The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)—A Next-Generation Sequencing and Bioinformatic Approach
Abstract
1. Introduction
2. Results
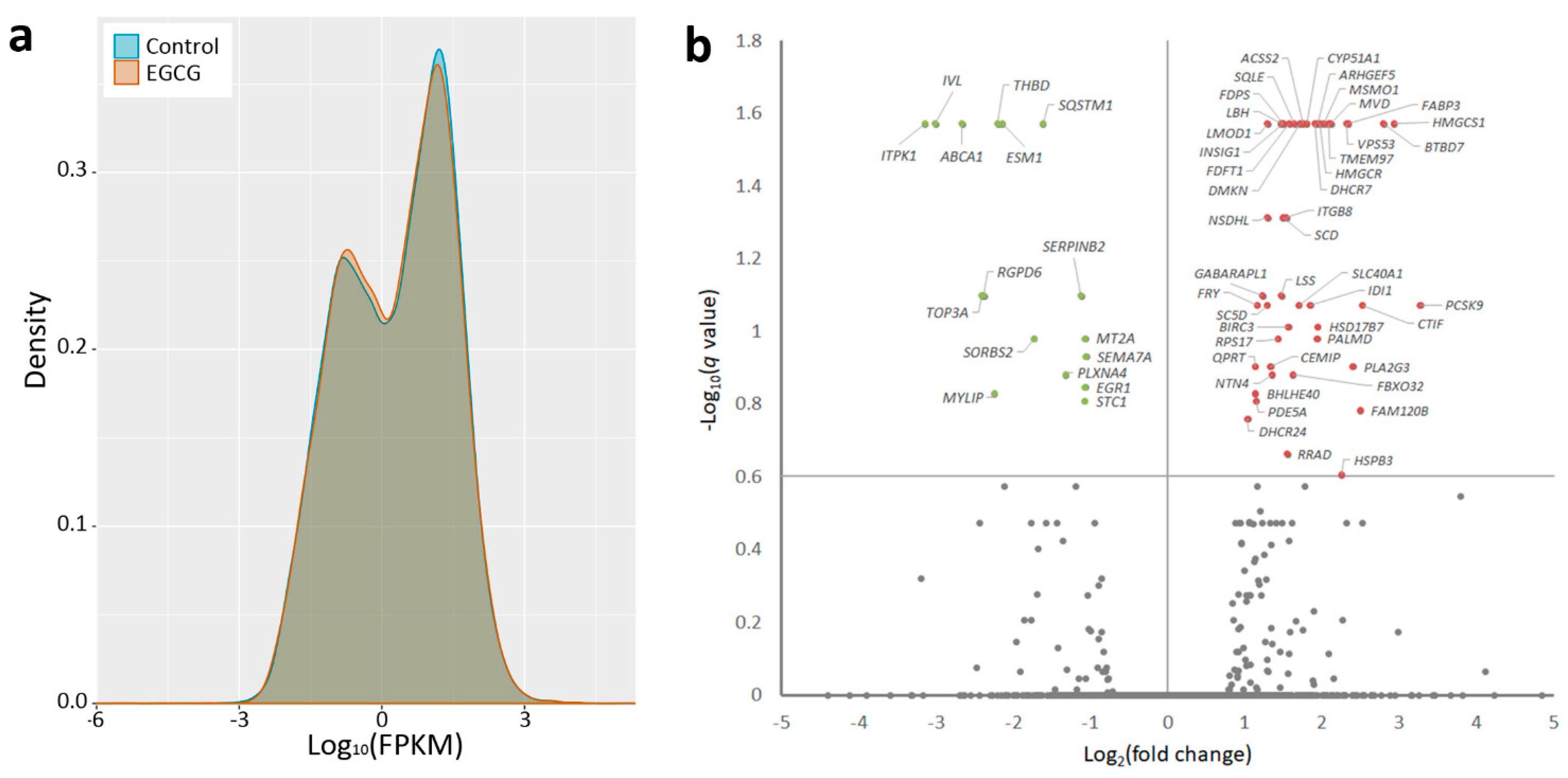
2.1. Gene Expression Profiling and Microrna Changes in IPF Fibroblasts Treated with EGCG
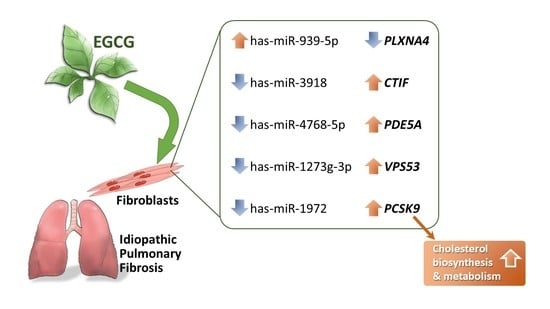
2.2. Discovering the Altered microRNA–mRNA Interactions in IPF Fibroblasts Treated with EGCG
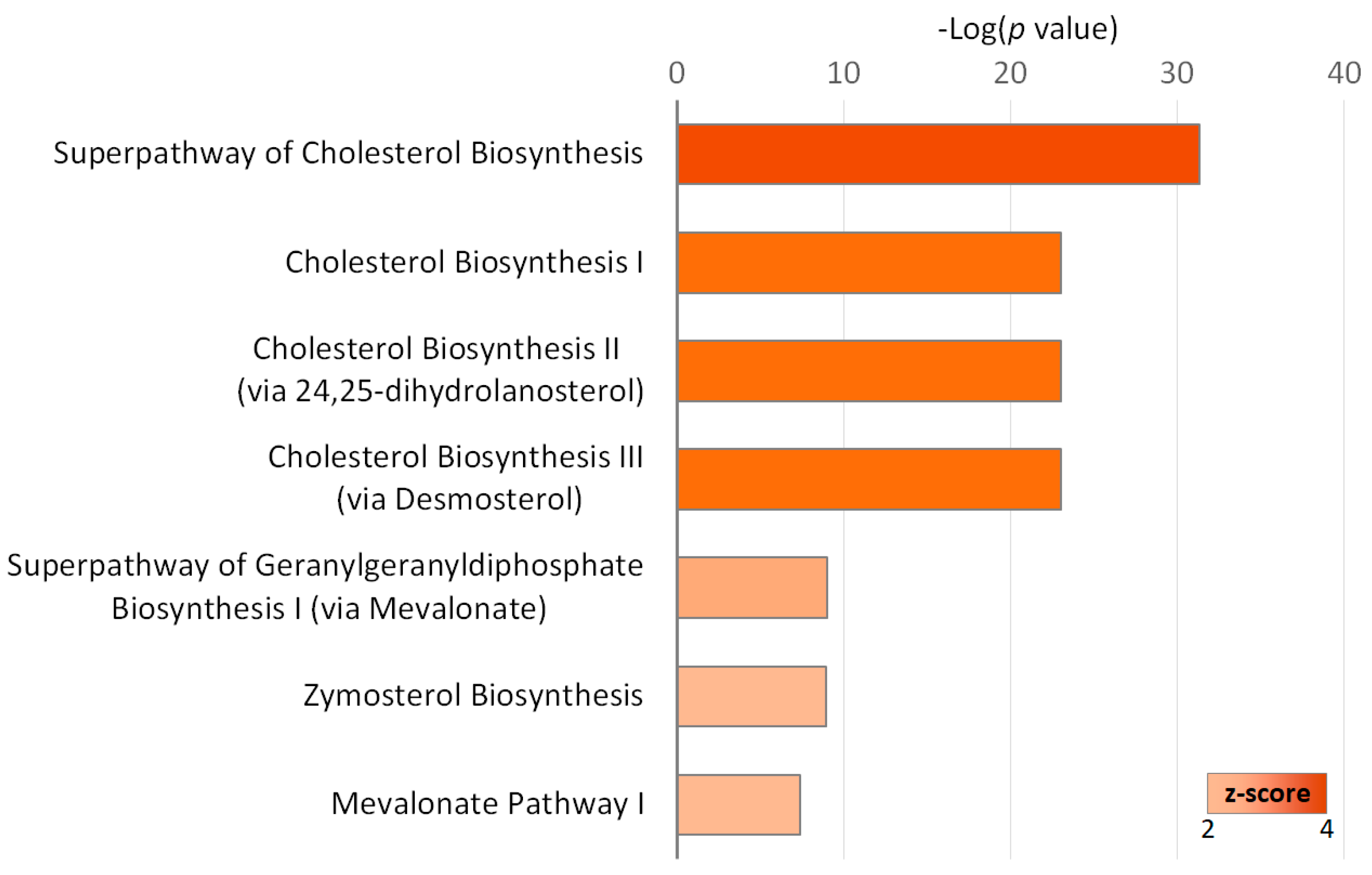
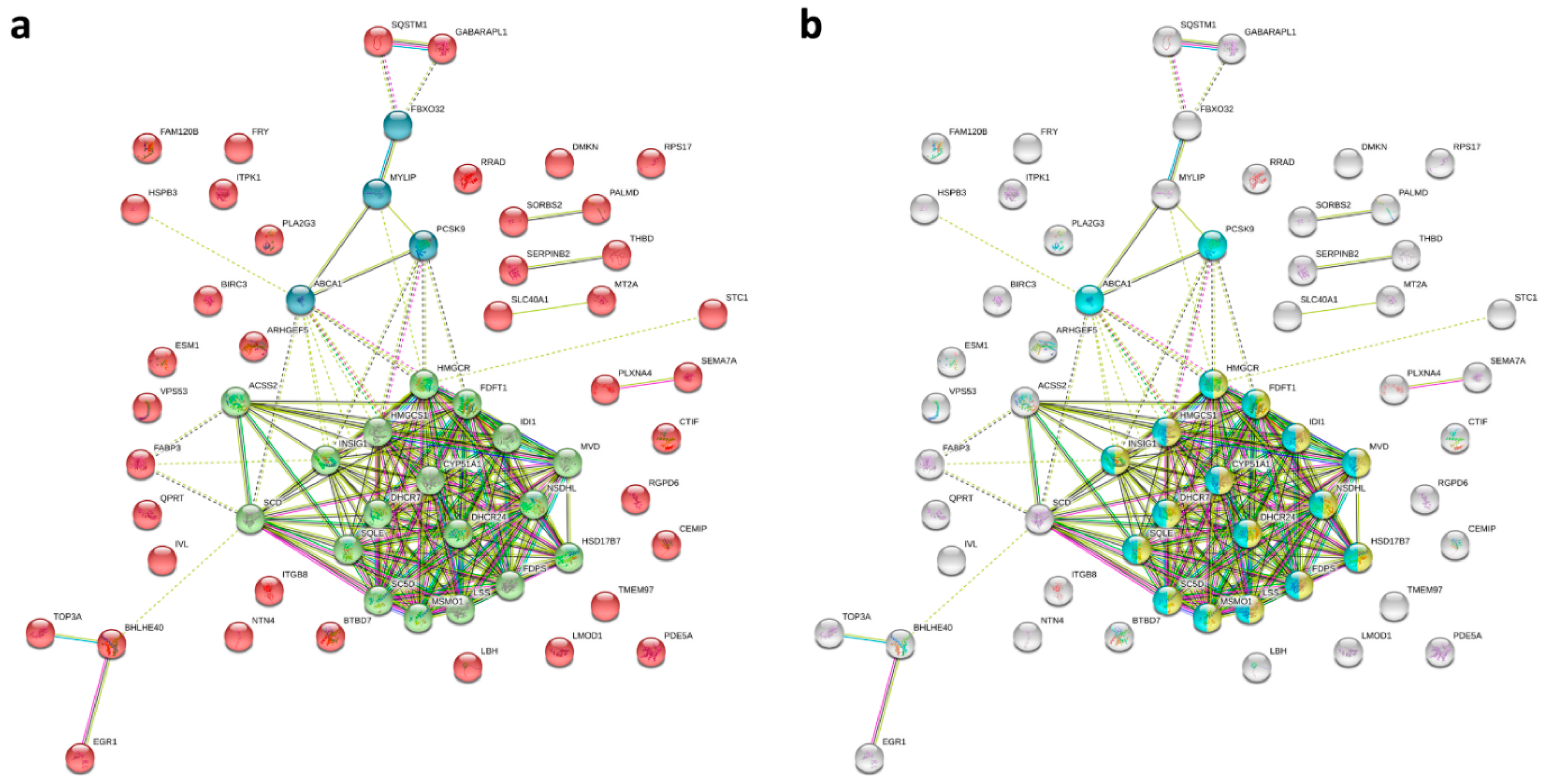
2.3. Gene Ontology Annotations of the Differentially Expressed Genes in IPF Fibroblasts Treated with EGCG
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Next-Generation Sequencing (NGS)
4.2. Analyses Using microRNA Target Predicting Databases
4.3. Database for Annotation, Visualization and Integrated Discovery (DAVID) Database Analysis
4.4. Ingenuity Pathway Analysis (IPA)
4.5. Search Tool for the Retrieval of Interacting Genes (STRING)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Official Gene Symbol | FPKM | Ratio (EGCG/Control) | Log2(ratio) | p-Value | q-Value * | |
|---|---|---|---|---|---|---|
| EGCG | Control | |||||
| PCSK9 | 0.85 | 0.09 | 9.65 | 3.27 | 0.0003 | 0.0846 |
| HMGCS1 | 441.43 | 57.81 | 7.64 | 2.93 | 0.0001 | 0.0268 |
| BTBD7 | 7.42 | 1.07 | 6.96 | 2.80 | 0.0001 | 0.0268 |
| CTIF | 5.12 | 0.89 | 5.75 | 2.52 | 0.0003 | 0.0846 |
| FAM120B | 2.44 | 0.43 | 5.64 | 2.50 | 0.0007 | 0.1650 |
| PLA2G3 | 1.29 | 0.24 | 5.29 | 2.40 | 0.0005 | 0.1250 |
| FABP3 | 117.28 | 23.12 | 5.07 | 2.34 | 0.0001 | 0.0268 |
| VPS53 | 10.80 | 2.16 | 4.99 | 2.32 | 0.0001 | 0.0268 |
| HSPB3 | 5.34 | 1.12 | 4.76 | 2.25 | 0.0011 | 0.2481 |
| MVD | 96.80 | 22.27 | 4.35 | 2.12 | 0.0001 | 0.0268 |
| TMEM97 | 140.13 | 32.98 | 4.25 | 2.09 | 0.0001 | 0.0268 |
| MSMO1 | 224.89 | 55.13 | 4.08 | 2.03 | 0.0001 | 0.0268 |
| HMGCR | 136.04 | 34.55 | 3.94 | 1.98 | 0.0001 | 0.0268 |
| ARHGEF5 | 3.48 | 0.90 | 3.86 | 1.95 | 0.0001 | 0.0268 |
| HSD17B7 | 11.98 | 3.12 | 3.85 | 1.94 | 0.0003 | 0.0973 |
| PALMD | 3.68 | 0.96 | 3.82 | 1.93 | 0.0004 | 0.1047 |
| DHCR7 | 84.31 | 22.39 | 3.76 | 1.91 | 0.0001 | 0.0268 |
| IDI1 | 185.79 | 51.74 | 3.59 | 1.84 | 0.0003 | 0.0846 |
| CYP51A1 | 329.34 | 94.32 | 3.49 | 1.80 | 0.0001 | 0.0268 |
| ACSS2 | 25.91 | 7.69 | 3.37 | 1.75 | 0.0001 | 0.0268 |
| DMKN | 2.32 | 0.71 | 3.28 | 1.71 | 0.0001 | 0.0268 |
| SLC40A1 | 7.87 | 2.43 | 3.24 | 1.70 | 0.0003 | 0.0846 |
