Melatonin Levels in Preterm and Term Infants and Their Mothers
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of Patients
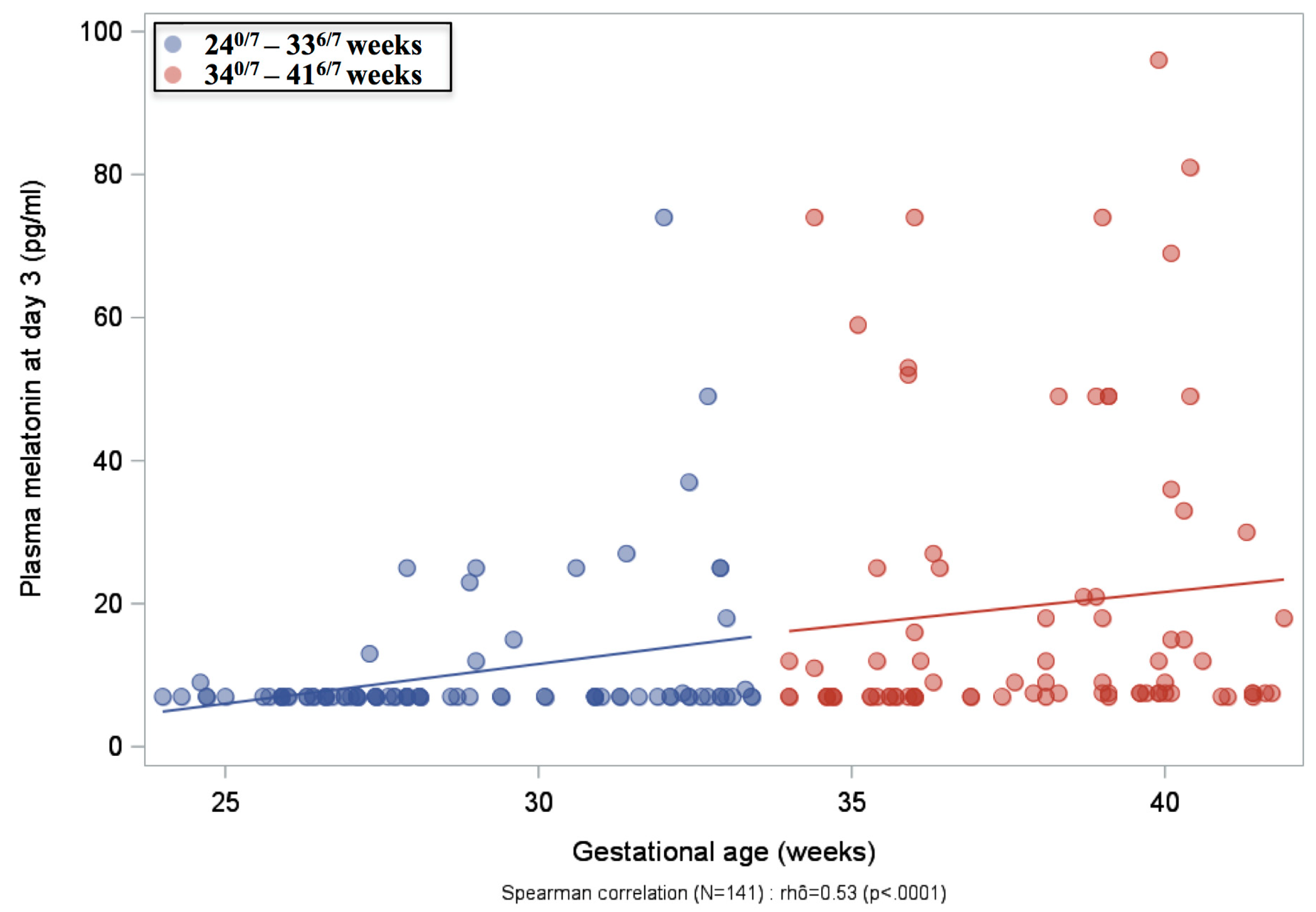
2.2. Plasma Melatonin Concentrations
2.3. Urine aMT6s Excretion
2.4. Milk Melatonin Concentrations
2.5. Multivariate Analyses of Factors Associated with Melatonin Deficiency
3. Discussion
4. Methods
4.1. Study Design
4.2. Blood Samples for Melatonin Measurement
4.3. Urine Samples for aMT6s Measurement
4.4. Collection of Breast Milk Samples
4.5. Melatonin and aMT6s Assays
4.6. Main Outcomes and Sample Size Determination
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Claustrat, B.; Brun, J.; Chazot, G. The Basic Physiology and Pathophysiology of Melatonin. Sleep Med. Rev. 2005, 99, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kennaway, D.J. Melatonin and Development: Physiology and Pharmacology. Semin. Perinatol. 2000, 24, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and Pharmacology of Melatonin in Relation to Biological Rhythms. Pharmacol. Rep. 2009, 61, 383–410. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress. A Review. J. Biomed. Sci. 2000, 77, 444–458. [Google Scholar] [CrossRef]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I.; et al. Melatonin Reduces Oxidative Stress in Surgical Neonates. J. Pediatr. Surg. 2004, 39, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Reiter, R.J.; Sabatino, G.; Buonocore, G.; Romeo, C.; Gitto, P.; Buggé, C.; Trimarchi, G.; Barberi, I. Correlation among Cytokines, Bronchopulmonary Dysplasia and Modality of Ventilation in Preterm Newborns: Improvement with Melatonin Treatment. J. Pineal Res. 2005, 39, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Tan, D.X.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of Melatonin Treatment in Septic Newborns. Pediatr. Res. 2001, 50, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased Levels of Malondialdehyde and Nitrite/Nitrate in the Blood of Asphyxiated Newborns: Reduction by Melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Biran, V.; Baud, O. Melatonin and the Newborn Brain. Early Hum. Dev. 2016, 102, 1–3. [Google Scholar] [CrossRef]
- Biran, V.; Phan Duy, A.; Decobert, F.; Bednarek, N.; Alberti, C.; Baud, O. Is Melatonin Ready to Be Used in Preterm Infants as a Neuroprotectant? Dev. Med. Child Neurol. 2014, 56, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Commentz, J.C.; Henke, A.; Dammann, O.; Hellwege, H.H.; Willig, R.P. Decreasing Melatonin and 6-Hydroxymelatonin Sulfate Excretion with Advancing Gestational Age in Preterm and Term Newborn Male Infants. Eur. J. Endocrinol. 1996, 135, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Hoyos, A.; Rodriguez-Cabezas, T.; Molina-Carballo, A.; Martinez-Sempere, J.J.; Ruiz-Cosano, C.; Acuña-Castroviejo, D. Melatonin Concentration in the Umbilical Artery and Vein in Human Preterm and Term Neonates and Neonates with Acute Fetal Distress. J. Pineal Res. 1992, 13, 184–191. [Google Scholar] [CrossRef]
