Inhaled Cisplatin for NSCLC: Facts and Results
Abstract
1. Introduction
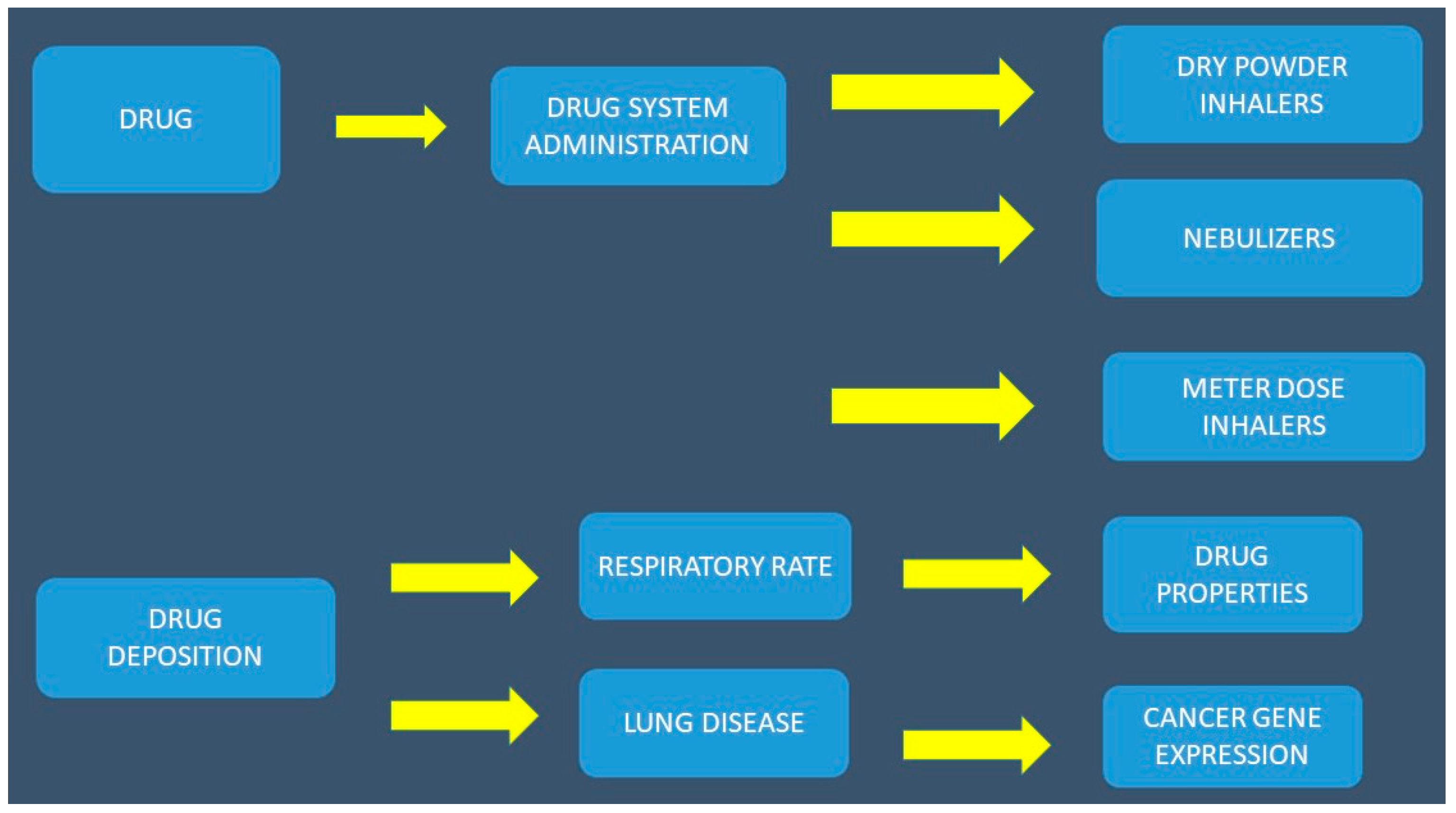
2. Aerosol Administration
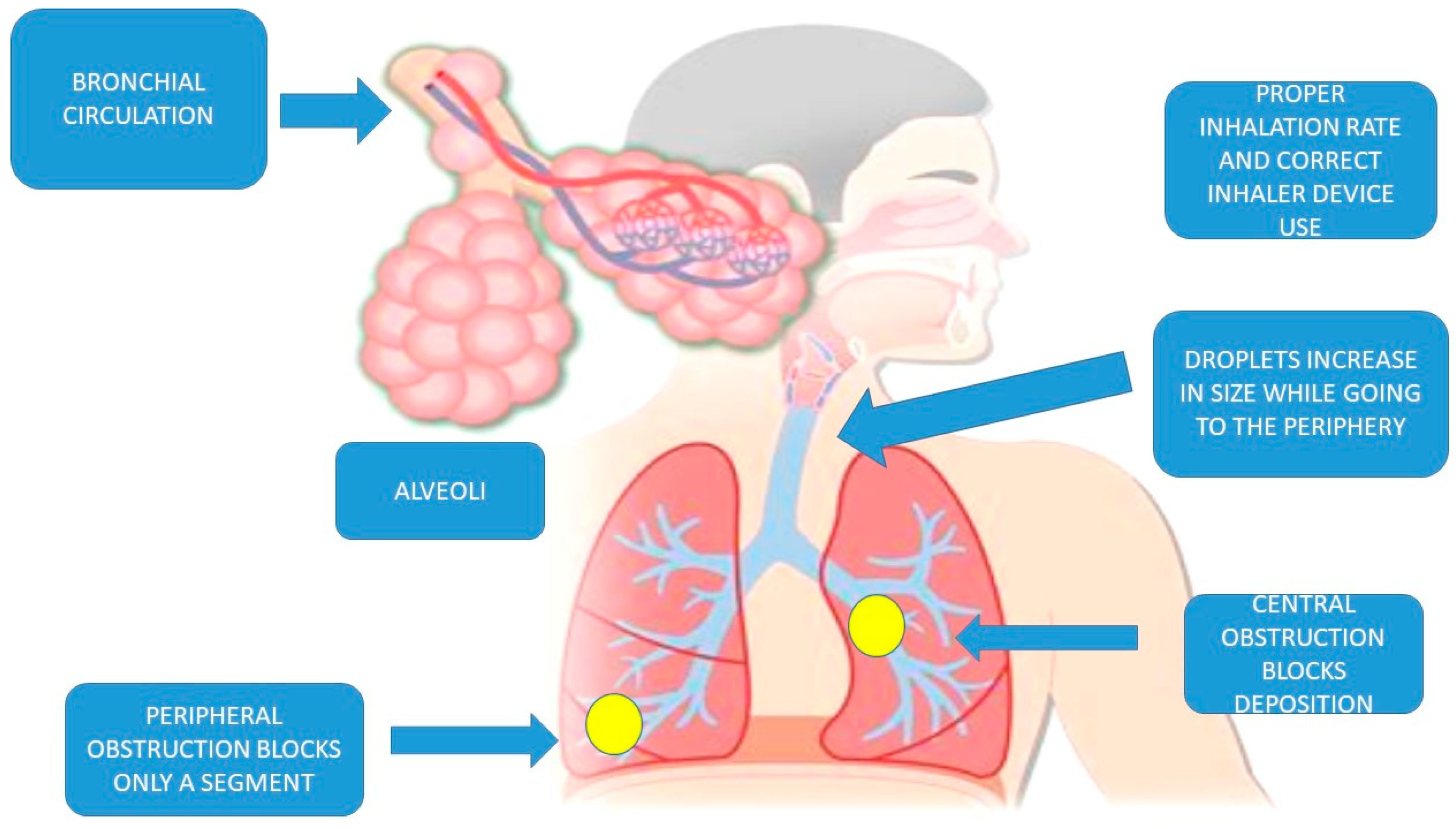
2.1. Lung Anatomy
2.2. Factors Affecting the Efficacy and Bioavailability of Aerolized Drug Formulations
2.2.1. Inhaled Particle Size
2.2.2. Airway Geometry and Humidity
2.2.3. Lung Clearance Mechanisms
2.2.4. Lung Disease
2.2.5. Bronchial Circulation
2.2.6. Duration of Inhalation, Drugs, Breathing Frequency (F) and Tidal Volume (TV), Local Cells, the Local Environment, and Aerosol Production Systems
3. Cisplatin Studies
4. Current and Future Administration Strategies
5. Conclusions
Conflicts of Interest
References
- Zaric, B.; Stojsic, V.; Carapic, V.; Kovacevic, T.; Stojanovic, G.; Panjkovic, M.; Kioumis, I.; Darwiche, K.; Zarogoulidis, K.; Stratakos, G.; et al. Radial Endobronchial Ultrasound (EBUS) Guided Suction Catheter-Biopsy in Histological Diagnosis of Peripheral Pulmonary Lesions. J. Cancer 2016, 7, 7–13. [Google Scholar] [CrossRef]
- Oezkan, F.; Khan, A.; Zarogoulidis, P.; Hohenforst-Schmidt, W.; Theegarten, D.; Yasufuku, K.; Nakajima, T.; Freitag, L.; Darwiche, K. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. OncoTargets Ther. 2014, 7, 2061–2065. [Google Scholar] [CrossRef][Green Version]
- Zaric, B.; Kovacevic, T.; Stojsic, V.; Milovancev, A. New technologies in diagnostic bronchoscopy—an age of meta-analyses. Expert Rev. Med. Devices 2016, 13, 789–791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zarogoulidis, P.; Papadopoulos, V.; Maragouli, E.; Papatsibas, G.; Sardeli, C.; Man, Y.G.; Bai, C.; Huang, H. Nivolumab as first-line treatment in non-small cell lung cancer patients-key factors: tumor mutation burden and PD-L1 >/=50. Transl. Lung Cancer Res. 2018, 7, S28–S30. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Caglevic, C.; Santarpia, M.; Araujo, A.; Giovannetti, E.; Gallardo, C.D.; Pauwels, P.; Mahave, M. Immunotherapy in NSCLC: A Promising and Revolutionary Weapon. Adv. Exp. Med. Biol. 2017, 995, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, A.A.; Iqbal, F. CTLA-4 blockade: therapeutic potential in cancer treatments. OncoTargets Ther. 2010, 3, 15–25. [Google Scholar] [CrossRef]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bahr, O.; Eigentler, T.K.; Grimm, M.O.; Grunwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Manegold, C.; Dingemans, A.C.; Gray, J.E.; Nakagawa, K.; Nicolson, M.; Peters, S.; Reck, M.; Wu, Y.L.; Brustugun, O.T.; Crino, L.; et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J. Thoracic Oncol. 2017, 12, 194–207. [Google Scholar] [CrossRef]