| SQLE | 108.28 | 34.77 | 3.11 | 1.64 | 0.0001 | 0.0268 |
| FBXO32 | 5.71 | 1.85 | 3.08 | 1.62 | 0.0005 | 0.1319 |
| FDFT1 | 206.04 | 69.04 | 2.98 | 1.58 | 0.0001 | 0.0268 |
| BIRC3 | 12.03 | 4.07 | 2.95 | 1.56 | 0.0003 | 0.0973 |
| RRAD | 4.19 | 1.43 | 2.94 | 1.55 | 0.0010 | 0.2174 |
| ITGB8 | 6.31 | 2.17 | 2.91 | 1.54 | 0.0001 | 0.0486 |
| FDPS | 260.06 | 91.77 | 2.83 | 1.50 | 0.0001 | 0.0268 |
| SCD | 377.97 | 134.01 | 2.82 | 1.50 | 0.0001 | 0.0486 |
| INSIG1 | 268.58 | 95.40 | 2.82 | 1.49 | 0.0001 | 0.0268 |
| LSS | 129.93 | 46.81 | 2.78 | 1.47 | 0.0002 | 0.0798 |
| LBH | 128.19 | 46.39 | 2.76 | 1.47 | 0.0001 | 0.0268 |
| RPS17 | 60.77 | 22.54 | 2.70 | 1.43 | 0.0004 | 0.1047 |
| NTN4 | 12.06 | 4.72 | 2.56 | 1.35 | 0.0005 | 0.1319 |
| CEMIP | 216.43 | 85.86 | 2.52 | 1.33 | 0.0005 | 0.1250 |
| LMOD1 | 15.53 | 6.33 | 2.46 | 1.30 | 0.0001 | 0.0268 |
| NSDHL | 53.96 | 22.04 | 2.45 | 1.29 | 0.0001 | 0.0486 |
| SC5D | 93.06 | 38.02 | 2.45 | 1.29 | 0.0003 | 0.0846 |
| GABARAPL1 | 20.49 | 8.74 | 2.34 | 1.23 | 0.0002 | 0.0798 |
| FRY | 6.83 | 3.06 | 2.23 | 1.16 | 0.0003 | 0.0846 |
| PDE5A | 278.27 | 125.88 | 2.21 | 1.14 | 0.0007 | 0.1556 |
| BHLHE40 | 16.48 | 7.50 | 2.20 | 1.13 | 0.0006 | 0.1482 |
| QPRT | 12.85 | 5.85 | 2.20 | 1.13 | 0.0005 | 0.1250 |
| DHCR24 | 151.61 | 73.96 | 2.05 | 1.04 | 0.0008 | 0.1742 |
| SEMA7A | 26.51 | 54.97 | 0.48 | -1.05 | 0.0004 | 0.1174 |
| MT2A | 352.79 | 738.65 | 0.48 | -1.07 | 0.0004 | 0.1047 |
| EGR1 | 17.36 | 36.40 | 0.48 | -1.07 | 0.0006 | 0.1426 |
| STC1 | 41.69 | 87.70 | 0.48 | -1.07 | 0.0007 | 0.1556 |
| SERPINB2 | 131.57 | 286.07 | 0.46 | -1.12 | 0.0002 | 0.0798 |
| PLXNA4 | 2.83 | 7.07 | 0.40 | -1.32 | 0.0005 | 0.1319 |
| SQSTM1 | 11.91 | 36.40 | 0.33 | -1.61 | 0.0001 | 0.0268 |
| SORBS2 | 0.12 | 0.41 | 0.30 | -1.73 | 0.0004 | 0.1047 |
| ESM1 | 10.28 | 45.34 | 0.23 | -2.14 | 0.0001 | 0.0268 |
| THBD | 3.13 | 14.37 | 0.22 | -2.20 | 0.0001 | 0.0268 |
| MYLIP | 0.61 | 2.91 | 0.21 | -2.24 | 0.0006 | 0.1482 |
| RGPD6 | 0.57 | 2.99 | 0.19 | -2.38 | 0.0002 | 0.0798 |
| TOP3A | 0.42 | 2.21 | 0.19 | -2.40 | 0.0002 | 0.0798 |
| ABCA1 | 2.09 | 13.21 | 0.16 | -2.66 | 0.0001 | 0.0268 |
| IVL | 0.13 | 1.03 | 0.12 | -3.01 | 0.0001 | 0.0268 |
| ITPK1 | 0.85 | 7.50 | 0.11 | -3.14 | 0.0001 | 0.0268 |
| miRNA | Precursor | Normalized Read Count (rpm) | Fold Change | Up/Down | |
|---|---|---|---|---|---|
| EGCG | Control | ||||
| hsa-miR-491-3p | hsa-mir-491 | 1.39 | 0.08 | 17.38 | Up |