- Muñoz-Hoyos, A.; Bonillo-Perales, A.; Avila-Villegas, R.; González-Ripoll, M.; Uberos, J.; Florido-Navío, J.; Molina-Carballo, A. Melatonin Levels during the First Week of Life and Their Relation with the Antioxidant Response in the Perinatal Period. Neonatology 2007, 92, 209–216. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Takayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of Serum Melatonin Level and Its Relationship to Feto-Placental Unit during Pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of Melatonin Production in Infants and the Impact of Prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and Pregnancy in the Human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Soliman, A.; Lacasse, A.-A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental Melatonin System Is Present throughout Pregnancy and Regulates Villous Trophoblast Differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef]
- Bojkowski, C.J.; Arendt, J.; Shih, M.C.; Markey, S.P. Melatonin Secretion in Humans Assessed by Measuring Its Metabolite, 6-Sulfatoxymelatonin. Clin. Chem. 1987, 33, 1343–1348. [Google Scholar]
- Kennaway, D.J.; Rowe, S.A. Melatonin Binding Sites and Their Role in Seasonal Reproduction. J. Reprod. Fertil. Suppl. 1995, 49, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and Developmental Toxicity Evaluation of Melatonin Administered Orally to Pregnant Sprague-Dawley Rats. Toxicol. Sci. 1999, 50, 271–279. [Google Scholar] [CrossRef]
- Jan, J.E.; Wasdell, M.B.; Freeman, R.D.; Bax, M. Evidence Supporting the Use of Melatonin in Short Gestation Infants. J. Pineal Res. 2007, 42, 22–27. [Google Scholar] [CrossRef]
- Siegrist, C.; Benedetti, C.; Orlando, A.; Beltrán, J.M.; Tuchscherr, L.; Noseda, C.M.; Brusco, L.I.; Cardinali, D.P. Lack of Changes in Serum Prolactin, FSH, TSH, and Estradiol after Melatonin Treatment in Doses That Improve Sleep and Reduce Benzodiazepine Consumption in Sleep-Disturbed, Middle-Aged, and Elderly Patients. J. Pineal Res. 2001, 30, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Palm, L.; Blennow, G.; Wetterberg, L. Long-Term Melatonin Treatment in Blind Children and Young Adults with Circadian Sleep-Wake Disturbances. Dev. Med. Child Neurol. 1997, 39, 319–325. [Google Scholar] [CrossRef]
- Merchant, N.M.; Azzopardi, D.V.; Hawwa, A.F.; McElnay, J.C.; Middleton, B.; Arendt, J.; Arichi, T.; Gressens, P.; Edwards, A.D. Pharmacokinetics of Melatonin in Preterm Infants. Br. J. Clin. Pharmacol. 2013, 76, 725–733. [Google Scholar] [CrossRef]