- Insinga, R.P.; Vanness, D.J.; Feliciano, J.L.; Vandormael, K.; Traore, S.; Ejzykowicz, F.; Burke, T. Cost-effectiveness of pembrolizumab in combination with chemotherapy versus chemotherapy and pembrolizumab monotherapy in the first-line treatment of squamous non-small-cell lung cancer in the US. Curr. Med. Res. Opin. 2019, 1–16. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Chinelis, P.; Athanasiadou, A.; Porpodis, K.; Kallianos, A.; Rapti, A.; Trakada, G.; Velentza, L.; Huang, H.; Tsiouda, T.; et al. “Liquid elbows” due to afatinib administration. Respir. Med. Case Rep. 2017, 22, 64–66. [Google Scholar] [CrossRef]
- Sapalidis, K.; Kosmidis, C.; Michalopoulos, N.; Koulouris, C.; Mantalobas, S.; Giannakidis, D.; Munteanu, A.; Surlin, V.; Laskou, S.; Zarogoulidis, P.; et al. Psoriatic arthritis due to nivolumab administration a case report and review of the literature. Respir. Med. Case Rep. 2018, 23, 182–187. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Huang, H.; Tsiouda, T.; Sardeli, C.; Trakada, G.; Veletza, L.; Kallianos, A.; Kosmidis, C.; Rapti, A.; Papaemmanouil, L.; et al. Immunotherapy “Shock” with vitiligo due to nivolumab administration as third line therapy in lung adenocarcinoma. Respir. Med. Case Rep. 2017, 22, 283–286. [Google Scholar] [CrossRef]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. North Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Mandal, A.; Mitra, A.K. Recent Patents in Pulmonary Delivery of Macromolecules. Recent Pat. Drug Deliv. Formul. 2015, 9, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Chatzaki, E.; Porpodis, K.; Domvri, K.; Hohenforst-Schmidt, W.; Goldberg, E.P.; Karamanos, N.; Zarogoulidis, K. Inhaled chemotherapy in lung cancer: future concept of nanomedicine. Int. J. Nanomed. 2012, 7, 1551–1572. [Google Scholar] [CrossRef] [PubMed]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharm. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharm. 2003, 56, 600–612. [Google Scholar] [CrossRef]
- Phipps, P.R.; Gonda, I.; Anderson, S.D.; Bailey, D.; Bautovich, G. Regional deposition of saline aerosols of different tonicities in normal and asthmatic subjects. Eur. Respir. J. 1994, 7, 1474–1482. [Google Scholar] [CrossRef]
- Swift, D.L. Aerosols and humidity therapy. Generation and respiratory deposition of therapeutic aerosols. Am. Rev. Respir. Dis. 1980, 122, 71–77. [Google Scholar] [CrossRef]
- Houtmeyers, E.; Gosselink, R.; Gayan-Ramirez, G.; Decramer, M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999, 13, 1177–1188. [Google Scholar] [CrossRef]
