 16, 15–24. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Glim, J.E.; van Egmond, M.; Niessen, F.B.; Everts, V.; Beelen, R.H. Detrimental dermal wound healing: What can we learn from the oral mucosa? Wound Repair. Regen. 2013, 21, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Das, A.; Sen, C.K. Disorder of Localized Inflammation in Wound Healing: A Systems Perspective. In Complex Systems and Computational Biology Approaches to Acute Inflammation; Vodovotz, Y., An, G., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Karamanos, E.; Osgood, G.; Siddiqui, A.; Rubinfeld, I. Wound healing in plastic surgery: Does age matter? An American college of surgeons national surgical quality improvement program study. Plast. Reconstr. Surg. 2015, 135, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Amerio, P.; Pesce, M.; Vianale, G.; Di Luzio, S.; Tulli, A.; Franceschelli, S.; Grilli, A.; Muraro, R.; Reale, M. Extremely low frequency electromagnetic fields modulate expression of inducible nitric oxide synthase, endothelial nitric oxide synthase and cyclooxygenase-2 in the human keratinocyte cell line HaCat: Potential therapeutic effects in wound healing. Br. J. Derm. 2010, 162, 258–266. [Google Scholar] [CrossRef]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef]
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, J.O. Wound Healing Problems in the Mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Buskermolen, J.K.; Roffel, S.; Gibbs, S. Stimulation of oral fibroblast chemokine receptors identifies CCR3 and CCR4 as potential wound healing targets. J. Cell Physiol. 2017, 232, 2996–3005. [Google Scholar] [CrossRef]
- Mah, W.; Jiang, G.; Olver, D.; Cheung, G.; Kim, B.; Larjava, H.; Häkkinen, L. Human gingival fibroblasts display a non-fibrotic phenotype distinct from skin fibroblasts in three-dimensional cultures. PLoS ONE 2014, 9, e90715. [Google Scholar] [CrossRef][Green Version]
- Susin, C.; Fiorini, T.; Lee, J.; de Stefano, J.A.; Dickinson, D.P.; Wikesjo, U.M. Wound healing following surgical and regenerative periodontal therapy. Periodontology 2000 2015, 68, 83–98. [Google Scholar] [CrossRef]
- Cáceres, M.; Oyarzun, A.; Smith, P.C. Defective Wound-healing in Aging Gingival Tissue. J. Dent. Res. 2014, 93, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Vodovnic, L.; Karba, R. Treatment of chronic wounds by means of electric and electromagnetic fields. Med. Biol. Eng. Comput. 1992, 30, 257–266. [Google Scholar] [CrossRef]
- Aaron, R.K.; Ciombor, D.M. Therapeutic effects of electromagnetic fields in stimulation of connective tissue repair. J. Cell. Biochem. 1993, 52, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Patruno, A.; Pesce, M.; Grilli, A.; Speranza, L.; Franceschelli, S.; De Lutiis, M.A.; Vianale, G.; Costantini, E.; Amerio, P.; Muraro, R.; et al. mTOR Activation by PI3K/Akt and ERK Signaling in Short ELF-EMF Exposed Human Keratinocytes. PLoS ONE 2015, 10, e0139644. [Google Scholar] [CrossRef] [PubMed]
- Irwin, C.R.; Myrillas, T.T.; Traynor, P.; Leadbetter, N.; Cawston, T.E. The role of soluble interleukin (IL)-6 receptor in mediating the effects of IL-6 on matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 expression by gingival fibroblasts. J. Periodontol. 2002, 73, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Miyzono, K.; Heldin, C.H. Structure, function and possible clinical application of TGF-beta 1. Dermatol. 1992, 19, 644–647. [Google Scholar] [CrossRef]
- Rees, P.A.; Greaves, N.S.; Baguneid, M.; Bayat, A. Chemokines in wound healing and as potential therapeutic targets for reducing cutaneous scarring. Adv. Wound Care 2015, 4, 687–703. [Google Scholar] [CrossRef]