- Carloni, S.; Proietti, F.; Rocchi, M.; Longini, M.; Marseglia, L.; D’Angelo, G.; Balduini, W.; Gitto, E.; Buonocore, G. Melatonin Pharmacokinetics Following Oral Administration in Preterm Neonates. Molecules 2017, 22, 2115. [Google Scholar] [CrossRef]
- Katzer, D.; Pauli, L.; Mueller, A.; Reutter, H.; Reinsberg, J.; Fimmers, R.; Bartmann, P.; Bagci, S. Melatonin Concentrations and Antioxidative Capacity of Human Breast Milk According to Gestational Age and the Time of Day. J. Hum. Lact. 2016, 32, NP105–NP110. [Google Scholar] [CrossRef]
- van Faassen, M.; Bischoff, R.; Kema, I.P. Relationship between Plasma and Salivary Melatonin and Cortisol Investigated by LC-MS/MS. Clin. Chem. Lab. Med. 2017, 55, 1340–1348. [Google Scholar] [CrossRef]
- Claustrat, B.; Chazot, G.; Brun, J.; Jordan, D.; Sassolas, G. A Chronobiological Study of Melatonin and Cortisol Secretion in Depressed Subjects: Plasma Melatonin, a Biochemical Marker in Major Depression. Biol. Psychiatry 1984, 19, 1215–1228. [Google Scholar]
- Harthé, C.; Claustrat, B.; Brun, J.; Chazot, G. Direct Radioimmunoassay of 6-Sulfatoxymelatonin in Plasma with Use of an Iodinated Tracer. Clin. Chem. 1991, 37, 536–539. [Google Scholar]

| Sample | 240/7–336/7 Weeks | 340/7–416/7 Weeks | p-Value * |
|---|---|---|---|
| Mother at delivery Median (IQR) Min; Max | n = 77 7 (7–20) 7; 213 | n = 75 11 (7–50) 7; 158 | 0.02 |
| Newborn at birth Median (IQR) Min; Max | n = 81 7 (7–7) 7; 83 | n = 86 7 (7–24) 7; 184 | 0.002 |
| Newborn on Day 3 Median (IQR) Min; Max | n = 90 7 (7–7) 7; 74 | n = 83 8 (7–21) 7; 96 | <0.0001 |
| Newborn on Day 10 Median (IQR) Min; Max | n = 85 7 (7–7) 7; 74 | - | - |
| Newborn on Day 25 Median (IQR) Min; Max | n = 73 7 (7–7) 7; 25 | - | - |
| Newborn on Day 55 Median (IQR) Min; Max | n = 47 7 (7–7) 7; 38 | - | - |
| Sample | 240/7–336/7 Weeks | 340/7–416/7 Weeks | p Value * |
|---|---|---|---|
| Day 1 | |||
| 24 h Median (IQR) Min; Max | n = 97 230 (137–425) 48; 1917 | n = 62 533 (241–830) 48; 4876 | <0.0001 |
| Daytime (08:00–19:59) Median (IQR) Min; Max | n = 84 192 (103–361) 22; 2358 | n = 46 538 (195–828) 48; 4458 | <0.0001 |
| Nighttime (20:00–07:59) Median (IQR) Min; Max | n = 87 222 (124–456) 48; 1836 | n = 53 413 (210–745) 48; 5294 | 0.002 |
| Day 3 | |||
| 24 h Median (IQR) Min; Max | n = 104 197 (114–336) 16; 4302 | n = 63 359 (211–647) 48; 6527 | 0.0001 |
| Daytime (08:00–19:59) Median (IQR) Min; Max | n = 93 187 (119;401) 16; 5862 | n = 49 329 (172;612) 48; 6641 | 0.003 |
| Nighttime (20:00–07:59) Median (IQR) Min; Max | n = 94 178 (112–298) 48; 4252 | n = 50 350 (204–495) 48; 9469 | 0.0003 |