- Summers, Q.A. Inhaled drugs and the lung. Clin. Exp. Allergy 1991, 21, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Wirkes, A.; Jung, K.; Ochs, M.; Muhlfeld, C. Allometry of the mammalian intracellular pulmonary surfactant system. J. Appl. Physiol. 2010, 109, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, H.G.; Matthay, M.A.; Westrom, B.R.; Kim, K.J.; Karlsson, B.W.; Hastings, R.H. Alveolar epithelial clearance of protein. J. Appl. Physiol. 1996, 80, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Martonen, T.B. Mathematical model for the selective deposition of inhaled pharmaceuticals. J. Pharm. Sci. 1993, 82, 1191–1199. [Google Scholar] [CrossRef]
- Hastings, R.H.; Grady, M.; Sakuma, T.; Matthay, M.A. Clearance of different-sized proteins from the alveolar space in humans and rabbits. J. Appl. Physiol. 1992, 73, 1310–1316. [Google Scholar] [CrossRef]
- Deffebach, M.E.; Charan, N.B.; Lakshminarayan, S.; Butler, J. The bronchial circulation. Small, but a vital attribute of the lung. Am. Rev. Respir. Dis. 1987, 135, 463–481. [Google Scholar] [CrossRef]
- Shinohara, H. Distribution of lymphatic stomata on the pleural surface of the thoracic cavity and the surface topography of the pleural mesothelium in the golden hamster. Anat. Rec. 1997, 249, 16–23. [Google Scholar] [CrossRef]
- Lai-Fook, S.J. Mechanical factors in lung liquid distribution. An. Rev. Physiol. 1993, 55, 155–179. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Knight, V.; Gilbert, B.E.; Golunski, E.; Roberts, L.; Waldrep, J.C. Improved respiratory delivery of the anticancer drugs, camptothecin and paclitaxel, with 5% CO2-enriched air: Pharmacokinetic studies. Cancer Chemother. Pharm. 2001, 47, 451–456. [Google Scholar] [CrossRef]
- Davis, J.N.; Stagg, D. Interrelationships of the volume and time components of individual breaths in resting man. J. Physiol. 1975, 245, 481–498. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.; Vincken, W.; Virchow, J.C.; Roche, N.; Agusti, A.; Lavorini, F.; van Aalderen, W.M.; Price, D. Prescription of inhalers in asthma and COPD: Towards a rational, rapid and effective approach. Respir. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Mogayzel, P.J., Jr.; Naureckas, E.T.; Robinson, K.A.; Mueller, G.; Hadjiliadis, D.; Hoag, J.B.; Lubsch, L.; Hazle, L.; Sabadosa, K.; Marshall, B. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am. J. Respir. Crit. Care Med. 2013, 187, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.B.; Marsh, R.L.; Smith-Vaughan, H.C.; Hoffman, L.R. Emerging drugs for bronchiectasis. Expert Opin. Emerg. Drugs 2012, 17, 361–378. [Google Scholar] [CrossRef]