- Vianale, G.; Reale, M.; Amerio, P.; Stefanachi, M.; Di Luzio, S.; Muraro, R. Extremely low frequency electromagnetic field enhances human keratinocyte cell growth and decreases proinflammatory chemokine production. Br. J. Derm. 2008, 158, 1189–1196. [Google Scholar] [CrossRef]
- Sul, A.R.; Park, S.N. Effects of sinusoidal electromagnetic field on structure and function of different kinds of cell lines. Yonsei Med. J. 2006, 47, 852–861. [Google Scholar] [CrossRef]
- Reale, M.; D’Angelo, C.; Costantini, E.; Tata, A.M.; Regen, F.; Hellmann-Regen, J. Effect of Environmental Extremely Low-Frequency Electromagnetic Fields Exposure on Inflammatory Mediators and Serotonin Metabolism in a Human Neuroblastoma Cell Line. CNS Neurol. Disord. Drug Targets 2016, 15, 1203–1215. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Lach, R.; Mis, J.; Skrzypek, K.; Gozdecka, M.; Sroczynska, P.; Dubiel, M.; Rutkowski, A.; Kozakowska, M.; Zagorska, A.; et al. Heme Oxygenase-1 Accelerates Cutaneous Wound Healing in Mice. PLoS ONE 2009, 4, e5803. [Google Scholar] [CrossRef]
- Reale, M.; Kamal, M.A.; Patruno, A.; Costantini, E.; D’Angelo, C.; Pesce, M.; Greig, N.H. Neuronal Cellular Responses to Extremely Low Frequency Electromagnetic Field Exposure: Implications Regarding Oxidative Stress and Neurodegeneration. PLoS ONE 2014, 9, e104973. [Google Scholar] [CrossRef]
- Patil, K.; Bellner, L.; Cullaro, G.; Gotlinger, K.H.; Dunn, M.W.; Schwartzman, M.L. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3379–3386. [Google Scholar] [CrossRef]
- Kido, D.; Mizutani, K.; Takeda, K.; Mikami, R.; Matsuura, T.; Iwasaki, K.; Izumi, Y. Impact of diabetes on gingival wound healing via oxidative stress. PLoS ONE 2017, 12, e0189601. [Google Scholar] [CrossRef]
- Moonen, C.G.J.; Alders, S.T.; Bontk, H.J.; Schoenmaker, T.; Nicu, E.A.; Loos, B.G.; de Vries, T.J. Survival, Retention, and Selective Proliferation of Lymphocytes Is Mediated by Gingival Fibroblasts. Front. Immunol. 2018, 9, 1725. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| 18s | CTTGCCATCACTGCCATTAAG | TCCATCCTTTACATCCTTCTGTC |
| TGF-b | AACAATTCCTGGCGATACCTC | GATGTGAACCCGTTGATGTCC |
| IL-6 | GTACATCCTCGACGGCATC | ACCTCAAACTCCAAAAGACCAG |
| MCP-1 | GGTCCCTGTCATGCTTCTGG | CCTGCTGCTGGTGATCCTCT |
| iNOS | GGTATCCTGGAGCGAGTGGT | CTCTCAGGCTCTTCTGTGGC |
| HO-1 | TCTTCACCTTCCCCAACATTG | CTCTGGTCCTTGGTGTCATG |
| MMP-2 | CAGTGACGGAAAGATGTGGT | TGGTGTAGGTGTAAATGGGTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, E.; Sinjari, B.; D’Angelo, C.; Murmura, G.; Reale, M.; Caputi, S. Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement. Int. J. Mol. Sci. 2019, 20, 2108. https://doi.org/10.3390/ijms20092108
Costantini E, Sinjari B, D’Angelo C, Murmura G, Reale M, Caputi S. Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement. International Journal of Molecular Sciences. 2019; 20(9):2108. https://doi.org/10.3390/ijms20092108
Chicago/Turabian StyleCostantini, Erica, Bruna Sinjari, Chiara D’Angelo, Giovanna Murmura, Marcella Reale, and Sergio Caputi. 2019. "Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement" International Journal of Molecular Sciences 20, no. 9: 2108. https://doi.org/10.3390/ijms20092108
APA StyleCostantini, E., Sinjari, B., D’Angelo, C., Murmura, G., Reale, M., & Caputi, S. (2019). Human Gingival Fibroblasts Exposed to Extremely Low-Frequency Electromagnetic Fields: In Vitro Model of Wound-Healing Improvement. International Journal of Molecular Sciences, 20(9), 2108. https://doi.org/10.3390/ijms20092108