| Day 10 | |||
| 24 h urine excretion Median (IQR) Min; Max | n = 92 168 (96–253) 47; 1167 | - | - |
| Daytime (08:00–19:59) Median (IQR) Min; Max | n = 91 168 (95–259) 48; 1597 | - | - |
| Nighttime (20:00–07:59) Median (IQR) Min; Max | n = 81 145 (94–247) 24; 944 | - | - |
| Day 25 | |||
| 24 h Median (IQR) Min; Max | n = 82 160 (119–217) 48; 2749 | - | - |
| Daytime (08:00–19:59) Median (IQR) Min; Max | n = 75 170 (118–243) 27; 2691 | - | - |
| Nighttime (20:00–07:59) Median (IQR) Min; Max | n = 76 147 (93–223) 48; 2807 | - | - |
| Day 55 | |||
| 24 h Median (IQR) Min; Max | n = 49 168 (119–257) 53; 1481 | - | - |
| Daytime (08:00–19:59) Median (IQR) Min; Max | n = 49 200 (118–249) 53; 1713 | - | - |
| Nighttime (20:00–07:59) Median (IQR) Min; Max | n = 45 133 (80–275) 48; 1249 | - | - |
| Variable | Odds Ratio [IC95%] | p Value |
|---|---|---|
| Plasma melatonin at birth ≤7 pg/mL | ||
| Nighttime sampling (00:00–05:59) | 0.41 [0.18; 0.90] | 0.03 |
| Plasma melatonin concentration ≤7 pg/mL in mother at birth | 0.46 [0.24; 0.87] | 0.02 |
| Epidural analgesia | 2.13 [1.03; 4.35] | 0.04 |
| Plasma melatonin on Day 3 ≤ 7 pg/mL | ||
| Lower gestational age group (240/7–336/7 weeks) | 5.56 [2.94; 11.11] | <0.0001 |
| Multiple gestation | 2.94 [1.47; 5.88] | 0.002 |
| Urine aMT6s on Day 1 | ||
| Lower gestational age group (240/7–336/7 weeks) | 2.08 [1.59; 2.70] | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.-F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. Int. J. Mol. Sci. 2019, 20, 2077. https://doi.org/10.3390/ijms20092077
Biran V, Decobert F, Bednarek N, Boizeau P, Benoist J-F, Claustrat B, Barré J, Colella M, Frérot A, Garnotel R, et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. International Journal of Molecular Sciences. 2019; 20(9):2077. https://doi.org/10.3390/ijms20092077
Chicago/Turabian StyleBiran, Valérie, Fabrice Decobert, Nathalie Bednarek, Priscilla Boizeau, Jean-François Benoist, Bruno Claustrat, Jérôme Barré, Marina Colella, Alice Frérot, Roselyne Garnotel, and et al. 2019. "Melatonin Levels in Preterm and Term Infants and Their Mothers" International Journal of Molecular Sciences 20, no. 9: 2077. https://doi.org/10.3390/ijms20092077
APA StyleBiran, V., Decobert, F., Bednarek, N., Boizeau, P., Benoist, J.-F., Claustrat, B., Barré, J., Colella, M., Frérot, A., Garnotel, R., Graesslin, O., Haddad, B., Launay, J.-M., Schmitz, T., Schroedt, J., Virlouvet, A.-L., Guilmin-Crépon, S., Yacoubi, A., Jacqz-Aigrain, E., ... Baud, O. (2019). Melatonin Levels in Preterm and Term Infants and Their Mothers. International Journal of Molecular Sciences, 20(9), 2077. https://doi.org/10.3390/ijms20092077