- Laube, B.L.; Janssens, H.M.; de Jongh, F.H.; Devadason, S.G.; Dhand, R.; Diot, P.; Everard, M.L.; Horvath, I.; Navalesi, P.; Voshaar, T.; et al. What the pulmonary specialist should know about the new inhalation therapies. Eur. Respir. J. 2011, 37, 1308–1331. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Spyratos, D.; Secen, N.; Hohenforst-Schmidt, W.; Katsikogiannis, N.; Huang, H.; Gschwendtner, A.; Zarogoulidis, K. Defense Mechanisms of the Respiratory System and Aerosol Production Systems. Med. Chem. 2014, 10, 123–136. [Google Scholar] [CrossRef]
- Clay, M.M.; Pavia, D.; Newman, S.P.; Clarke, S.W. Factors influencing the size distribution of aerosols from jet nebulisers. Thorax 1983, 38, 755–759. [Google Scholar] [CrossRef]
- Bier, M.; de Graaf, J.; Zwanikken, J.; van Roij, R. Curvature dependence of the electrolytic liquid-liquid interfacial tension. J. Chem. Physiol. 2009, 130, 024703. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Darwiche, K.; Spyratos, D.; Huang, H.; Goldberg, E.P.; Yarmus, L.; Li, Q.; Freitag, L.; et al. Establishing the optimal nebulization system for paclitaxel, docetaxel, cisplatin, carboplatin and gemcitabine: back to drawing the residual cup. Int. J. Pharm. 2013, 453, 480–487. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Petridis, D.; Ritzoulis, C.; Darwiche, K.; Kioumis, I.; Porpodis, K.; Spyratos, D.; Hohenforst-Schmidt, W.; Yarmus, L.; Huang, H.; et al. Internal mouthpiece designs as a future perspective for enhanced aerosol deposition. Comparative results for aerosol chemotherapy and aerosol antibiotics. Int. J. Pharm. 2013, 456, 325–331. [Google Scholar] [CrossRef]
- Mercer, T.T.; Goddard, R.F.; Flores, R.L. Effect of auxiliary air flow on the output characteristics of compressed-air nebulizers. Ann. Allergy 1969, 27, 211–217. [Google Scholar]
- Kendrick, A.H.; Smith, E.C.; Wilson, R.S. Selecting and using nebuliser equipment. Thorax 1997, 52, S92–S101. [Google Scholar] [CrossRef]
- Newman, S.P.; Pellow, P.G.; Clay, M.M.; Clarke, S.W. Evaluation of jet nebulisers for use with gentamicin solution. Thorax 1985, 40, 671–676. [Google Scholar] [CrossRef]
- Sterk, P.J.; Plomp, A.; van de Vate, J.F.; Quanjer, P.H. Physical properties of aerosols produced by several jet- and ultrasonic nebulizers. Bull Eur. Physiopathol. Respir. 1984, 20, 65–72. [Google Scholar] [PubMed]
- Ferron, G.A.; Kerrebijn, K.F.; Weber, J. Properties of aerosols produced with three nebulizers. Am. Rev. Respir. Dis. 1976, 114, 899–908. [Google Scholar] [PubMed]
- Steckel, H.; Eskandar, F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur. J. Pharm. Sci. 2003, 19, 443–455. [Google Scholar] [CrossRef]