| hsa-miR-4803 | hsa-mir-4803 | 1.21 | 0.08 | 15.13 | Up |
| hsa-miR-1322 | hsa-mir-1322 | 1.04 | 0.17 | 6.12 | Up |
| hsa-miR-939-5p | hsa-mir-939 | 1.65 | 0.34 | 4.85 | Up |
| hsa-miR-101-5p | hsa-mir-101-1 | 1.13 | 0.25 | 4.52 | Up |
| hsa-miR-141-3p | hsa-mir-141 | 1.82 | 0.42 | 4.33 | Up |
| hsa-miR-33a-3p | hsa-mir-33a | 3.03 | 0.84 | 3.61 | Up |
| hsa-miR-34a-3p | hsa-mir-34a | 6.5 | 1.93 | 3.37 | Up |
| hsa-miR-503-3p | hsa-mir-503 | 1.21 | 0.42 | 2.88 | Up |
| hsa-miR-200c-3p | hsa-mir-200c | 1.3 | 0.5 | 2.60 | Up |
| hsa-miR-145-5p | hsa-mir-145 | 93.71 | 37.01 | 2.53 | Up |
| hsa-miR-491-5p | hsa-mir-491 | 1.91 | 0.76 | 2.51 | Up |
| hsa-miR-770-5p | hsa-mir-770 | 2.51 | 1.01 | 2.49 | Up |
| hsa-miR-29b-2-5p | hsa-mir-29b-2 | 2.6 | 1.09 | 2.39 | Up |
| hsa-miR-548at-5p | hsa-mir-548at | 2.17 | 0.93 | 2.33 | Up |
| hsa-miR-4636 | hsa-mir-4636 | 2.51 | 1.09 | 2.30 | Up |
| hsa-miR-3684 | hsa-mir-3684 | 1.65 | 0.76 | 2.17 | Up |
| hsa-miR-6723-5p | hsa-mir-6723 | 1.65 | 0.76 | 2.17 | Up |
| hsa-miR-142-5p | hsa-mir-142 | 1.39 | 0.67 | 2.07 | Up |
| hsa-miR-655-3p | hsa-mir-655 | 4.85 | 2.35 | 2.06 | Up |
| hsa-miR-3130-5p | hsa-mir-3130-2 | 1.56 | 0.76 | 2.05 | Up |
| hsa-miR-3613-3p | hsa-mir-3613 | 1.21 | 0.59 | 2.05 | Up |
| hsa-miR-1273g-3p | hsa-mir-1273g | 0.87 | 1.77 | −2.03 | Down |
| hsa-miR-2116-3p | hsa-mir-2116 | 2.51 | 5.13 | −2.04 | Down |
| hsa-miR-3622a-5p | hsa-mir-3622a | 0.69 | 1.43 | −2.07 | Down |
| hsa-miR-5699-5p | hsa-mir-5699 | 1.65 | 3.45 | −2.09 | Down |
| hsa-miR-138-1-3p | hsa-mir-138-1 | 55.39 | 117.24 | −2.12 | Down |
| hsa-miR-340-3p | hsa-mir-340 | 3.81 | 8.24 | −2.16 | Down |
| hsa-miR-423-5p | hsa-mir-423 | 822.58 | 1779.79 | −2.16 | Down |
| hsa-miR-25-5p | hsa-mir-25 | 27.39 | 59.54 | −2.17 | Down |
| hsa-miR-3605-3p | hsa-mir-3605 | 12.05 | 26.66 | −2.21 | Down |
| hsa-miR-1910-5p | hsa-mir-1910 | 22.71 | 50.63 | −2.23 | Down |
| hsa-miR-4745-5p | hsa-mir-4745 | 0.95 | 2.19 | −2.31 | Down |
| hsa-miR-6840-5p | hsa-mir-6840 | 0.61 | 1.43 | −2.34 | Down |
| hsa-miR-92a-1-5p | hsa-mir-92a-1 | 8.06 | 19.09 | −2.37 | Down |
| hsa-miR-1914-5p | hsa-mir-1914 | 0.52 | 1.26 | −2.42 | Down |
| hsa-miR-937-3p | hsa-mir-937 | 4.33 | 10.85 | −2.51 | Down |
| hsa-miR-323a-5p | hsa-mir-323a | 0.43 | 1.09 | −2.53 | Down |
| hsa-miR-3648 | hsa-mir-3648-1 | 0.78 | 2.02 | −2.59 | Down |