- Lourenco, R.V.; Cotromanes, E. Clinical aerosols II. Therapeutic aerosols. Arch Intern. Med. 1982, 142, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Niven, R.W.; Ip, A.Y.; Mittelman, S.; Prestrelski, S.J.; Arakawa, T. Some factors associated with the ultrasonic nebulization of proteins. Pharm. Res. 1995, 12, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.H.; Stenton, S.C.; Beach, J.R.; Avery, A.J.; Walters, E.H.; Hendrick, D.J. Jet and ultrasonic nebuliser output: use of a new method for direct measurement of aerosol output. Thorax 1990, 45, 728–732. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Barry, P.W. The science of nebulised drug delivery. Thorax 1997, 52, S31–S44. [Google Scholar] [CrossRef]
- Darwiche, K.; Zarogoulidis, P.; Baehner, K.; Welter, S.; Tetzner, R.; Wohlschlaeger, J.; Theegarten, D.; Nakajima, T.; Freitag, L. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann. Oncol. 2013, 24, 2866–2870. [Google Scholar] [CrossRef]
- Wittgen, B.P.; Kunst, P.W.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. 2016, 25, 60–72. [Google Scholar] [CrossRef]
- Tseng, C.L.; Su, W.Y.; Yen, K.C.; Yang, K.C.; Lin, F.H. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials 2009, 30, 3476–3485. [Google Scholar] [CrossRef]
- Lee, H.Y.; Mohammed, K.A.; Goldberg, E.P.; Kaye, F.; Nasreen, N. Cisplatin loaded albumin mesospheres for lung cancer treatment. Am. J. Cancer Res. 2015, 5, 603–615. [Google Scholar]
- El-Gendy, N.; Berkland, C. Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. Pharm. Res. 2009, 26, 1752–1763. [Google Scholar] [CrossRef]
- Ishiguro, S.; Cai, S.; Uppalapati, D.; Turner, K.; Zhang, T.; Forrest, W.C.; Forrest, M.L.; Tamura, M. Intratracheal Administration of Hyaluronan-Cisplatin Conjugate Nanoparticles Significantly Attenuates Lung Cancer Growth in Mice. Pharm. Res. 2016, 33, 2517–2529. [Google Scholar] [CrossRef]
- Selting, K.; Essman, S.; Reinero, C.; Branson, K.R.; Henry, C.J.; Owen, N.; Guntur, V.P.; Waldrep, J.C.; Kim, D.Y.; Dhand, R. Targeted combined aerosol chemotherapy in dogs and radiologic toxicity grading. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 43–48. [Google Scholar] [CrossRef]
- Hao, Y.; Altundal, Y.; Moreau, M.; Sajo, E.; Kumar, R.; Ngwa, W. Potential for enhancing external beam radiotherapy for lung cancer using high-Z nanoparticles administered via inhalation. Physiol. Med. Biol. 2015, 60, 7035–7043. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Darwiche, K.; Krauss, L.; Huang, H.; Zachariadis, G.A.; Katsavou, A.; Hohenforst-Schmidt, W.; Papaiwannou, A.; Vogl, T.J.; Freitag, L.; et al. Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. Fut. Oncol. 2013, 9, 1307–1313. [Google Scholar] [CrossRef]
- Singh, D.J.; Lohade, A.A.; Parmar, J.J.; Hegde, D.D.; Soni, P.; Samad, A.; Menon, M.D. Development of Chitosan-based Dry Powder Inhalation System of Cisplatin for Lung Cancer. Indian J. Pharm. Sci. 2012, 74, 521–526. [Google Scholar] [CrossRef]
- Chou, A.J.; Gupta, R.; Bell, M.D.; Riewe, K.O.; Meyers, P.A.; Gorlick, R. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar] [CrossRef]
- Li, Z.; Song, M.; He, Z.; Zong, L.; Jiang, B.; Zhang, T.; Hu, Z. Comparison of quick recovery outcome of inhalable doxorubicin and cisplatin in lung cancer patients: a randomized, double-blind, single-center trial. Drug Deliv. Transl. Res. 2018, 8, 985–993. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Levet, V.; Rosiere, R.; Merlos, R.; Fusaro, L.; Berger, G.; Amighi, K.; Wauthoz, N. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 2016, 515, 209–220. [Google Scholar] [CrossRef]
- Nowacki, M.; Alyami, M.; Villeneuve, L.; Mercier, F.; Hubner, M.; Willaert, W.; Ceelen, W.; Reymond, M.; Pezet, D.; Arvieux, C.; et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur. J. Surg. Oncol. 2018, 44, 991–996. [Google Scholar] [CrossRef]
- Anderson, K.; Lawson, K.A.; Simmons-Menchaca, M.; Sun, L.; Sanders, B.G.; Kline, K. Alpha-TEA plus cisplatin reduces human cisplatin-resistant ovarian cancer cell tumor burden and metastasis. Exp. Biol. Med. 2004, 229, 1169–1176. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Jungst, F.; Jutte, H.; Tannapfel, A.; Hilal, Z.; Hefler, L.A.; Reymond, M.A.; Tempfer, C.B. Dynamic changes of tumor gene expression during repeated pressurized intraperitoneal aerosol chemotherapy (PIPAC) in women with peritoneal cancer. BMC Cancer 2016, 16, 654. [Google Scholar] [CrossRef]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I - pre-analytical and analytical validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef]
- Turgeon, G.A.; Weickhardt, A.; Azad, A.A.; Solomon, B.; Siva, S. Radiotherapy and immunotherapy: a synergistic effect in cancer care. Med. J. Austr. 2019, 210, 47–53. [Google Scholar] [CrossRef]
- McGaughey, D.S.; Nikcevich, D.A.; Long, G.D.; Vredenburgh, J.J.; Rizzieri, D.; Smith, C.A.; Broadwater, G.; Loftis, J.S.; McDonald, C.; Morris, A.K.; et al. Inhaled steroids as prophylaxis for delayed pulmonary toxicity syndrome in breast cancer patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Biol. Blood Marrow Transpl. 2001, 7, 274–278. [Google Scholar] [CrossRef]
- Darwiche, K.; Zarogoulidis, P.; Karamanos, N.K.; Domvri, K.; Chatzaki, E.; Constantinidis, T.C.; Kakolyris, S.; Zarogoulidis, K. Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: a future dilemma for micro-oncology. Fut. Oncol. 2013, 9, 505–525. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Giraleli, C.; Karamanos, N.K. Inhaled chemotherapy in lung cancer: safety concerns of nanocomplexes delivered. Ther. Deliv. 2012, 3, 1021–1023. [Google Scholar] [CrossRef]