| hsa-miR-3648 | hsa-mir-3648-2 | 0.78 | 2.02 | −2.59 | Down |
| hsa-miR-1228-3p | hsa-mir-1228 | 0.52 | 1.35 | −2.6 | Down |
| hsa-miR-1294 | hsa-mir-1294 | 0.69 | 1.85 | −2.68 | Down |
| hsa-miR-3177-5p | hsa-mir-3177 | 0.69 | 1.85 | −2.68 | Down |
| hsa-miR-4768-5p | hsa-mir-4768 | 0.52 | 1.43 | −2.75 | Down |
| hsa-miR-197-5p | hsa-mir-197 | 0.69 | 2.1 | −3.04 | Down |
| hsa-miR-1972 | hsa-mir-1972-1 | 0.35 | 1.09 | −3.11 | Down |
| hsa-miR-1972 | hsa-mir-1972-2 | 0.35 | 1.09 | −3.11 | Down |
| hsa-miR-3691-5p | hsa-mir-3691 | 1.13 | 3.7 | −3.27 | Down |
| hsa-miR-548al | hsa-mir-548al | 0.35 | 1.18 | −3.37 | Down |
| hsa-miR-4766-3p | hsa-mir-4766 | 0.43 | 1.77 | −4.12 | Down |
| hsa-miR-3918 | hsa-mir-3918 | 0.26 | 1.09 | −4.19 | Down |
| hsa-miR-548t-5p | hsa-mir-548t | 0.26 | 1.09 | −4.19 | Down |
| hsa-miR-6783-3p | hsa-mir-6783 | 0.26 | 1.09 | −4.19 | Down |
| hsa-miR-155-3p | hsa-mir-155 | 0.26 | 1.18 | −4.54 | Down |
| hsa-miR-184 | hsa-mir-184 | 0.61 | 2.78 | −4.56 | Down |
| hsa-miR-6859-5p | hsa-mir-6859-1 | 0.17 | 1.18 | −6.94 | Down |
| hsa-miR-6859-5p | hsa-mir-6859-2 | 0.17 | 1.18 | −6.94 | Down |
| hsa-miR-6859-5p | hsa-mir-6859-3 | 0.17 | 1.18 | −6.94 | Down |
| hsa-miR-6859-5p | hsa-mir-6859-4 | 0.17 | 1.18 | −6.94 | Down |
| hsa-miR-1304-5p | hsa-mir-1304 | 0.09 | 1.43 | −15.89 | Down |
| hsa-miR-891a-5p | hsa-mir-891a | 0.09 | 1.43 | −15.89 | Down |
References
- Sheu, C.C.; Chang, W.A.; Tsai, M.J.; Liao, S.H.; Chong, I.W.; Kuo, P.L. Bioinformatic analysis of nextgeneration sequencing data to identify dysregulated genes in fibroblasts of idiopathic pulmonary fibrosis. Int. J. Mol. Med. 2019, 4080, 1643–1656. [Google Scholar]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Barratt, S.L.; Creamer, A.; Hayton, C.; Chaudhuri, N. Idiopathic Pulmonary Fibrosis (IPF): An Overview. J. Clin. Med. 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef]
- Vancheri, C.; Failla, M.; Crimi, N.; Raghu, G. Idiopathic pulmonary fibrosis: A disease with similarities and links to cancer biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2016, 13, 417–421. [Google Scholar] [CrossRef]
- Ramos, C.; Montano, M.; Garcia-Alvarez, J.; Ruiz, V.; Uhal, B.D.; Selman, M.; Pardo, A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am. J. Respir. Cell Mol. Biol. 2001, 24, 591–598. [Google Scholar] [CrossRef]