| Preclinical | - The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. - Cisplatin loaded albumin mesospheres for lung cancer treatment. - Combination chemotherapeutic dry powder aerosols via controlled nanoparticle agglomeration. - Intratracheal administration of hyaluronan–cisplatin conjugate nanoparticles significantly attenuates lung cancer growth in mice. - Targeted combined aerosol chemotherapy in dogs and radiologic toxicity grading. - Development of chitosan-based dry powder inhalation system of cisplatin for lung cancer. - Innovative strategy for treatment of lung cancer: targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. - Development of controlled-release cisplatin dry powders for inhalation against lung cancers. |
| Clinical | - Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. - Potential for enhancing external beam radiotherapy for lung cancer using high-Z nanoparticles administered via inhalation. - Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. - Inhaled cisplatin deposition and distribution in lymph nodes in stage II lung cancer patients. - Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. - Comparison of quick recovery outcome of inhalable doxorubicin and cisplatin in lung cancer patients: a randomized, double-blind, single-center trial. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmidis, C.; Sapalidis, K.; Zarogoulidis, P.; Sardeli, C.; Koulouris, C.; Giannakidis, D.; Pavlidis, E.; Katsaounis, A.; Michalopoulos, N.; Mantalobas, S.; et al. Inhaled Cisplatin for NSCLC: Facts and Results. Int. J. Mol. Sci. 2019, 20, 2005. https://doi.org/10.3390/ijms20082005
Kosmidis C, Sapalidis K, Zarogoulidis P, Sardeli C, Koulouris C, Giannakidis D, Pavlidis E, Katsaounis A, Michalopoulos N, Mantalobas S, et al. Inhaled Cisplatin for NSCLC: Facts and Results. International Journal of Molecular Sciences. 2019; 20(8):2005. https://doi.org/10.3390/ijms20082005
Chicago/Turabian StyleKosmidis, Christoforos, Konstantinos Sapalidis, Paul Zarogoulidis, Chrysanthi Sardeli, Charilaos Koulouris, Dimitrios Giannakidis, Efstathios Pavlidis, Athanasios Katsaounis, Nikolaos Michalopoulos, Stylianos Mantalobas, and et al. 2019. "Inhaled Cisplatin for NSCLC: Facts and Results" International Journal of Molecular Sciences 20, no. 8: 2005. https://doi.org/10.3390/ijms20082005
APA StyleKosmidis, C., Sapalidis, K., Zarogoulidis, P., Sardeli, C., Koulouris, C., Giannakidis, D., Pavlidis, E., Katsaounis, A., Michalopoulos, N., Mantalobas, S., Koimtzis, G., Alexandrou, V., Tsiouda, T., Amaniti, A., & Kesisoglou, I. (2019). Inhaled Cisplatin for NSCLC: Facts and Results. International Journal of Molecular Sciences, 20(8), 2005. https://doi.org/10.3390/ijms20082005