- Pierce, E.M.; Carpenter, K.; Jakubzick, C.; Kunkel, S.L.; Evanoff, H.; Flaherty, K.R.; Martinez, F.J.; Toews, G.B.; Hogaboam, C.M. Idiopathic pulmonary fibrosis fibroblasts migrate and proliferate to CC chemokine ligand 21. Eur. Respir. J. 2007, 29, 1082–1093. [Google Scholar] [CrossRef]
- Vuga, L.J.; Ben-Yehudah, A.; Kovkarova-Naumovski, E.; Oriss, T.; Gibson, K.F.; Feghali-Bostwick, C.; Kaminski, N. WNT5A is a regulator of fibroblast proliferation and resistance to apoptosis. Am. J. Respir. Cell Mol. Biol. 2009, 41, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.J.; Boots, A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Dobashi, K.; Sano, T.; Yamada, M. ROCK activation in lung of idiopathic pulmonary fibrosis with oxidative stress. Int. J. Immunopathol. Pharmacol. 2014, 27, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Oldham, J.M.; Ma, S.F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Azuma, A.; Nukiwa, T.; Tsuboi, E.; Suga, M.; Abe, S.; Nakata, K.; Taguchi, Y.; Nagai, S.; Itoh, H.; Ohi, M.; et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Bradford, W.Z.; Castro-Bernardini, S.; Fagan, E.A.; Glaspole, I.; Glassberg, M.K.; Gorina, E.; Hopkins, P.M.; Kardatzke, D.; Lancaster, L.; et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Costabel, U.; Glaspole, I.; Glassberg, M.K.; Lancaster, L.H.; Lederer, D.J.; Pereira, C.A.; Trzaskoma, B.; Morgenthien, E.A.; Limb, S.L.; et al. Efficacy of Pirfenidone in the Context of Multiple Disease Progression Events in Patients With Idiopathic Pulmonary Fibrosis. Chest 2018, 155, 712–719. [Google Scholar] [CrossRef]
- Richeldi, L.; Costabel, U.; Selman, M.; Kim, D.S.; Hansell, D.M.; Nicholson, A.G.; Brown, K.K.; Flaherty, K.R.; Noble, P.W.; Raghu, G.; et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2011, 365, 1079–1087. [Google Scholar] [CrossRef]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef]
- Kolb, M.; Raghu, G.; Wells, A.U.; Behr, J.; Richeldi, L.; Schinzel, B.; Quaresma, M.; Stowasser, S.; Martinez, F.J.; Investigators, I. Nintedanib plus Sildenafil in Patients with Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 379, 1722–1731. [Google Scholar] [CrossRef]
- Kropski, J.A.; Blackwell, T.S. Progress in Understanding and Treating Idiopathic Pulmonary Fibrosis. Annu. Rev. Med. 2019, 70, 211–224. [Google Scholar] [CrossRef]
- Casanova, E.; Salvado, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods, Release 3; Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2011.
- Oz, H.S. Chronic Inflammatory Diseases and Green Tea Polyphenols. Nutrients 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 modified liposome encapsulating epigallocatechin-3-gallate in the presence of magnesium: Characterization and protective effect against oxidative damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chemico-Biol. Interact. 2009, 180, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, L.; Ding, Y.; Yang, K.; Chen, Z.; Fan, X.; Jiang, S.; Guan, Y.; Liu, Z.; Xu, D.; et al. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future Med. Chem. 2018, 10, 795–809. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-beta1 signalling. Clin. Exp. Pharm. Phys. 2015, 42, 849–859. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Enhancement of antioxidant defense system by epigallocatechin-3-gallate during bleomycin induced experimental pulmonary fibrosis. Biol. Pharm. Bull. 2008, 31, 1306–1311. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharm. Ther. 2009, 22, 221–236. [Google Scholar] [CrossRef]
- You, H.; Wei, L.; Sun, W.L.; Wang, L.; Yang, Z.L.; Liu, Y.; Zheng, K.; Wang, Y.; Zhang, W.J. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int. J. Mol. Med. 2014, 34, 92–102. [Google Scholar] [CrossRef]
- Dweep, H.; Gretz, N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin Gallate: A Review of Its Beneficial Properties to Prevent Metabolic Syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Javaid, M.S.; Latief, N.; Ijaz, B.; Ashfaq, U.A. Epigallocatechin Gallate as an anti-obesity therapeutic compound: An in silico approach for structure-based drug designing. Nat. Prod. Res. 2018, 32, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Mandal, A.K.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef]
- Pandima Devi, K.; Rajavel, T.; Daglia, M.; Nabavi, S.F.; Bishayee, A.; Nabavi, S.M. Targeting miRNAs by polyphenols: Novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017, 46, 146–157. [Google Scholar] [CrossRef]
- Sueoka, N.; Suganuma, M.; Sueoka, E.; Okabe, S.; Matsuyama, S.; Imai, K.; Nakachi, K.; Fujiki, H. A new function of green tea: Prevention of lifestyle-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gowdy, K.M.; Fessler, M.B. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm. Pharm. Therap. 2013, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Ishii, H.; Iwata, A.; Kushima, H.; Toba, S.; Hashinaga, K.; Umeki, K.; Tokimatsu, I.; Hiramatsu, K.; Kadota, J. Inhibitory effects of pitavastatin on fibrogenic mediator production by human lung fibroblasts. Life Sci. 2013, 93, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Watts, K.L.; Sampson, E.M.; Schultz, G.S.; Spiteri, M.A. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2005, 32, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Rhee, C.K.; Kim, T.J.; Kim, Y.H.; Lee, S.H.; Yoon, H.K.; Kim, S.C.; Lee, S.Y.; Kwon, S.S.; Kim, K.H.; et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin. Exp. Pharm. Phys. 2010, 37, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Podolanczuk, A.J.; Raghu, G.; Tsai, M.Y.; Kawut, S.M.; Peterson, E.; Sonti, R.; Rabinowitz, D.; Johnson, C.; Barr, R.G.; Hinckley Stukovsky, K.; et al. Cholesterol, lipoproteins and subclinical interstitial lung disease: The MESA study. Thorax 2017, 72, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cao, G.; Jing, L.; Lin, S.; Wang, X.; Zhang, J.; Wang, M.; Liu, W.; Lv, C. Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J. Cell Mol. Med. 2014, 18, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Zaiman, A.; Champion, H.C. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, 24–33. [Google Scholar] [CrossRef]
- Coriati, A.; Arslanian, E.; Bouvet, G.F.; Prat, A.; Seidah, N.G.; Rabasa-Lhoret, R.; Berthiaume, Y. Proprotein Convertase Subtilisin/Kexin type 9 affects insulin but not lipid metabolism in cystic fibrosis. Clin. Investig. Med. 2017, 40, 59–65. [Google Scholar] [CrossRef]
- Lai, Q.; Giralt, A.; Le May, C.; Zhang, L.; Cariou, B.; Denechaud, P.D.; Fajas, L. E2F1 inhibits circulating cholesterol clearance by regulating Pcsk9 expression in the liver. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Pandit, K.V.; Milosevic, J.; Kaminski, N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 2011, 157, 191–199. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rajaguru, P.; Sudhakar Gandhi, P.S. MicroRNAs as potential targets for progressive pulmonary fibrosis. Front. Pharm. 2015, 6, 254. [Google Scholar] [CrossRef]
- Pottier, N.; Maurin, T.; Chevalier, B.; Puissegur, M.P.; Lebrigand, K.; Robbe-Sermesant, K.; Bertero, T.; Lino Cardenas, C.L.; Courcot, E.; Rios, G.; et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: Implication in epithelial-mesenchymal interactions. PLoS ONE 2009, 4, e6718. [Google Scholar] [CrossRef]
- Cushing, L.; Kuang, P.P.; Qian, J.; Shao, F.; Wu, J.; Little, F.; Thannickal, V.J.; Cardoso, W.V.; Lu, J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 287–294. [Google Scholar] [CrossRef]
- Cui, H.; Ge, J.; Xie, N.; Banerjee, S.; Zhou, Y.; Antony, V.B.; Thannickal, V.J.; Liu, G. miR-34a Inhibits Lung Fibrosis by Inducing Lung Fibroblast Senescence. Am. J. Respir Cell Mol. Biol. 2017, 56, 168–178. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Yang, C.S.; Maliakal, P.; Meng, X. Inhibition of carcinogenesis by tea. Annu. Rev. Pharm. Toxicol. 2002, 42, 25–54. [Google Scholar] [CrossRef]
- Wu, Y.R.; Choi, H.J.; Kang, Y.G.; Kim, J.K.; Shin, J.W. In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int. J. Nanomed. 2017, 12, 7007–7013. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults - Results of a systematic review. Regul. Toxicol. Pharm. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar]
- Witschi, H.; Espiritu, I.; Ly, M.; Uyeminami, D.; Morin, D.; Raabe, O.G. Chemoprevention of tobacco smoke-induced lung tumors by inhalation of an epigallocatechin gallate (EGCG) aerosol: A pilot study. Inhal. Toxicol. 2004, 16, 763–770. [Google Scholar] [CrossRef]
- Sheu, C.C.; Tsai, M.J.; Chen, F.W.; Chang, K.F.; Chang, W.A.; Chong, I.W.; Kuo, P.L.; Hsu, Y.L. Identification of novel genetic regulations associated with airway epithelial homeostasis using next-generation sequencing data and bioinformatics approaches. Oncotarget 2017, 8, 82674–82688. [Google Scholar] [CrossRef]
- Chang, W.A.; Tsai, M.J.; Jian, S.F.; Sheu, C.C.; Kuo, P.L. Systematic analysis of transcriptomic profiles of COPD airway epithelium using next-generation sequencing and bioinformatics. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2387–2398. [Google Scholar] [CrossRef]
- Tsai, M.J.; Chang, W.A.; Jian, S.F.; Chang, K.F.; Sheu, C.C.; Kuo, P.L. Possible mechanisms mediating apoptosis of bronchial epithelial cells in chronic obstructive pulmonary disease—A next-generation sequencing approach. Pathol. Res. Pract. 2018, 214, 1489–1496. [Google Scholar] [CrossRef]
- Tsai, M.J.; Tsai, Y.C.; Chang, W.A.; Lin, Y.S.; Tsai, P.H.; Sheu, C.C.; Kuo, P.L.; Hsu, Y.L. Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease-A Next-Generation Sequencing-Guided Bioinformatic Approach. Int. J. Mol. Sci. 2019, 20, 553. [Google Scholar] [CrossRef]
- Lee, W.H.; Tsai, M.J.; Chang, W.A.; Wu, L.Y.; Wang, H.Y.; Chang, K.F.; Su, H.M.; Kuo, P.L. Deduction of novel genes potentially involved in hypoxic AC16 human cardiomyocytes using next-generation sequencing and bioinformatics approaches. Int. J. Mol. Med. 2018, 42, 2489–2502. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Siren, J.; Valimaki, N.; Makinen, V. Indexing Graphs for Path Queries with Applications in Genome Research. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 11, 375–388. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 1, 284–287. [Google Scholar] [CrossRef]
- Galipon, J.; Ishii, R.; Suzuki, Y.; Tomita, M.; Ui-Tei, K. Differential Binding of Three Major Human ADAR Isoforms to Coding and Long Non-Coding Transcripts. Genes 2017, 8, 68. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Vejnar, C.E.; Zdobnov, E.M. MiRmap: Comprehensive prediction of microRNA target repression strength. Nucleic Acids Res. 2012, 40, 11673–11683. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, 362–368. [Google Scholar] [CrossRef]
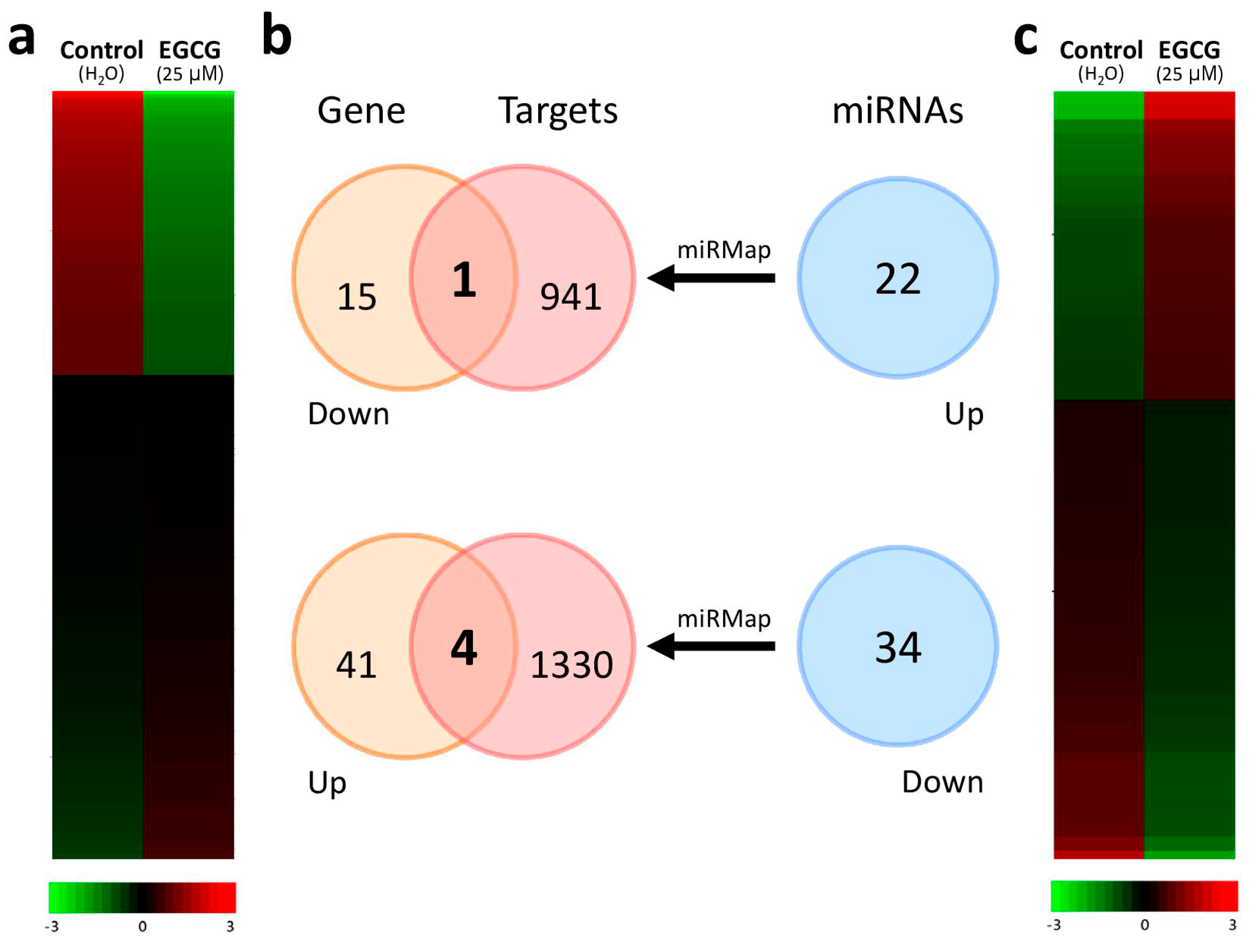
| Gene | miRNA | microRNA-Target Gene Prediction in Various Databases * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Official Symbol | Gene Name | Log2 (ratio) | miRNA Name | Fold Change | mirmap Score | miRWalk | Microt4 | miRanda | miRDB | RNA22 | RNAhybrid | TargetScan |
| PLXNA4 | plexin A4 | −1.32 | hsa-miR-939-5p | 4.85 | 99.97 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| CTIF | cap binding complex dependent translation initiation factor | 2.52 | hsa-miR-3918 | −4.19 | 99.97 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| PDE5A | phosphodiesterase 5A | 1.14 | hsa-miR-4768-5p | −2.75 | 99.34 | Yes | Yes | Yes | No | No | Yes | Yes |
| VPS53 | VPS53, GARP complex subunit | 2.32 | hsa-miR-1273g-3p | −2.03 | 99.76 | Yes | Yes | Yes | No | No | Yes | Yes |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 | 3.27 | hsa-miR-1972 | −3.11 | 99.58 | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Category and Term | Gene Count | Genes | Fold Enrichment | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|
| Cellular components | |||||
| Endoplasmic reticulum | 15 | GABARAPL1, MSMO1, HMGCR, CYP51A1, SCD, FDFT1, SQSTM1, SQLE, DHCR7, INSIG1, CEMIP, PCSK9, HSD17B7, NSDHL, DHCR24 | 5.41 | 3.54 × 10−7 | 3.75 × 10−5 |
| Endoplasmic reticulum membrane | 14 | SC5D, MSMO1, CYP51A1, SQLE, HMGCR, SCD, DHCR7, INSIG1, LSS, ABCA1, HSD17B7, NSDHL, FDFT1, DHCR24 | 4.85 | 3.55 × 10−6 | 1.88 × 10−4 |
| Biological processes | |||||
| Cholesterol biosynthetic process | 15 | MSMO1, MVD, HMGCR, CYP51A1, HMGCS1, FDPS, LSS, FDFT1, SQLE, DHCR7, INSIG1, IDI1, HSD17B7, NSDHL, DHCR24 | 108.66 | 9.71 × 10−26 | 4.19 × 10−23 |
| Isoprenoid biosynthetic process | 6 | MVD, HMGCR, FDPS, HMGCS1, IDI1, FDFT1 | 117.98 | 9.59 × 10−10 | 2.07 × 10−7 |
| Oxidation-reduction process | 11 | SC5D, MSMO1, SQLE, HMGCR, CYP51A1, SCD, DHCR7, HSD17B7, NSDHL, FDFT1, DHCR24 | 5.11 | 4.23 × 10−5 | 0.0061 |
| Cholesterol biosynthetic process via lathosterol | 3 | SC5D, DHCR7, DHCR24 | 206.46 | 7.50 × 10−5 | 0.0080 |
| Cholesterol biosynthetic process via desmosterol | 3 | SC5D, DHCR7, DHCR24 | 206.46 | 7.50 × 10−5 | 0.0080 |
| Cholesterol homeostasis | 5 | TMEM97, FABP3, PCSK9, MYLIP, ABCA1 | 21.51 | 7.97 × 10−5 | 0.0068 |
| Steroid biosynthetic process | 4 | CYP51A1, LSS, NSDHL, FDFT1 | 34.41 | 0.0002 | 0.0143 |
| Description | Count | p-value | Adjusted p-value * | Genes | Fold Enrichment |
|---|---|---|---|---|---|
| Steroid biosynthesis | 10 | 2.11 × 10−16 | 1.42 × 10−14 | SC5D, MSMO1, SQLE, CYP51A1, DHCR7, LSS, HSD17B7, NSDHL, FDFT1, DHCR24 | 90.51 |
| Biosynthesis of antibiotics | 14 | 2.91 × 10−11 | 9.31 × 10−10 | SC5D, MSMO1, MVD, CYP51A1, SQLE, HMGCR, FDPS, HMGCS1, LSS, IDI1, ACSS2, HSD17B7, NSDHL, FDFT1 | 11.95 |
| Terpenoid backbone biosynthesis | 5 | 4.84 × 10−6 | 1.03 × 10−4 | MVD, HMGCR, FDPS, HMGCS1, IDI1 | 41.14 |
| Metabolic pathways | 19 | 1.53 × 10−5 | 2.45 × 10−4 | SC5D, MSMO1, MVD, CYP51A1, HMGCR, HMGCS1, FDPS, LSS, ACSS2, FDFT1, SQLE, DHCR7, QPRT, PLA2G3, ITPK1, IDI1, HSD17B7, NSDHL, DHCR24 | 2.82 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-J.; Chang, W.-A.; Liao, S.-H.; Chang, K.-F.; Sheu, C.-C.; Kuo, P.-L. The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)—A Next-Generation Sequencing and Bioinformatic Approach. Int. J. Mol. Sci. 2019, 20, 1958. https://doi.org/10.3390/ijms20081958
Tsai M-J, Chang W-A, Liao S-H, Chang K-F, Sheu C-C, Kuo P-L. The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)—A Next-Generation Sequencing and Bioinformatic Approach. International Journal of Molecular Sciences. 2019; 20(8):1958. https://doi.org/10.3390/ijms20081958
Chicago/Turabian StyleTsai, Ming-Ju, Wei-An Chang, Ssu-Hui Liao, Kuo-Feng Chang, Chau-Chyun Sheu, and Po-Lin Kuo. 2019. "The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)—A Next-Generation Sequencing and Bioinformatic Approach" International Journal of Molecular Sciences 20, no. 8: 1958. https://doi.org/10.3390/ijms20081958
APA StyleTsai, M.-J., Chang, W.-A., Liao, S.-H., Chang, K.-F., Sheu, C.-C., & Kuo, P.-L. (2019). The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)—A Next-Generation Sequencing and Bioinformatic Approach. International Journal of Molecular Sciences, 20(8), 1958. https://doi.org/10.3390/ijms20081958